Association between ADAMTS13 activity–VWF antigen imbalance and the therapeutic effect of HAIC in patients with hepatocellular carcinoma
Hiroaki Takaya, Tadashi Namisaki, Kei Moriya, Naotaka Shimozato, Kosuke Kaji, Hiroyuki Ogawa, Koji Ishida, Yuki Tsuji, Daisuke Kaya, Hirotestu Takagi, Yukihisa Fujinaga, Norihisa Nishimura, Yasuhiko Sawada, Hideto Kawaratani, Takemi Akahane, Masanori Matsumoto, Hitoshi Yoshiji
Abstract
Key Words: ADAMTS13; Von Willebrand factor; Vascular endothelial growth factor; Biomarkers; Hepatocellular carcinoma; HAIC
INTRODUCTION
Hepatocellular carcinoma (HCC) has one of the highest mortality rates of any cancer[1,2]. In Japan, HCC management follows the consensus-based clinical practice guidelines of the Japan Society of Hepatology (JSH)[3]. JSH recommends that advanced HCC patients with vascular invasion or more than four tumors should undergo chemotherapy such as HAIC or molecular-targeted drugs[3]. However, it is important to predict HAIC response before deciding on the appropriate chemotherapy protocol for improving the prognosis in patients with HCC.
ADAMTS13 is a metalloproteinase that is exclusively produced from hepatic stellate cells adjacent to endothelial cells (ECs)[4-8]. It specifically cleaves multimeric von Willebrand factor (VWF) between the Tyr1605 and Met1606 residues in the A2 domain[4-7]. VWF is synthesized in vascular ECs and released into the plasma as unusually large multimers[9]. During ADAMTS13 enzyme–VWF substrate imbalance, VWF is improperly cleaved, resulting in the accumulation of multimers and the induction of platelet thrombi formation in the microvasculature under high shearstress conditions[10]. In other words, ADAMTS13 enzyme–VWF substrate balance is related to hypercoagulability. Furthermore, the blood coagulation cascade is related to cancer progression[11,12], and our previous study has reported that ADAMTS13 enzyme–VWF substrate imbalance becomes worse based on HCC progression[13,14].
Angiogenesis plays an important role in HCC progression[15]. A recent study has reported that ADAMTS13 enzyme–VWF substrate imbalance is related to angiogenesis[16]as well as hypercoagulability and is associated with the prognosis in patients with various kinds of cancer undergoing chemotherapy[14,17].
In the present study, we investigated the relationship between ADAMTS13 enzyme–VWF substrate balance and HCC in patients undergoing HAIC treatment. In addition, we sought to determine whether ADAMTS13 and VWF may become predictive biomarkers of treatment response in HCC patients before starting HAIC treatment.
MATERIALS AND METHODS
Patients
This retrospective observational study included patients with HCC who underwent HAIC treatment from December 2009 to March 2019. Patients with HCC had no vascular invasion or less than four tumors were excluded. A total of 72 patients with HCC were included in this study. HAIC treatment was performed according to the Moriya method[18,19], which features a bi-monthly protocol that is simple and easy to manage. The patients underwent dynamic computed tomographic scanning or dynamic magnetic resonance imaging at various points, namely before starting HAIC treatment, 1 mo after commencement of the treatment, and every 2 mo thereafter. HAIC treatment responses were evaluated according to modified response evaluation criteria in solid tumors. This study had no patient with infection, uncontrolled hepatic encephalopathy, ascites, or gastroesophageal varices. This study was approved by the local ethics committee in Nara Medical University and was performed according to the ethical standards laid down in the Declaration of Helsinki. Informed consent was obtained from all patients included in the study.
Determination of ADAMTS13 activity and VWF antigen levels
We collected blood samples from each patient at the time of admission, during their hospital stay, or during regular outpatient treatment before starting HAIC treatment. The plastic tubes with 0.38% sodium citrate was used to store these samples. We centrifuged these samples at 3000 ×gat 4 ℃ for 15 min to prepare the plasma and stored the plasma at -80 ℃ until analysis. Plasma ADAMTS13 activity (ADAMTS13:AC) was determined using a sensitive chromogenic enzyme-linked immunosorbent assay (ELISA) (Kainos Laboratories Inc., Tokyo, Japan)[20]to show a normal value of 99% ± 22%. Plasma VWF antigen (VWF:Ag) levels were measuredviasandwich ELISA using a rabbit anti-human VWF polyclonal antiserum (Dako, Glostrup, Denmark). The normal VWF:Ag value is 102% ± 33%[21].
VEGF measurements
VEGF levels were determined using a commercially available kit (Immunoassay Kits, RayBiotech Inc., United States). The detection limit of VEGF was < 10 pg/mL.
Statistical analysis
The Mann–WhitneyU-test and the Fisher’s exact test were performed to analyze differences between study groups and categorical data, respectively. Univariable and multivariable analysis were performed to evaluate HAIC response for HCC. Logistic regression analysis was performed to determine independent response factors, and data were expressed as median (interquartile range). A two-tailedPvalue of < 0.05 was considered significant. Analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Japan), a graphical user interface of R version 2.13.0 (The R Foundation for Statistical Computing, Vienna, Austria), and a modified version of R commander (version 1.6-3) that includes statistical functions that are frequently used in biostatistics[22].
RESULTS
Clinical characteristics of the HCC patients
Table 1 showed the clinical characteristics of HCC patients. The median period of HAIC treatment was 121 (range 41-218) d and the median age of HCC patients was 70.5 (range 64.2-76.1) years. Of the study population (57 males, 15 females), 17 patients had hepatitis B virus infection, 36 had hepatitis C virus infection, 10 had alcohol abuse, 4 had non-alcoholic steatohepatitis, and 5 had others. The median maximum tumor size was 3.3 (range 2.2-5.0) cm. Tumors numbering 1, 2, 3, 4, or > 4 were 9, 5, 6, 2, and 50, respectively. Thirty-one patients had vascular invasion and no patient had distant metastasis. Serum alpha-fetoprotein (AFP), des-γ-carboxy prothrombin, and lensculinaris agglutinin-reactive fraction of AFP levels were 95.3 (17.9–1162.5) ng/mL, 359.5 (58.0–5277.5) mAU/mL, and 33.7% (7.7%–73.4%), respectively. We investigated the HAIC treatment response between stable disease (SD) + partial response (PR) and progressive disease (PD). No significant differences were observed in HCC patients’ characteristics between SD + PR and PD, except for treatment periods.
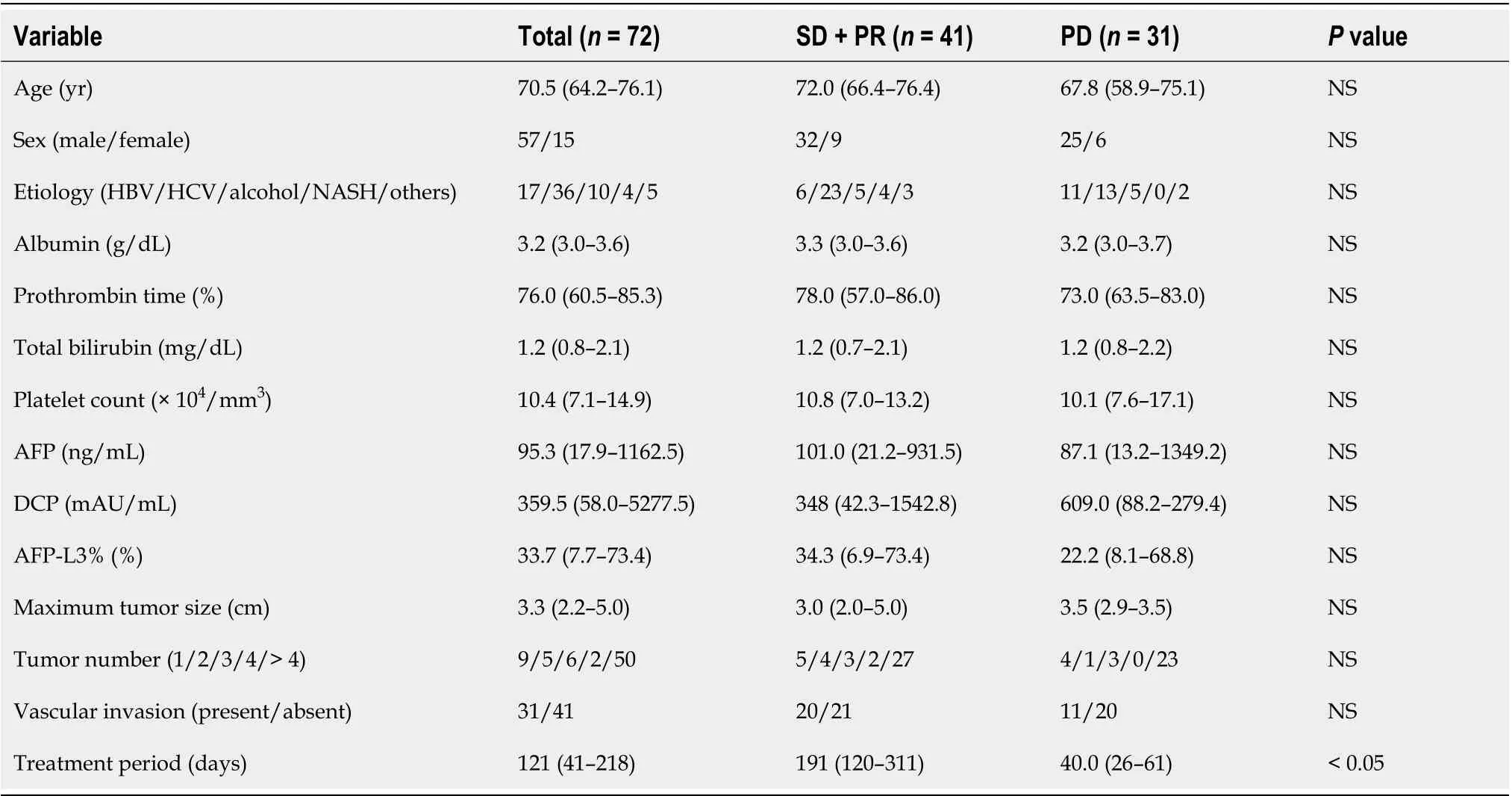
Table 1 Hepatocellular carcinoma patients’ characteristics between stable disease + partial response and progressive disease with hepatic arterial infusion chemotherapy
Plasma ADAMTS13:AC and VWF:Ag levels
ADAMTS13:AC levels in HCC patients with SD + PR were significantly higher than those with PD (P< 0.05) (Figure 1A). VWF:Ag levels were no different between patients with SD + PR and PD (Figure 1B). The ratio of VWF:Ag to ADAMTS13:AC (VWF:Ag/ADAMTS13:AC ratio) in patients with SD + PR was significantly lower than those with PD (P< 0.05) (Figure 1C).
Plasma VEGF levels
VEGF levels in HCC patients with SD + PR were significantly lower than those with PD (P< 0.05) (Figure 2A). Patients were categorized into two groups according to receiver operating characteristic (ROC) cut-off VEGF: Low, ≤ 100 and high, > 100. Patients with high VEGF levels also had higher platelet levels than those with low VEGF (Figure 2B). Patients were categorized into two groups according to ROC cut-off VWF:Ag/ADAMTS13:AC ratio: Low, ≤ 2.7 and high, > 2.7. Patients with high VWF:Ag/ADAMTS13:AC ratio had higher VEGF levels than those with low ratio (Figure 2C).
總之,無人機測繪技術目前已運用到多個領域中,國家大力支持此技術的研究,鼓勵在民用領域中運用,以發揮更大作用。在工程項目測量過程中,為了綜合了解整個工程項目的基本情況,完善工程規劃,使用無人機進行測繪時,監測到的范圍和尺度也較大,具有極高的監測效率,高空作業效果顯著,同時可與多項技術設備結合使用,大幅提高工程測量的質量和效率。因此,在工程測量過程中,在復雜的環境下合理設置無人機基本參數,定向分析采集數據信息,及時反饋高清圖像,確保工程測量中無人機測繪作業的安全性,可提高工程測量的效率。
Predictive factors for HAIC response
Patients were categorized into two groups according to ROC cut-off. Univariable analysis showed that HAIC treatment response is associated with prothrombin time (PT), VEGF, and VWF:Ag/ADAMTS13:AC ratio (Table 2). To determine the predictive factors of HAIC response, multivariable analysis was performed using PT, VEGF, and VWF:Ag/ADAMTS13:AC ratio, with these factors showingP< 0.05 in univariable analysis. VWF:Ag/ADAMTS13:AC ratio was significantly associated with HAIC treatment responseviamultivariable analysis (Table 2). ROC analysis showed that VWF:Ag/ADAMTS13:AC ratio is sensitivity of 53.7%, specificity of 87.1%, and area under the curve of 0.715.

Table 2 Predictive factors for response of hepatic arterial infusion chemotherapy in patients with hepatocellular carcinoma
DISCUSSION
We suggests that VWF:Ag/ADAMTS13:AC ratio is a potential biomarker for HAIC treatment response in the present study. It is well-known that this ratio is related to the coagulation cascade[10], which in turn plays an important role in the cancer development, including HCC[11,12]. Previous studies have reported that ADAMTS13 enzyme–VWF substrate imbalance is associated with cancer progression, prognosis of patients with various kinds of cancer, and response to chemotherapy[17,23]. Our previous study reported that VWF:Ag[7]and VWF:Ag/ADAMTS13:AC ratio[13]are predictive and detective factors of HCC in patients with cirrhosis, respectively. Moreover, a study has reported that the association between ADAMTS13:AC and VWF:Ag, and the treatment efficiency of molecular-targeted drugs[14].
It is well-known that angiogenesis is related to the pathophysiology of HCC development[15]and that VEGF plays an important role in angiogenesis[15]. Recently, studies have reported that VWF reduces VEGF-dependent angiogenesisviamultiple intracellular and extracellular pathways involving integrin avβ3 and angiopoietin-2[16,24,25]and that ADAMTS13 cleaves VWF and promotes VEGFR-2 phosphorylation, as the result, induces angiogenesis. This in turn results in enhancement of VEGF expression[26]. Xu have reported that the important role of ADAMTS13 enzyme–VWF substrate balance in the regulation of blood vessel formation[16]. A previous study has reported that HAIC treatment decreases VEGF levels in patients with advanced HCC[27]. Therefore, VWF:Ag/ADAMTS13:AC ratio may be associated with HAIC treatment responseviaVEGF and angiogenesis.
Furthermore, anti-platelet therapy inhibits VEGF that induces HCC development[28]. A recent study has reported that anti-platelet therapy for cirrhotic patients prevents HCC development[29]and prolongs survival time in hepatitis B virus mouse model of chronic liver disease[28]. ADAMTS13 enzyme–VWF substrate imbalance induces platelet thrombi formation[10]. In other words, ADAMTS13 enzyme–VWF substrate imbalance, VEGF, angiogenesis, and hypercoagulability are closely related to the cancer progression, including HCC. A previous study has found that VEGF is associated with HAIC treatment response and prognosis[30]. Our study reported the association between VWF:Ag/ADAMTS13:AC ratio and HAIC treatment response; however, our analysis indicated that VEGF is not a predictive factor of HAIC treatment response. VWF:Ag/ADAMTS13:AC ratio may become a more useful to predict HAIC treatment response than VEGF.
Our study has some limitations that include a small sample size and short observation. Cirrhotic patients with advanced HCC occasionally develop thrombosis or inflammation (e.g., portal thrombosis, and bacterial overgrowth and translocation). When VWF:Ag/ADAMTS13:AC ratio is used as a biomarker of HAIC treatment response, thrombosis and inflammation may affect the values[4,23,31]. In addition, VWF:Ag/ADAMTS13:AC ratio has high specificity but moderate sensitivity to predict HAIC treatment response. Therefore, we should continue to investigate highsensitivity biomarkers.
CONCLUSION
In summary, VWF:Ag/ADAMTS13:AC ratio is an independent predictive factor for response in patients with HCC undergoing HAIC treatment.
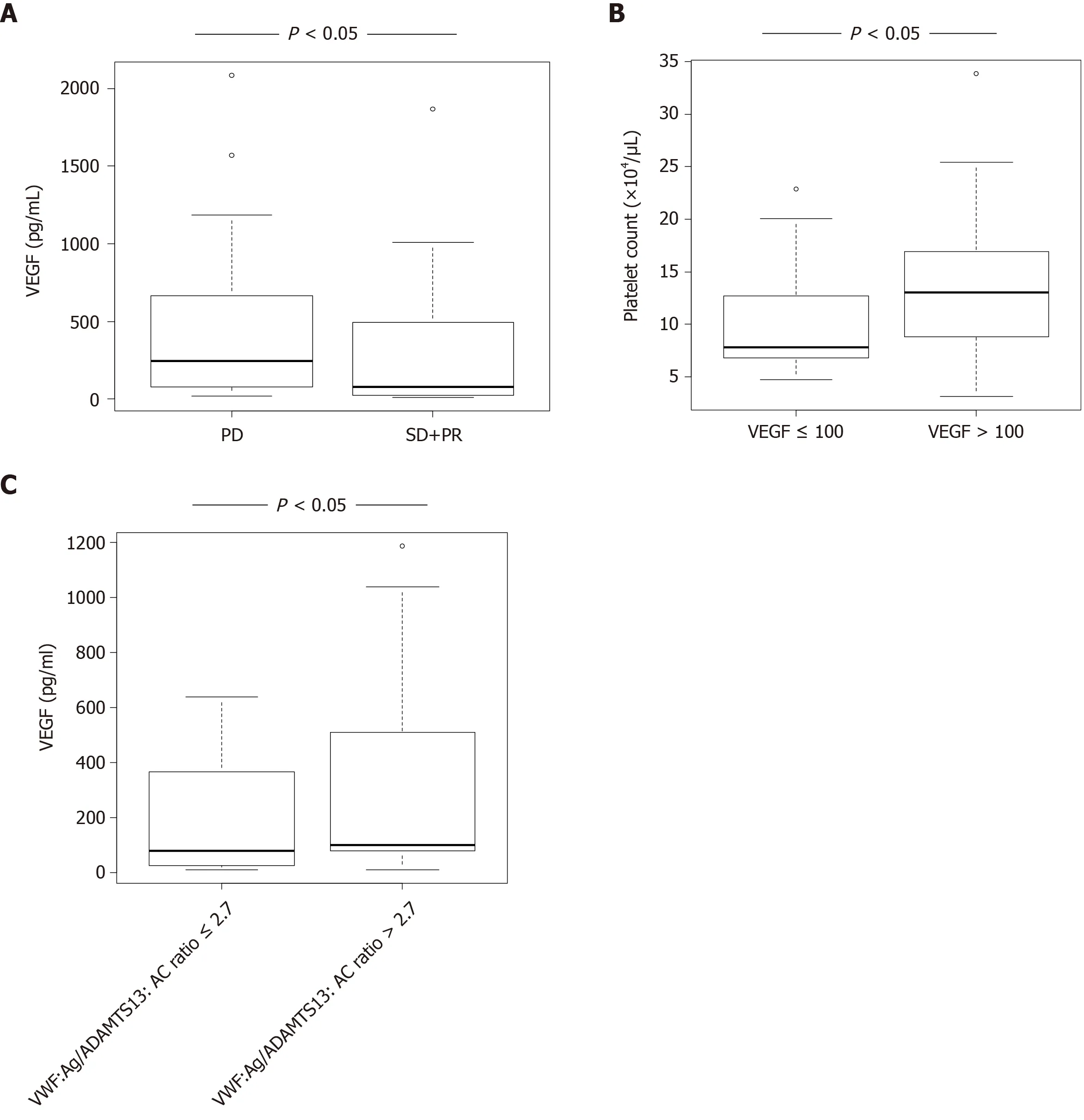
Figure 2 Plasma vascular endothelial growth factor levels in patients with hepatocellular carcinoma receiving hepatic arterial infusion chemotherapy treatment. A: Vascular endothelial growth factor (VEGF) levels were significantly lower in hepatocellular carcinoma patients with stable disease + partial response than in those with progressive disease (P < 0.05); B: Patients with high VEGF levels (> 100) had higher platelet levels than those with low VEGF levels (≤ 100) (P < 0.05); C: Patients with high Von Willebrand factor antigen (VWF:Ag)/ADAMTS13 activity (ADAMTS13:AC) ratio (> 2.7) had higher VEGF levels than those with low VWF:Ag/ADAMTS13:AC ratio (≤ 2.7). VEGF: Vascular endothelial growth factor; SD: Stable disease; PR: Partial response; PD: Progressive disease; ADAMTS13: A disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13; ADAMTS13:AC: ADAMTS13 activity; VWF: Von Willebrand factor; VWF:Ag: VWF antigen; VWF:Ag/ADAMTS13:AC ratio: Ratio of VWF:Ag to ADAMTS13:AC.
ARTICLE HIGHLIGHTS
Research background
Predicting HAIC treatment response is important for improving the prognosis of hepatocellular carcinoma (HCC) patients.
Research motivation
ADAMTS13 and von Willebrand factor (VWF) have been associated with the prognosis in patients with various kinds of cancer receiving chemotherapy.
Research objectives
The present study was investigated whether ADAMTS13 and VWF become useful biomarkers of treatment response in HCC patients before the initiation of HAIC treatment.
Research methods
Multivariable analysis was performed to determine the predictive factors of HAIC treatment response in patients with HCC.
Research results
VWF antigen (VWF:Ag)/ADAMTS13 activity (ADAMTS13:AC) ratio predicted HAIC treatment response in multivariable analysis.
Research conclusions
VWF:Ag/ADAMTS13:AC ratio may be a useful biomarker of treatment response in patients with HCC before HAIC treatment.
Research perspectives
VWF:Ag/ADAMTS13:AC ratio has high specificity to predict HAIC treatment response. On the other hand, this biomarker has moderate sensitivity. Therefore, we should continue to investigate high-sensitivity biomarkers.
ACKNOWLEDGEMENTS
This work was helped by Ms. Yoshie Nakai, Prof. Masahito Uemura, and Professor Hiroshi Fukui.
 World Journal of Gastroenterology2020年45期
World Journal of Gastroenterology2020年45期
- World Journal of Gastroenterology的其它文章
- Discovery of unique African Helicobacter pylori CagAmultimerization motif in the Dominican Republic
- Diagnosis and treatment of iron-deficiency anemia in gastrointestinal bleeding: A systematic review
- Liver fibrosis index-based nomograms for identifying esophageal varices in patients with chronic hepatitis B related cirrhosis
- High mortality associated with gram-negative bacterial bloodstream infection in liver transplant recipients undergoing immunosuppression reduction
- Nimbolide inhibits tumor growth by restoring hepatic tight junction protein expression and reduced inflammation in an experimental hepatocarcinogenesis
- Role of pancreatography in the endoscopic management of encapsulated pancreatic collections – review and new proposed classification
