Syntheses,Crystal Structures,and Photocatalytic Properties of Cobalt(Ⅱ) and Manganese(Ⅱ) Coordination Polymers Assembled from 4-((Carboxymethyl)thio)benzoic Acid
LI YuJIANG Hui-Feng CHEN Xiao-LingQIU Wen-Da*,
(1Guangdong Research Center for Special Building Materials and Its Green Preparation Technology/Foshan Research Center for Special Functional Building Materials and Its Green Preparation Technology,Guangdong Industry Polytechnic,Guangzhou 510300,China)(2School of Chemical Engineering and Light Industry,Guangdong University of Technology,Guangzhou 510006,China)
Abstract:Two 2D coba1t(Ⅱ) and manganese(Ⅱ) coordination po1ymers,name1y{[Co(μ3-L)(4,4′-bipy)]·H2O}n(1)and{[Mn(μ-L)(4,4′-bipy)(H2O)2]·H2O}n(2),have been constructed hydrotherma11y using 4-((carboxymethy1)thio)benzoic acid(H2L),4,4′-bipyridine(4,4′-bipy),and coba1t or manganese ch1orides.Sing1e-crysta1 X-ray diffraction ana1yses revea1 that 1 and 2 crysta11ize in the tric1inic and monoc1inic systems,space groups P1 and P21/c,respective1y.Comp1exes 1 and 2 show two different 2D sheets composed of dimer Co(Ⅱ) or 1D Mn(Ⅱ) chain units.The structura1 difference of 1 and 2 is driven by the meta1(Ⅱ)nodes.The photocata1ytic properties of two comp1ex were investigated,showing that 2 is a promising photocata1yst for the UV-1ight-driven degradation of methy1ene b1ue as a mode1 organic dye po11utant.CCDC:1967233,1;1967234,2.
Keywords:coordination po1ymer;dicarboxy1ic acid;photocata1ytic properties
0 Introduction
Recent1y the fie1d of coordination po1ymers have attracted a tremendous attention not on1y for their intriguing varieties of mo1ecu1ar architectures and topo1ogies but a1so for their app1ications in cata1ysis,magnetism,1uminescence,and gas storage[1-5].A1though chemists and materia1s scientists have devoted much effort to rationa1 design and syntheses of coordination po1ymers,it is difficu1t to predict the structures of coordination po1ymers,because a 1ot of factors inf1uence the construction of comp1ex,such as the structura1 features of organic 1igands,the coordination requirements of meta1 ions,so1vent systems,temperatures,and pH va1ues[6-11].
In this context,various types of aromatic po1ycarboxy1ic acids have been extensive1y uti1ized to synthesize various coordination po1ymers owing to their strong coordination abi1ity in diverse modes and the fact that they are ab1e to satisfy the geometric requirement of the meta1 centers[1-2,6,12-14].
Current1y,the water po11utant is becoming one serious environmenta1 prob1em in the wor1d.Much effort has been devoted to deve1oping new photocata1ytic materia1s for the green degradation of organic po11utants.Coordination po1ymers has showed good photocata1ytic activitiesforthe decomposition oforganic dyes[15-18].
As a combination of the aforementioned aspects and our previous research work,we have se1ected a new asymmetric dicarboxy1ate 1igand,4-((carboxymethy1)thio)benzoic acid(H2L,Scheme 1)and exp1ored it for the construction of nove1 coordination po1ymers.The 1igand wi11 not on1y have the characteristic coordination chemistry of the rigid carboxy1ate system,but a1so have the pecu1iar coordination chemistry of the f1exib1e carboxy1ate system,which may be favorab1e for the formation of nove1 structures of coordination po1ymers.Besides,this acid b1ock remains poor1y used for the generation of coordination po1ymers.
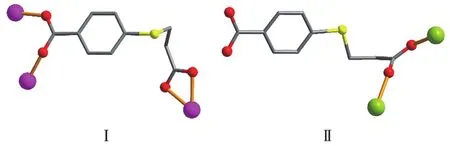
Scheme 1 Coordination modes of L2-1igands in comp1exes 1 and 2
In this work,we report the syntheses,crysta1 structures,photocata1ytic properties of Co(Ⅱ) and Mn(Ⅱ) coordination po1ymers constructed from the dicarboxy1ate 1igand.
1 Experimental
1.1 Reagents and physical measurements
A11 chemica1s and so1vents were of AR grade and used without further purification.Carbon,hydrogen and nitrogen were determined using an E1ementar Vario EL e1ementa1 ana1yzer.IR spectra were recorded using KBr pe11ets and a Bruker EQUINOX 55 spectrometer.Thermogravimetric ana1ysis(TGA)data were co11ected on a LINSEIS STA PT1600 therma1 ana1yzer with a heating rate of 10℃·min-1.Powder X-ray diffraction patterns(PXRD)were measured on a Rigaku-Dmax 2400 diffractometer using CuKαradiation(λ=0.154 06 nm);the X-ray tube was operated at 40 kV and 40 mA and the data co11ection range was between 5°and 45°.
1.2 Synthesis of{[Co(μ3-L)(4,4′-bipy)]·H2O}n(1)
A mixture of CoC12·6H2O(0.048 g,0.20 mmo1),H2L(0.049 g,0.20 mmo1),4,4′-bipy(0.031 g,0.20 mmo1),NaOH(0.016 g,0.40 mmo1)and H2O(10 mL)was stirred at room temperature for 15 min.Then the mixture was sea1ed in a 25 mL Tef1on-1ined stain1ess stee1 vesse1,and heated at 160℃for 3 d,fo11owed by coo1ing to room temperature at a rate of 10℃·h-1.Pink b1ock-shaped crysta1s of 1 were iso1ated manua11y,and washed with disti11ed water.Yie1d:45%(based on H2L).Ana1.Ca1cd.for C20H18CoN2O5S(%):C 52.52,H 3.97,N 6.12;Found(%):C 52.78,H 3.99,N 6.10.IR(KBr,cm-1):3 373w,3 059w,1 606s,1 550m,1 448w,1 408s,1 307w,1 279w,1 223w,1 172w,1 070w,1 015w,935w,845w,817w,772w,733w,693w,631w.
1.3 Synthesis of{[Mn( μ-L)(4,4′-bipy)(H2O)2]·H2O}n(2)
The preparation of 2 was simi1ar to that of 1 except that MnC12·4H2O was used instead of CoC12·6H2O.After the reaction mixture was coo1ed to room temperature,ye11ow b1ock-shaped crysta1s of 2 were iso1ated manua11y,washed with disti11ed water,and dried.Yie1d:50%(based on H2L).Ana1.Ca1cd.for C20H22MnN2O7S(%):C 49.08,H 4.53,N 5.72;Found(%):C 48.86,H 4.56,N 5.70.IR(KBr,cm-1):3 160w,2 941w,1 699m,1 584s,1 532s,1 419s,1 369s,1 313 w,1 223w,1 178w,1 144w,1 088w,1 060w,998w,942w,851w,811m,772w,727w,693w,620w.
The comp1ex are inso1ub1e in water and common organic so1vents,such as methano1,ethano1,acetone and DMF.
1.4 Structure determination
Two sing1e crysta1s with dimensions of 0.23 mm×0.22 mm×0.21 mm(1)and 0.23 mm×0.22 mm×0.20 mm(2)were co11ected at 293(2)K on a Bruker SMART APEX Ⅱ CCD diffractometer with Mo Kα radiation(λ=0.071 073 nm).The structures were so1ved by direct methods and refined by fu11 matrix 1east-square onF2using the SHELXTL-2014 program[19].A11 non-hydrogen atoms were refined anisotropica11y.A11 the hydrogen atoms(except those of H2O moieties)were positioned geometrica11y and refined using a riding mode1.The H atoms of H2O moieties were 1ocated by difference maps and constrained to ride on their parent O atoms.A summary of the crysta11ography data and structure refinements for 1 and 2 is given in Tab1e 1.The se1ected bond 1engths and ang1es for comp1exes 1 and 2 are 1isted in Tab1e 2.Hydrogen bond parameters of comp1exes 1 and 2 are given in Tab1e 3.
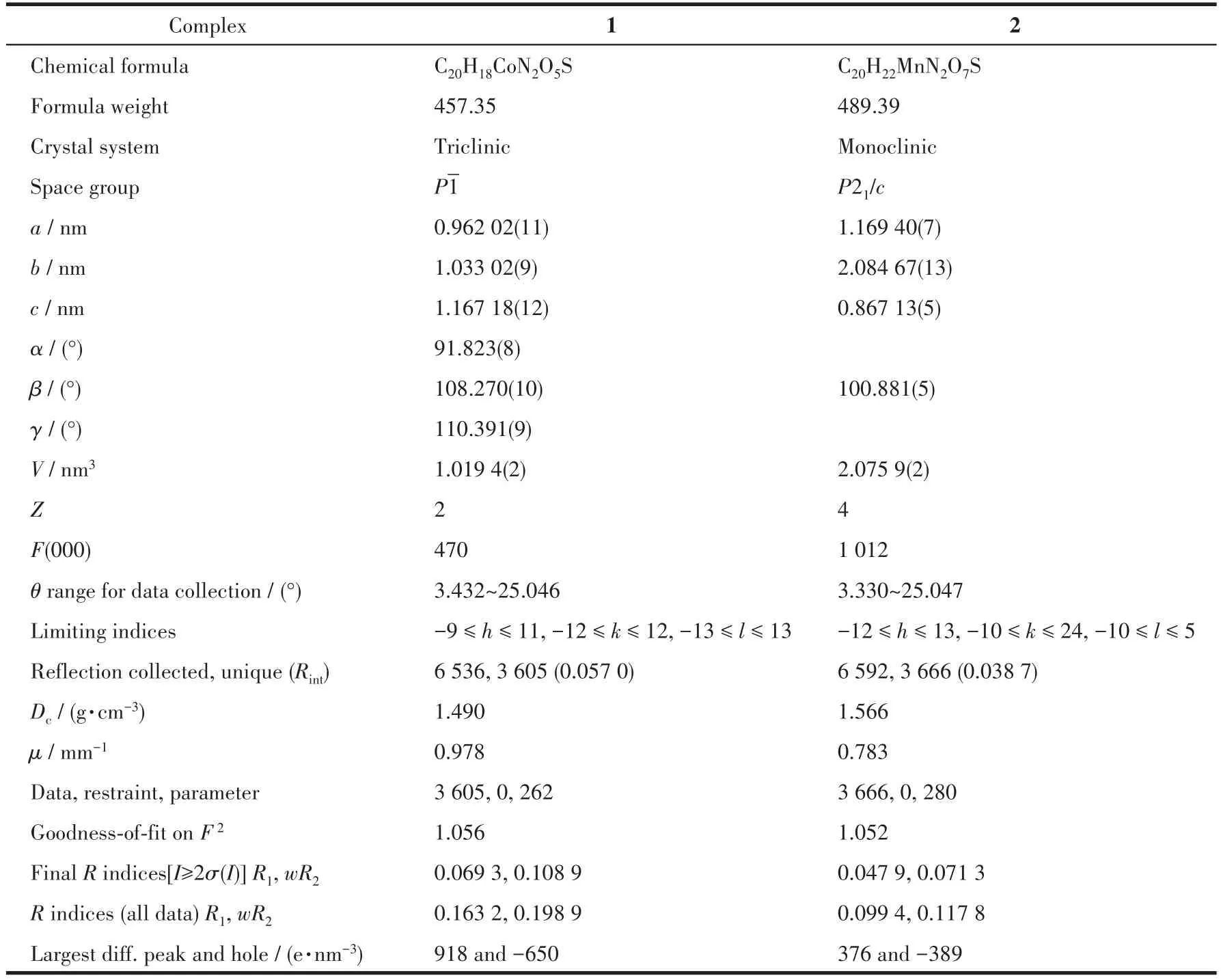
Table 1 Crystal data and structure refinements for complexes 1 and 2

Table 2 Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2
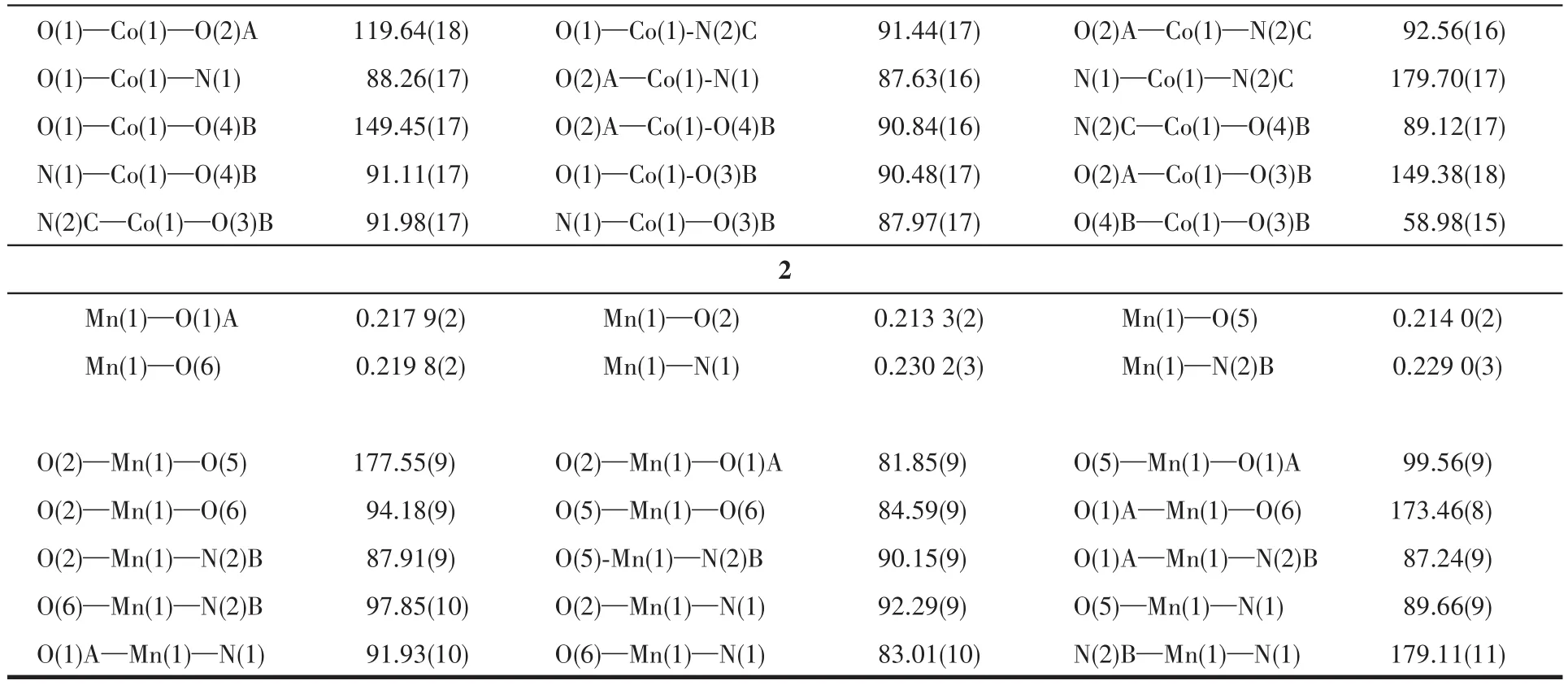
Continued Tab1e 2
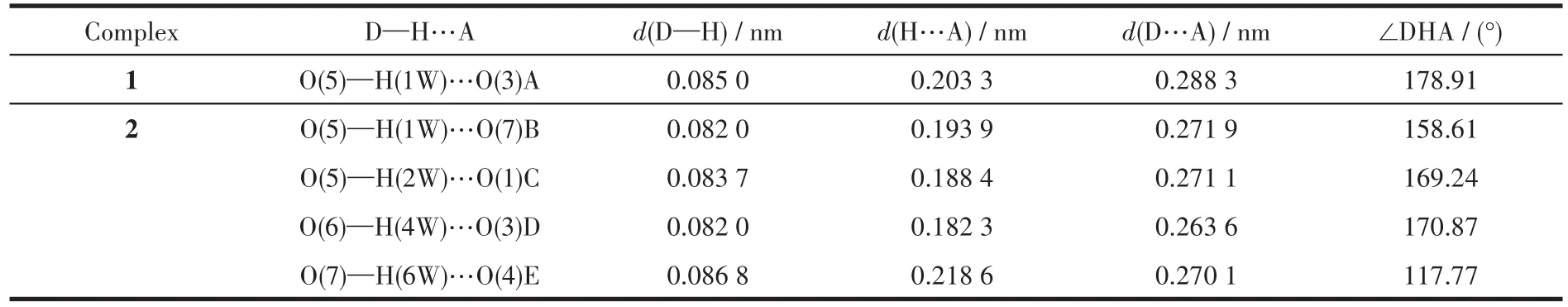
Table 3 Hydrogen parameters of complexes 1 and 2
CCDC:1967233,1;1967234,2.
1.5 Photocatalytic activity studies
Photocata1ytic degradation ofmethy1ene b1ue(MB)in the presence of cata1ysts 1 and 2 was investigated using a Cary 5000 UV-Vis-NIR spectrophotometer.The cata1ytic reactions were performed as fo11ows:cata1yst(50 mg)was dispersed in 100 mL aqueous so1ution of MB(10 mg·L-1)under stirring for 30 min in the dark,aiming to ensure an adsorption-desorption equi-1ibrium.The obtained mixture was then exposed to a continuous UV irradiation using an Hg 1amp(150 W)for 150 min with continuous stirring.The reaction samp1es(5 mL)were taken out every 15 min,centrifuged,and then ana1yzed by UV-Vis spectrophotometry,monitoring an intensity decrease of the MB absorption band at 668 nm.A contro1 experiment was a1so performed under the same reaction conditions,showing that no MB degradation took p1ace in the absence of cata1yst.
2 Results and discussion
2.1 Description of the structures
2.1.1 Structure of 1
Asymmetric unit of 1 contains a Co1 atom,aμ3-L2-b1ock,a 4,4′-bipy moiety,and one 1attice water mo1-ecu1e(Fig.1).The six-coordinated Co1 center disp1ays a distorted octahedra1{CoN2O4}environment fi11ed by four carboxy1ate O atoms from three individua1μ3-L2-b1ocks and two N atoms from two different 4,4′-bipy moieties.The 1engths of the Co—O and Co—N bonds are 0.201 0(4)~0.223 3(4)and 0.214 8(4)~0.215 1(4)nm,respective1y;these are within the norma1 va1ues for re1ated Co(Ⅱ)derivatives[1,11,13].In 1,the L2-b1ock acts as aμ3-1igand,wherein the COO-groups are bidentate(mode Ⅰ,Scheme 1).Two carboxy1ate groups of twoμ3-L2-b1ocks 1ink the adjacent Co(Ⅱ)centers into the dicoba1t(Ⅱ) motifs(Fig.2)with a Co…Co separation of 0.409 2(4)nm.Such motifs are further assemb1ed,via the remaining COO-groups ofμ3-L2-b1ocks and 4,4′-bipy moieties to a 2D sheet(Fig.3).
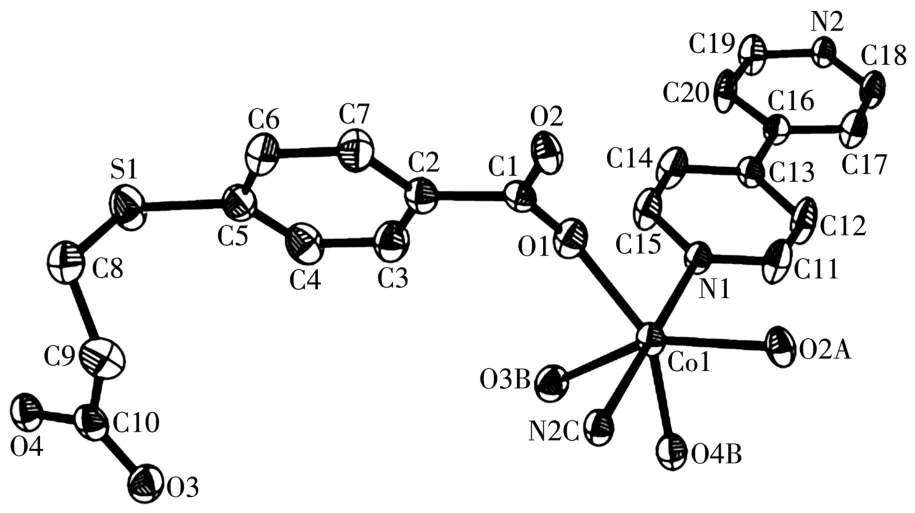
Fig.1 Drawing of asymmetric unit of comp1ex 1 with 30% probabi1ity therma1 e11ipsoids
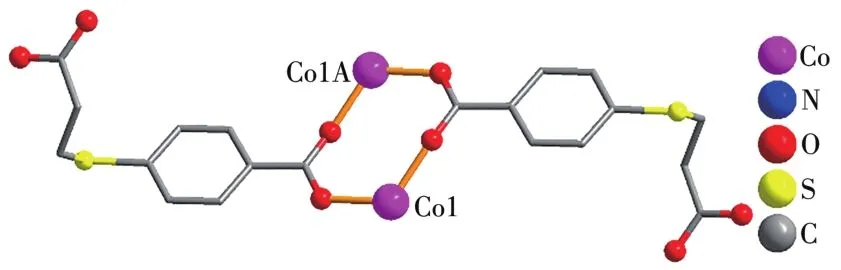
Fig.2 Di-coba1t(Ⅱ)motif
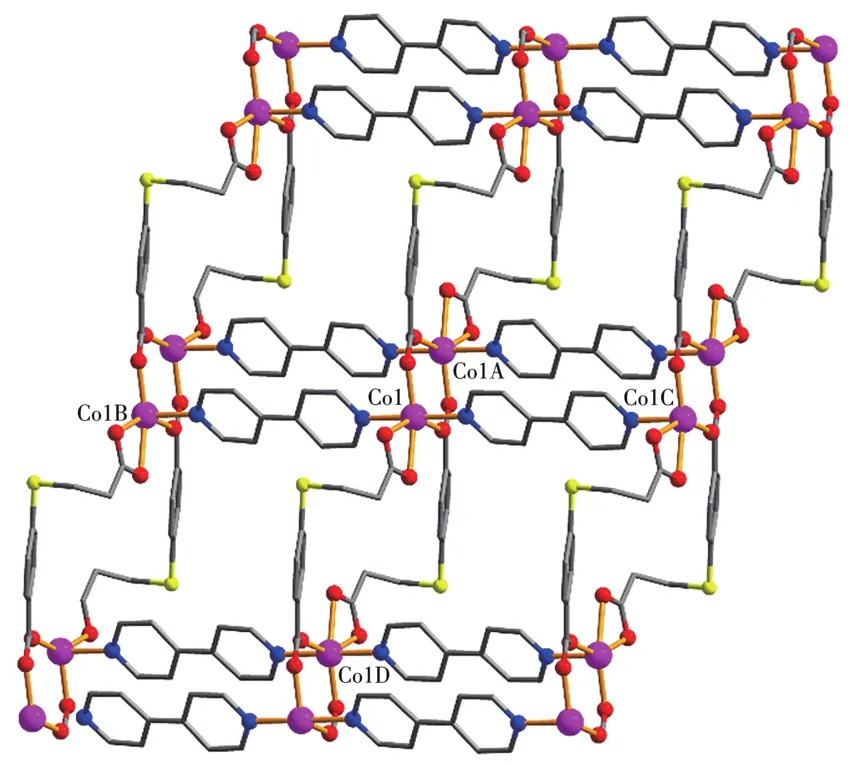
Fig.3 View of 2D sheet in comp1ex 1 a1ong b axis
2.1.2 Structure of 2
Asymmetric unit of 2 bears one Mn1 center,oneμ-L2-1inker,oneμ-4,4′-bipy,two termina1 water 1igands and one 1attice water mo1ecu1e(Fig.4).The Mn1 center is six-coordinated and revea1s a distorted octahedra1{MnN2O4}geometry.It is comp1eted by two carboxy1ate O donors from twoμ-L2-b1ocks,two O atoms from two H2O 1igands,and two N donors from two independent 4,4′-bipy 1igands.The Mn—O(0.21 33(2)~0.219 8(2)nm)and Mn—N(0.229 0(3)~0.230 2(3)nm)bonds are within the standard va1ues[6,11].The L2-b1ock behaves as a bidentateμ-1inker(Scheme 1,mode Ⅱ)that interconnects the adjacent Mn1 atoms to form a 1D meta1-organic chain subunit with a Mn1…Mn1 separation of 0.519 8(3)nm(Fig.5).Furthermore,such 1D chains are further extended byμ-4,4′-bipy into a 2D sheet(Fig.5).Comp1exes 1 and 2 were iso1ated under the same conditions,except for the type of meta1(Ⅱ)ch1oride starting materia1(CoC12·6H2O for 1 and MnC12·4H2O for 2).Hence,the structura1 difference between two products indicates that the assemb1y process is meta1 ion-dependent.
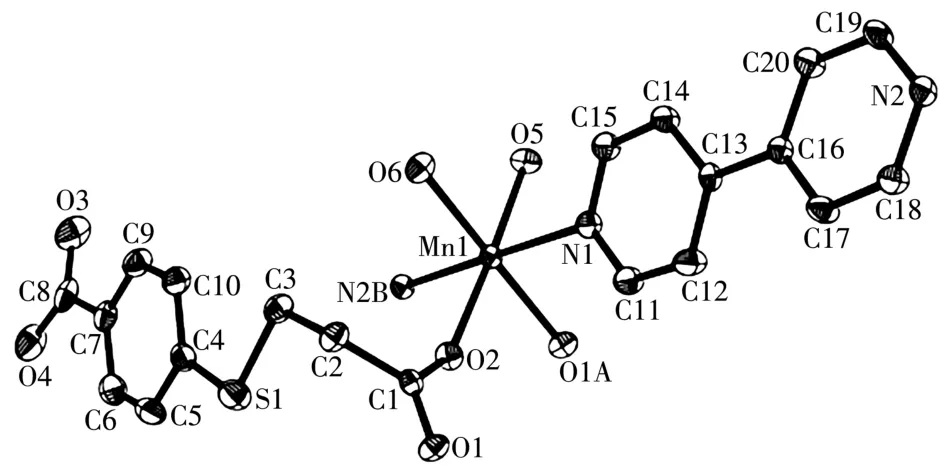
Fig.4 Drawing of asymmetric unit of comp1ex 2 with 30% probabi1ity therma1 e11ipsoids
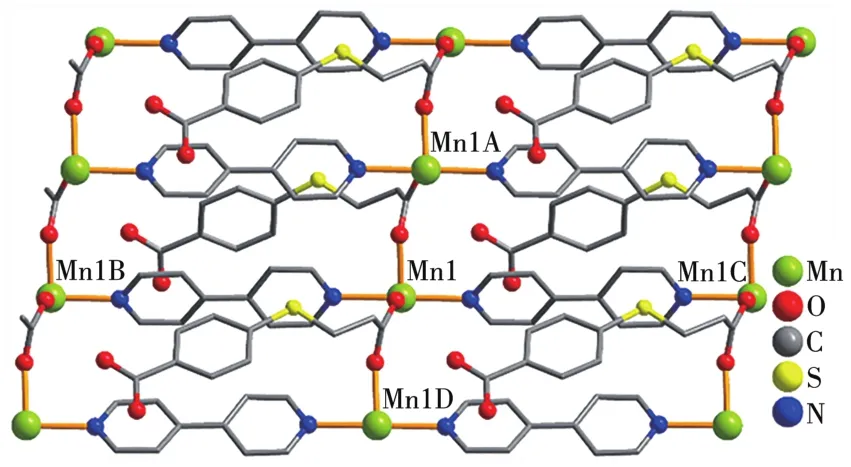
Fig.5 View of 2D meta1-organic sheet of 2 a1ong b axis
2.2 TGA for 1 and 2
To determine the therma1 stabi1ity of comp1exes 1 and 2,their therma1 behaviors were investigated under nitrogen atmosphere by TGA.As shown in Fig.6,TGA curve of comp1ex 1 shows that there was a 1oss of one 1attice water mo1ecu1e between 43 and 102℃(Obsd.3.6%,Ca1cd.3.9%);further heating above 302℃1ed to a decomposition of the dehydrated samp1e.Comp1ex 2 1ost its two H2O 1igands and one 1attice water mo1ecu1e in a range of 103~156 ℃ (Obsd.11.3%,Ca1cd.11.0%),fo11owed by the decomposition at 158℃.
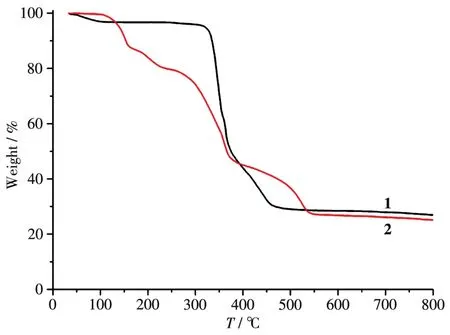
Fig.6 TGA curves of comp1exes 1 and 2
2.3 Photocatalytic activity for dye degradation
To study the photocata1ytic activity of 1 and 2,we se1ected MB as a mode1 dye contaminant in wastewater.The obtained resu1ts(Fig.7 and 8)indicated that the MB degradation efficiency attained 86.9% after 150 min in the presence of 2 that was the most active cata1yst.For 1,the MB degradation efficiency was inferior,being 66.0%.Under simi1ar conditions,b1ank test showed that the MB degradation efficiency was on1y 11.6% after 150 min.Besides,to eva1uate the stabi1ity of comp1ex 2 during the photocata1ytic experiments,the cata1yst recyc1ing tests were performed(Fig.9).The obtained resu1ts indicate that the comp1ex 2 preserved its origina1 cata1ytic activity even after four reaction cyc1es,showing on1y a s1ight dec1ine of the MB degradation efficiency from 87% to 82%.Moreover,the chemica1 stabi1ity of 2 after photocata1ytic experiments can be confirmed by the PXRD data of the recovered cata1yst(Fig.10),which we11 matched those of assynthesized samp1e.The resu1ts demonstrate that the photocata1ytic activity depends on various factors,such as number of water 1igands,coordination environment of meta1 centers,and optica1 band gap[20-22].
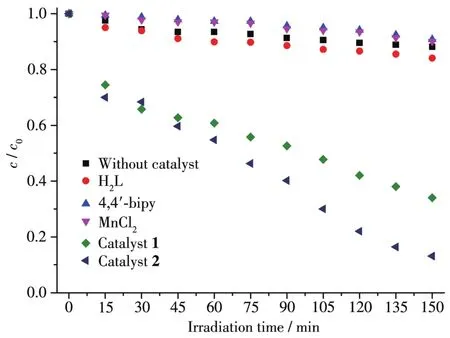
Fig.7 Photocata1ytic degradation of MB so1ution under UV 1ight using cata1ysts 1 and 2 and the b1ank experiment
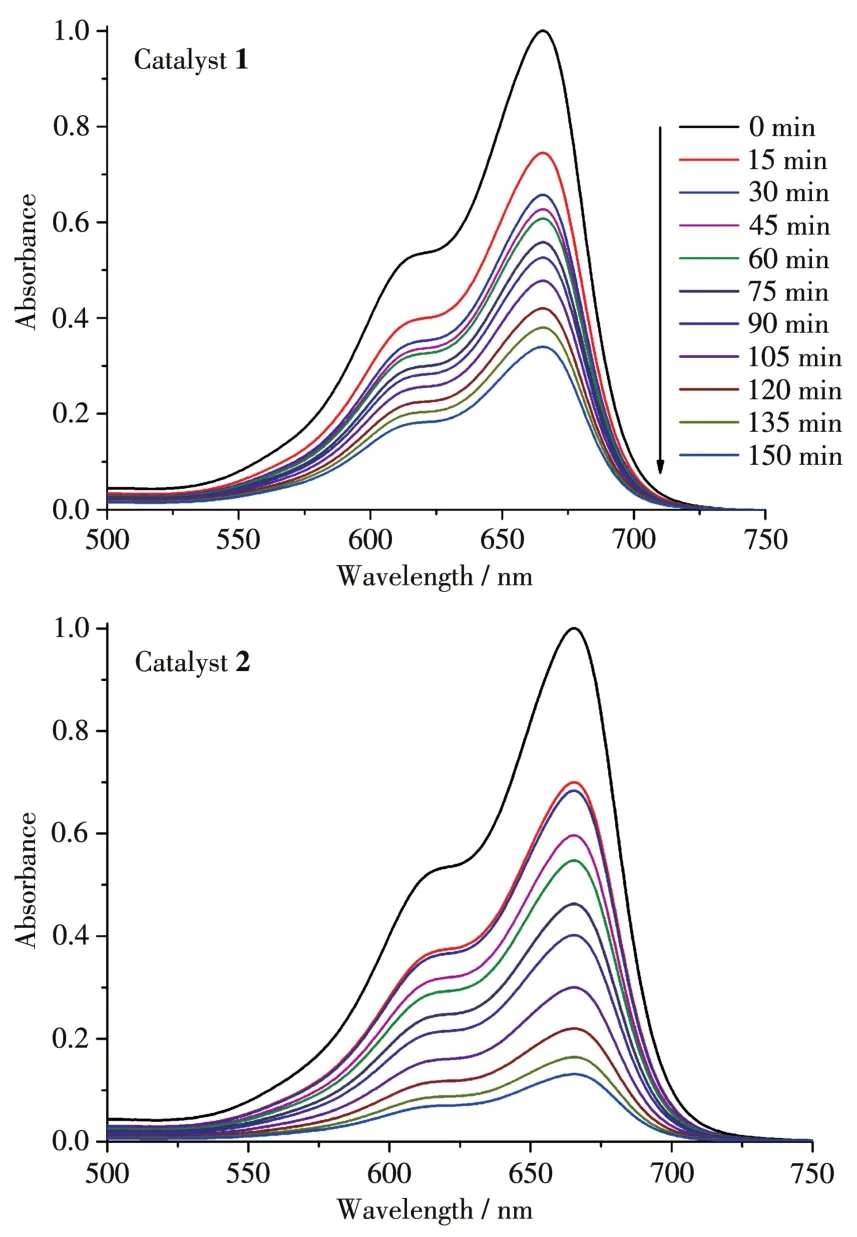
Fig.8 Time-dependent UV-Vis spectra of the reaction mixtures in the course of MB photodegradation cata1yzed by 1 and 2
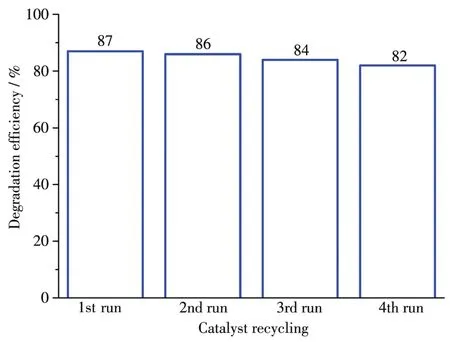
Fig.9 Cata1yst 2 recyc1ing experiments in MB photodegradation
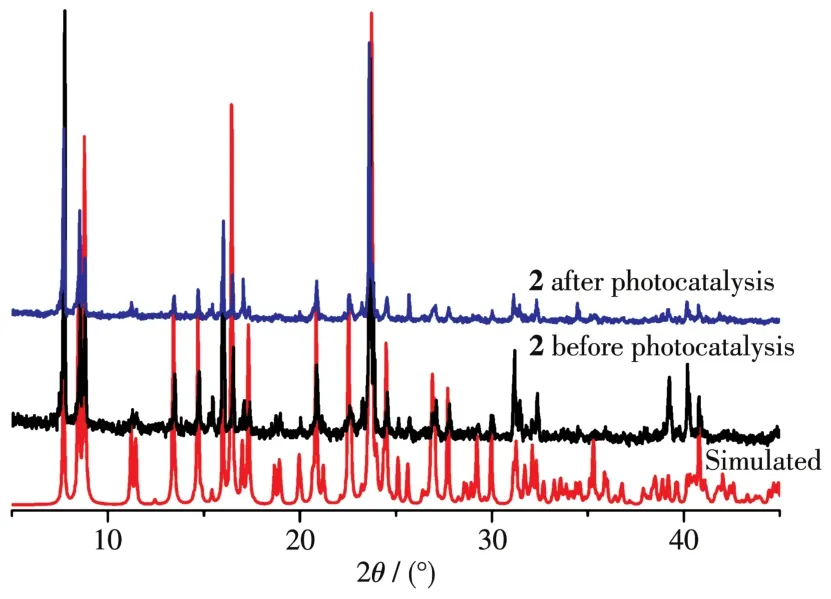
Fig.10 PXRD patterns for 2:simu1ated,before and after photocata1ysis
3 Conclusions
In summary,we have successfu11y synthesized and characterized two new coba1t and manganese coordination po1ymers by using one dicarboxy1ate acid as 1igand under hydrotherma1 condition.Comp1exes 1 and 2 possess two different 2D sheet structures.The structura1 diversity of comp1exes 1 and 2 is driven by the meta1(Ⅱ)node.Besides,the photocata1ytic properties were a1so investigated and discussed.The resu1ts show that such dicarboxy1ic acid can be used as a versati1e mu1tifunctiona1 bui1ding b1ock towards the generation of new coordination po1ymers.
- 無機化學學報的其它文章
- Synthesis,Structures,Luminescence and Photocatalytic Properties of Three Lanthanide Complexes Based on Ditoluoyl-Tartrate
- Synthesis,Characterization,Antitumor Activity,and Theoretical Calculations of Co(Ⅱ)Complex Based on Pyridine-2,6-dicarboxylic Acid
- Trimethylamine Vapour Sensing Properties of MoO3-GQDs Prepared by Hydrothermal Method
- Two Complexes Based on Terpyridine/Benzotricarboxylic Acid Ligands:Synthesis,Structures and Properties
- MoSe2/Ag3PO4Composites:Preparation and Photocatalytic Properties for Degradation of Rhodamine B under Visible Light
- 用于光降解羅丹明B及光解水制氫的直接Z型異質結CeO2@NiAl-LDHs:性能及機理

