Synthesis,Spectroscopic Characterization,Thermogravimetric and Biological Activity Evaluation of Te(Ⅳ),Se(Ⅳ),V(Ⅲ),Nb(Ⅴ),Ta(Ⅴ) Complexes With Indole-3-Acetic Acid Plant Hormone Ligand
Jehan Y.Al-Humaidi,Foziah A.Al-Saif,Dalal N.Binjawhar,Hanan A.Bakhsh,Moamen S.Refat
1.Department of Chemistry,College of Science,Princess Nourah bint Abdulrahman University,Riyadh 11671,Saudi Arabia 2.Department of Chemistry,Faculty of Science,Taif University,Al-Haweiah 21974,Taif,Saudi Arabia
Abstract Te(Ⅳ),Se(Ⅳ),V(Ⅲ),Nb(Ⅴ) and Ta(Ⅴ) complexes of indole-3-acetic acid (IAAH) ligand were synthesized,characterized by elemental analysis and various spectroscopic techniques like,IR,1H-NMR,X-ray powder diffraction,UV-Visible,thermogravimetry analysis,magnetic measurements,molar conductance and surface morphology using SEM.All the synthesized complexes of IAAH ligand have 1∶2 stoichiometry of the types [Te(IAA)2(NH3)2]·2Cl (Ⅰ),[Se(IAA)2(NH3)2]·2Cl (Ⅱ),[V(IAA)2(NH3)(Cl)] (Ⅲ),[Nb(IAA)2(Cl)3] (Ⅳ),and [Ta(IAA)2(Cl)3] (Ⅴ).Spectral analysis indicates octahedral geometry for the Te(Ⅳ),Se(Ⅳ) and V(Ⅲ) complexes,whereas both Nb(Ⅴ) and Ta(Ⅴ) have a seven-coordination.The bonding sites are the oxygen atoms of carboxylate group for the deprotonated indole-3-acetic acid (IAA) ligand.The thermogravimetry analysis studies gave evidence for the presence of other coordinated molecules (Cl or NH3) in the composition of IAA complexes,which were further supported by IR and micro analytical measurements.The higher molar conductance data of tellurium and selenium (Ⅳ) complexes reveal that these chelates are electrolytes,while low conductivity values for the vanadium(Ⅲ),niobium and tantalum(Ⅴ) chelates indicated a non-electrolytes.To test the antibacterial property of the five complexes in this study,four bacterial strains Klebsiella (G-),Escherichia coli (G-),Staphylococcus aureus (G+) and Staphylococcus epidermidis (G+) were used in the investigation.The effects of the five complexes in the cytotoxicity of Caco-2 and Mcf-7 human cancer cell lines were studied Neutral red uptake assay for the estimation of cell viability/cytotoxicity protocol.
Keywords Indole-3-acetic acid;chelation;IR;Metal ions;TEM;Antimicrobial test
Introduction
The indole-3-acetic acid (IAAH;Fig.1) is a dominant type of auxin found in plants and is involved in the growth responses of plants for the regulation of cell elongation,cell division,cell differentiation,root initiation and synthetic auxins are an important class of selective herbicides[1-2].IAAH has been produced not only in plants,but also by plant-associated microorganisms including ectomycorrhizal,endophytic,pathogenic,phyllosphere and rhizospheric microbes[3-6].
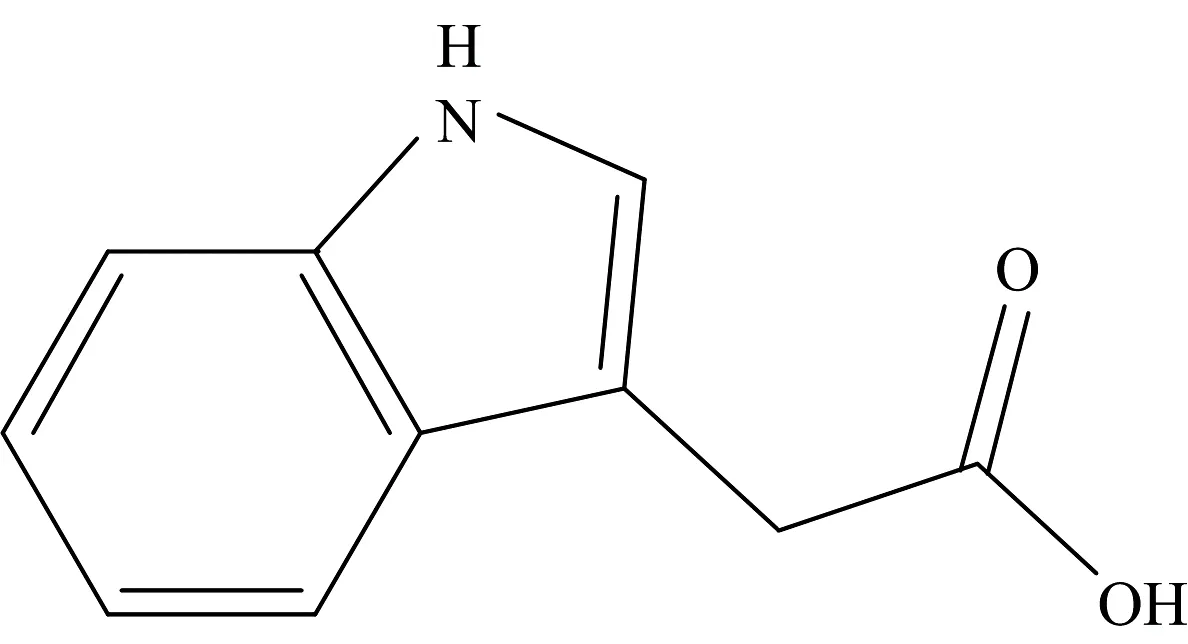
Fig.1 Structure of indole-3-acetic acid (IAAH)
Indole-3-acetic acid is a monocarboxylic acid that is acetic acid in which one of the methyl hydrogens has been replaced by a1H-indol-3-yl group.It has a role as a plant hormone,a human metabolite,a plant metabolite,a mouse metabolite and an auxin.It is a monocarboxylic acid and a member of indole-3-acetic acids.Metal complexes have important applications in many aspects of life.In recent years,the successful development of metal-based pharmaceuticals has attracted more attention on metal complexes in microbial treatment[7-9].Carboxylate ligands can bind to metal ions in several ways,such as monodentate,bidentate,bridging etc.These binding modes complicate selective synthesis of metal carboxylate complexes[10-12].A literature survey reveals that few research papers has been reported on the metal chelation of indole-3-acetic acid[10-12],so it seems important to conduct investigations of IAAH metal complexes.In continuation of our published work[7-9]on the coordination chemistry of pharmaceutical and bioactive ligands,we report in the present article a synthesis,spectroscopic characterization,and thermogravimetric measurements of Te(Ⅳ),Se(Ⅳ),V(Ⅲ),Nb(Ⅴ),Ta(Ⅴ) complexes with indole-3-acetic acid plant hormone ligand.The study also includes an antimicrobial and anticancer investigations.
1 Experimental
1.1 Chemicals and instruments
The chemicals presented in this article are of analytical grade and were used without further purification.These chemicals (Indole-3-acetic acid,TeCl4,SeCl4,VCl3,NbCl5and TaCl5) were received from Sigma-Aldrich Chemical Company,USA.The analyses and the corresponding models are summarized as follows:
1.2 Synthesis
The five IAA solid complexes were prepared by mixing 1.0 mmol of TeCl4,SeCl4,VCl3,NbCl5or TaCl5in 25 mL of methanol solvent with 2.0 mmol IAAH (0.350 g) in 25 mL of methanol.The reaction mixtures were neutralized between pH=7~8 by 5% ammonia solution,then refluxed in a water bath for about 2 h to give the precipitate.After cooling to room temperature,the solid complexes were filtered as fine precipitates.The precipitates were washed with hot methanol.Then they were dried and stored in a desiccators containing dry calcium chloride.The yield of the products was about 80%~87%.The solid products have a higher melting point >250 ℃.The micro analytical (calculated/Calc.and experimental/Found) and physical data of the five synthesized complexes are summarized in Table 1.
1.3 Biological tests
Based on disc diffusion method[13],an antibacterial screening for the five IAA complexes was performed against four bacterial strainsKlebsiella,Escherichiacoli,Staphylococcusaureus,andStaphylococcusepidermidis.Inhibition zones diameters around the disc were determined.In vitro the cytotoxic activity of the tested IAA complexes against two human cancer cell lines (colorectal adenocarcinoma (Caco-2) and breast cancer (MCF-7)) was evaluated using the standard neutral red uptake assay[14].
2 Results and discussions
2.1 Elemental analysis and conductance measurements
Elemental analysis was applied to further confirm the composition of these IAA complexes.The contents of carbon,hydrogen,nitrogen and chloride in each complex were consistent with the calculated values (Table 1),which supported the proposed structure of these complexes (Fig.2).The IAAH ligand and its complexes were stable in air.Furthermore,Te(Ⅳ),Se(Ⅳ),V(Ⅲ),Nb(Ⅴ) and Ta(Ⅴ) complexes were soluble in DMSO with gently heating and their molar conductivities (Λm) were 96,105,14,10,and 12 ohm-1·cm2·mol-1,respectively.Consequently,the V(Ⅲ),Nb(Ⅴ) and Ta(Ⅴ) IAA complexes were considered as non-electrolytes while the Te(Ⅳ) and Se(Ⅳ) complexes have an electrolyte feature[15].The obtained complexes were characterized by IR,1H-NMR,TG analysis and elemental analysis.Because these compounds were not crystallized,X-ray crystal structure was not performed.
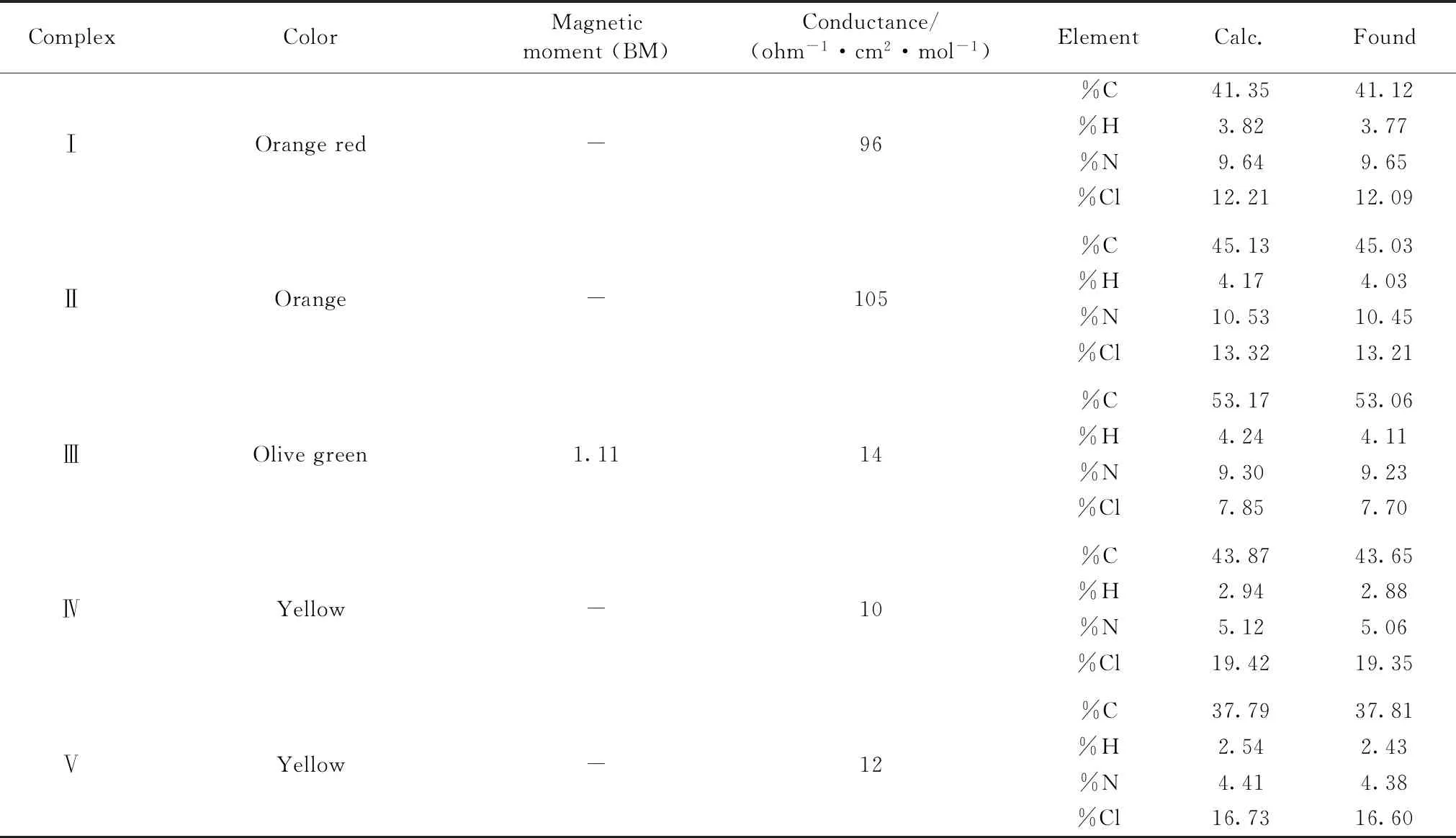
Table 1 Micro analytical and physical data of IAA complexes
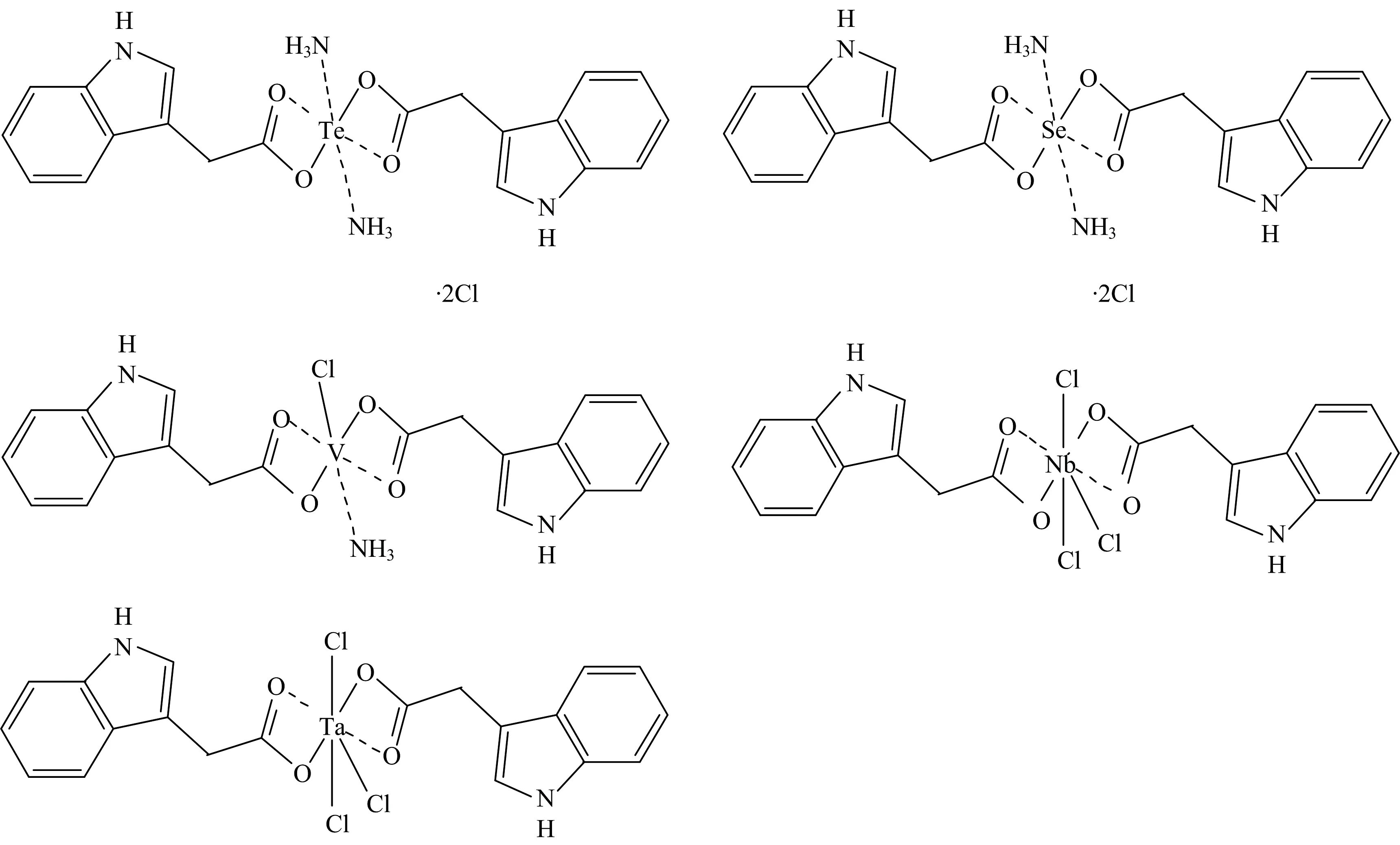
Fig.2 Suggested structures of IAA complexes
2.2 FTIR spectra
The IR spectra of IAA complexes are shown in Fig.3.The important IR spectral bands of IAA complexes are presented in Table 2.According to the IR spectra data of IAAH ligand and complexes (IAA-Te,IAA-Se,IAA-V,IAA-Nb and IAA-Ta),the strong absorption peaks at ~3 400 cm-1belong to the —NH group.No significant shifts were observed on the peaks among this ligand,suggesting that nitrogen atom in the indole ring didn’t coordinate with metal ions.Compared with the IR spectra of the IAA ligand,two new peaks at 1 549 and 1 403 cm-1were assigned toνas(COO-) andνs(COO-),respectively[16].According to Deacon and Phillips[17],a difference larger than >200 cm-1indicates monodentate coordination,whereas one smaller than <200 cm-1indicates a bidentate coordination mode.Regarding IAA complexes (Ⅰ—Ⅴ),Δν=νasym-νsym<200 cm-1,which is confirms that the coordination is bidentate.The absorption vibration frequencies appeared within the 630~500 cm-1range in these complexes were attributed to the formation of M—O bond.A new peak located in the range of 500~400 cm-1in case of tellurium(Ⅳ),selenium(Ⅳ) and vanadium(Ⅲ) complexes,suggesting the formation of M—N bond because of presence NH3group inside the coordination sphere.
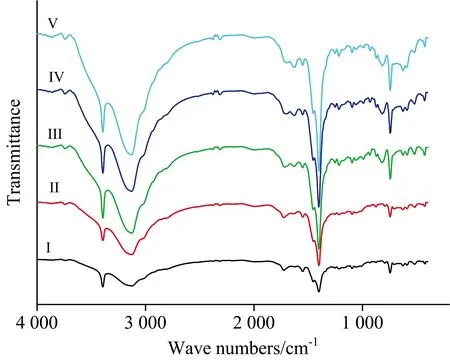
Fig.3 FTIR spectra of IAA complexes Ⅰ—Ⅴ
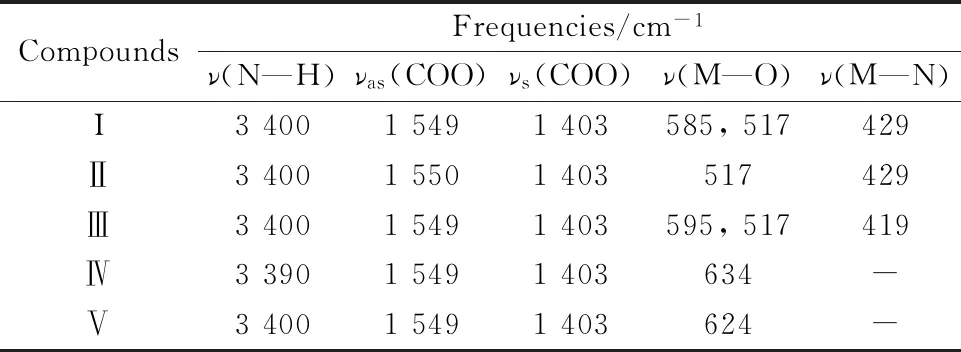
Table 2 Infrared spectral data (cm-1) of IAA complexes Ⅰ—Ⅴ
2.3 Electronic spectra and magnetic susceptibility
The UV-Vis spectrum of indol-3-acetic acid dissolved in DMSO solvent has displayed a two distinguish peaks at 219 and 280 nm that assigned to π—π*electronic transitions[10].For the Te(Ⅳ)-IAA,Se(Ⅳ)-IAA,Nb(Ⅴ)-IAA,and Ta(Ⅴ)-IAA complexes,both the π—π*electronic transition peaks showed a hyperchromic effect with red shifted to 227 and 280~293 nm.This data revealed the involvement of indol-3-acetic acid in the chelation with metal ions.In addition,the IAA complexes show two electronic transition peaks at 330 and 380 nm related to charge transfer MLCT.The effective magnetic moments (μeff) data regarding to Te(Ⅳ),Se(Ⅳ),Nb(Ⅴ),and Ta(Ⅴ) IAA complexes were less than 1.00 B.M.These results deduced that all of these complexes are a diamagnetic properties with a six-coordination for Te(Ⅳ) and Se(Ⅳ) complexes,whereas Nb(Ⅴ) and Ta(Ⅴ) have a seven-coordination mode[18].Concerning the vanadium(Ⅲ) IAA complex,theμeffdata is 1.11 B.M.due to a six-coordination structure for the speculated chelation[19-20].
2.4 1HNMR spectra
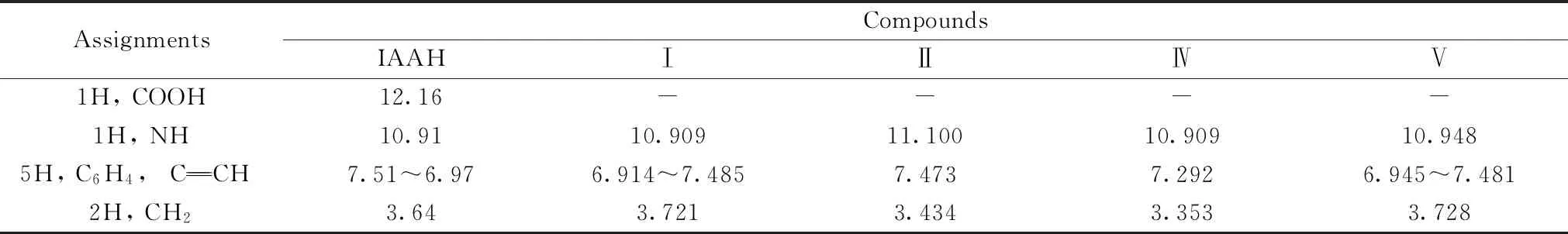
Table 3 1HNMR spectral data (cm-1) of IAA and its complexes (Ⅰ,Ⅱ,Ⅳ,and Ⅴ)
2.5 Thermo gravimetric analysis
The TG analyses of IAA complexes Ⅰ—Ⅴ were performed in the temperature range of 30~700 ℃.The TG curves of the five IAA complexes are difference,further indicating that the five complexes possess a difference metal ions and structures.For clarity’s sake,only the TG curve of complex Ⅲ has been shown in Fig.4.From 30 to 700 ℃,the weight loss of complex Ⅲ is 71%,corresponding to the decomposition of IAA,and release of NH3and HCl molecules,in a good accordance with the calculated value (71.02%).It was found that the five complexes (Ⅰ—Ⅴ) are thermally decomposed with DrTGA at 260,307,257,450,and 254 ℃,respectively.Thermal analysis (TG) of IAA complexes IAA-Te,IAA-Se,IAA-V,IAA-Nb,and IAA-Ta were recorded in the range from 30-to-700 ℃.These complexes were all decomposed actually in one or two steps and the residue rates of them were 21.97%,0.0%,28.98%,16.97%,and 28.47%,respectively,which were assigned to tellurium metal,sublimated selenium,VO2polluted with few carbon atoms,niobium metal,and tantalum metal.
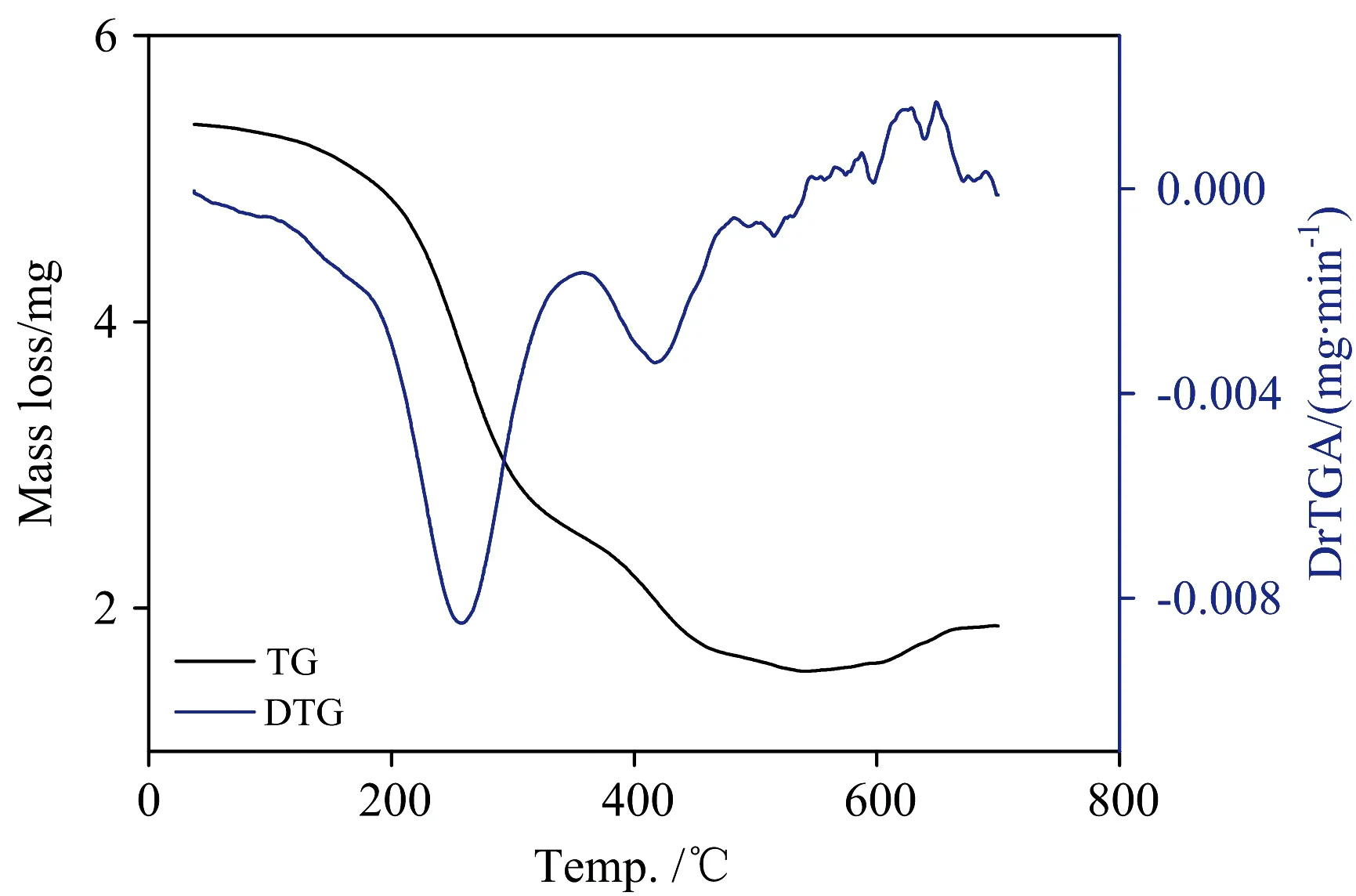
Fig.4 TG-DrTGA curve of [V(IAA)2(NH3)(Cl)] complex(Ⅴ) and tantalum(Ⅴ) complexes have an amorphous structure (Fig.5).The particle size was calculated dependent on Scherrer relationship[21] using full-width at half-maximum (FWHM).
2.6 Morphological analysis
The X-ray powder diffraction patterns of IAA complexes (Ⅰ—Ⅴ) at the range of 2θ=5°~90° are shown in Fig.5,these patterns have three types of structures as semi-crystalline for tellurium(Ⅳ) complex (Ⅰ),crystalline nature for selenium(Ⅳ) and vanadium(Ⅲ) complexes while both niobium
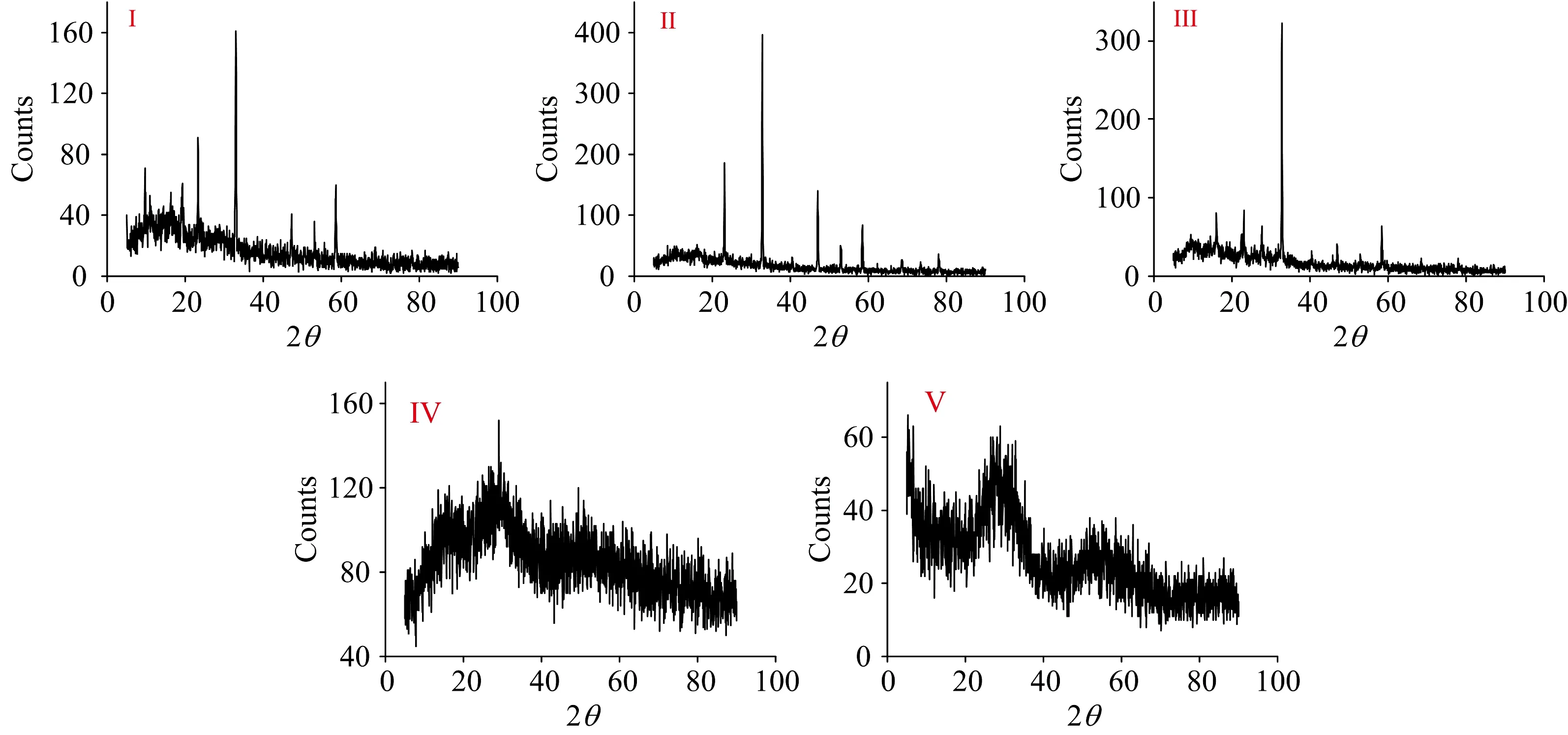
Fig.5 XRD pattern of IAA complexes Ⅰ—Ⅴ
TEM photos of the [Te(IAA)2(NH3)2]·2Cl (Ⅰ) and [Se(IAA)2(NH3)2]·2Cl (Ⅳ) complexes are displayed in Fig.6,the complexes refer to a black spots with spherical shape nanoparticles within 7~12 and 50~90 nm,respectively.SEM images of all IAA complexes (Fig.7) were scanned at energy 30 kV with magnification ×5 000~40 000.Figure 7 show the aggregation of tremendous micro-size fine powders and homogeneous uniform.
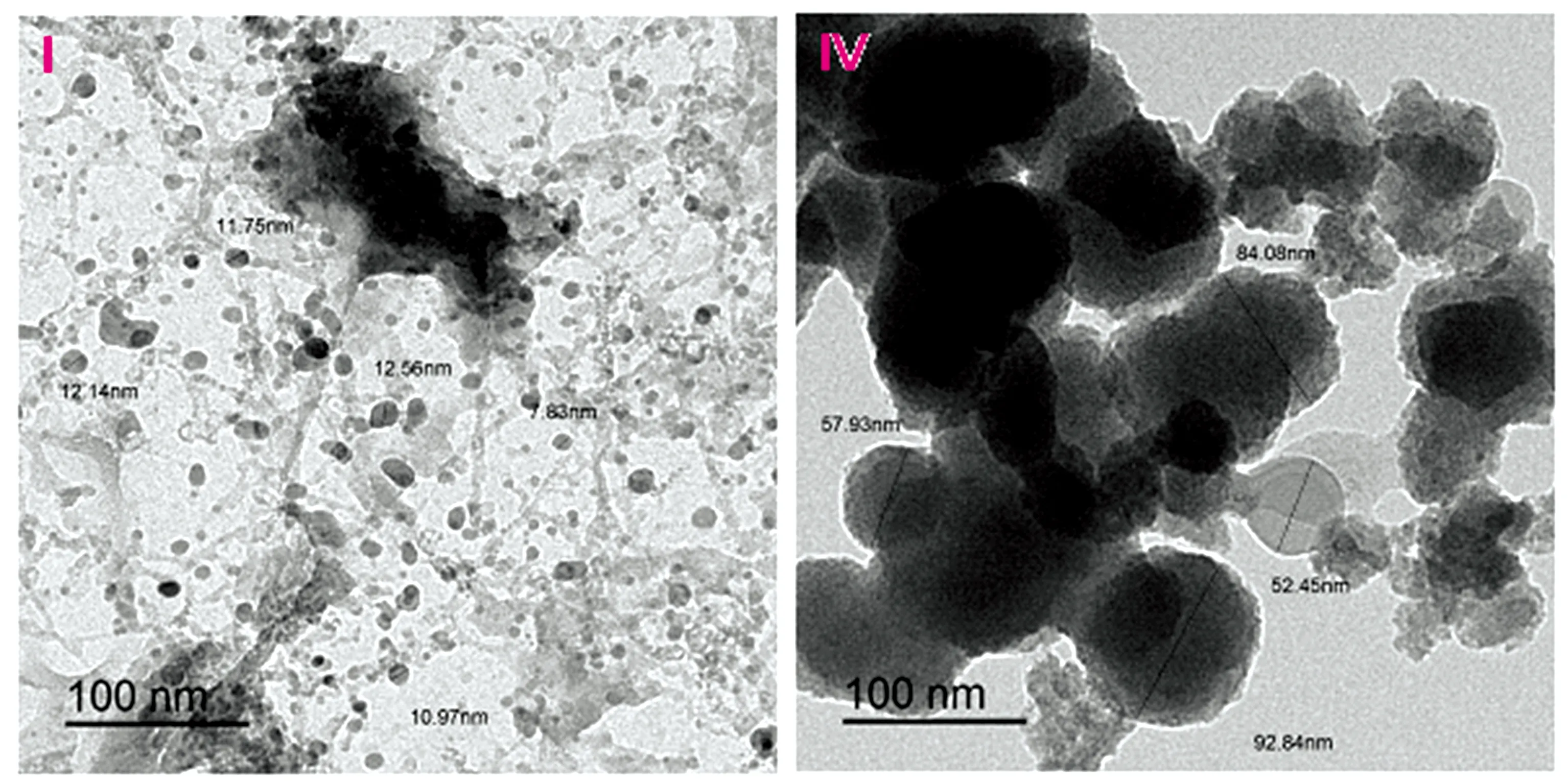
Fig.6 TEM images of (Ⅰ):tellurium and (Ⅳ):niobium complexes
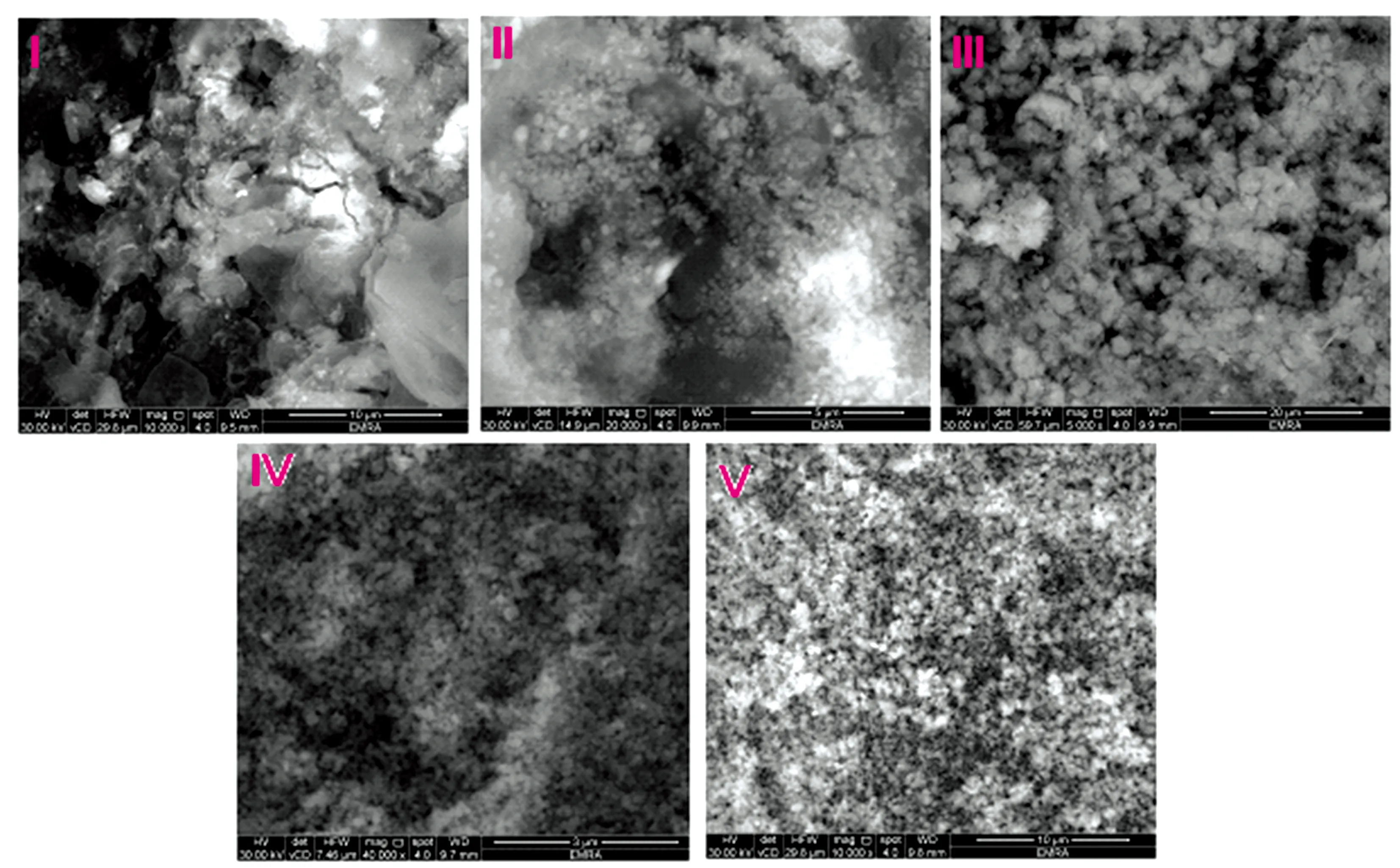
Fig.7 SEM images of IAA complexes Ⅰ—Ⅴ
2.7 Biological results
To test the antibacterial property of the five IAA complexes in this study,four bacterial strainsKlebsiella(g-),Escherichiacoli(g-),Staphylococcusaureus(g+) andStaphylococcusepidermidis(g+) were used in the investigation.Disc diffusion method was used to evaluate the antibacterial activity for the selected strains.Gentamicin and Ceftriaxone were used as positive controls and DMSO,the solvent,was used as negative control in this test.As shown in Table 4 marginal antibacterial activity was observed in all complexes againstKlebsiellastrain only,whereas no antibacterial effect was seen in all complexes against the rest of the tested bacterial strains.
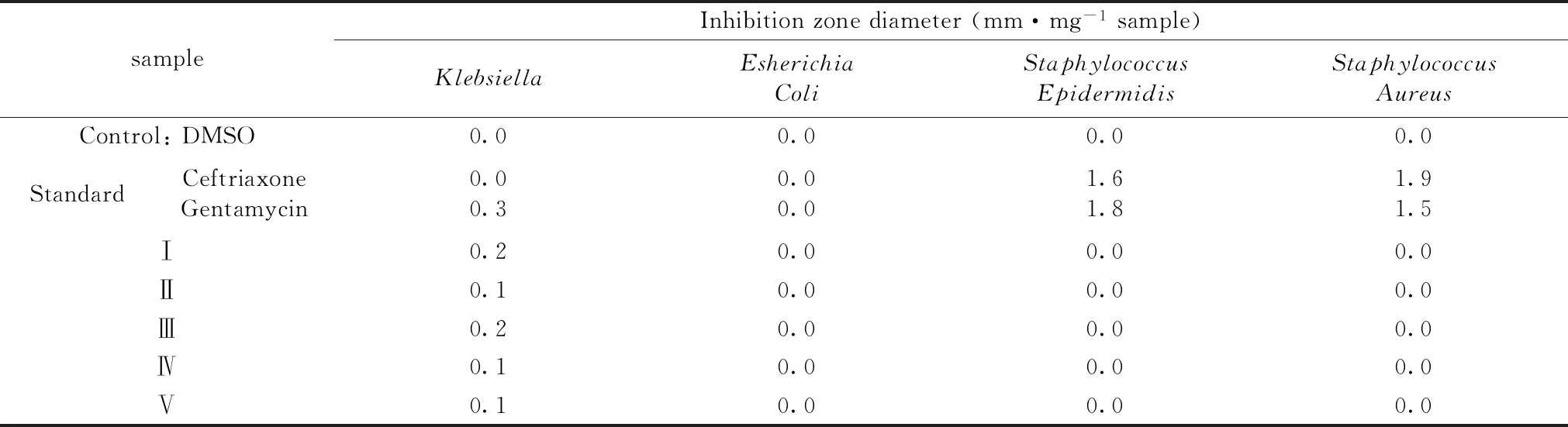
Table 4 Inhibition zone diameter of IAA complexes Ⅰ—Ⅴ against four bacterial strains
The effects of the five IAA complexes in the cytotoxicity of Caco-2 and Mcf-7 human cancer cell lines were studied by NR assay method.The percentage of cellular viability (IC50) for the five IAA complexes against two tumour cell lines Caco-2 and Mcf-7 deduced that only [V(IAA)2(NH3)Cl)] complex can be considered as a potential anticancer agent against colorectal adenocarcinoma cell line with IC50 value of 21.4 μg·mL-1.The IC50values for the rest of IAA complexes against the two tumour cell lines were higher than >100 μg·mL-1.

