發泡法制備二維材料泡沫體的進展
高天, 肖慶林, 許晨陽, 王學斌
發泡法制備二維材料泡沫體的進展
高天, 肖慶林, 許晨陽, 王學斌
(南京大學 現代工程與應用科學學院, 固體微結構物理國家重點實驗室, 人工微結構科學與技術協同創新中心, 江蘇省功能材料設計原理與應用技術重點實驗室, 南京 210093)
以石墨烯為代表的二維材料具有優異的本征性質, 例如高表面積和電導率, 但其宏觀塊體材料的性質仍不理想。這是由于石墨烯片層堆疊損失了有效的表面; 片層之間聯結較弱導致接觸電阻和熱阻增大。原則上二維材料的三維化設計能避免上述問題, 將納米尺度的優異性質傳遞到宏觀尺度, 獲得高表面積、高導電、貫通孔道和優良機械性能的塊體材料。二維材料多孔塊體可用于電極、吸附劑和彈性體等。發泡法工藝簡單、成本低, 是近年來制備二維材料泡沫體的主要方法。本文系統總結了發泡法的基本原理, 綜述了石墨烯、氮化硼等二維材料泡沫體的研究進展, 展望了二維材料泡沫體在能源、環境等方面的應用前景。
二維材料; 發泡法; 石墨烯; 氮化硼; 泡沫材料; 綜述
石墨烯是一種由碳原子通過sp2雜化構成的二維新材料[1], 具有超薄結構和獨特物性, 如高載流子遷移率、表面積、熱導率[2-5], 在電子、儲能和催化等領域具有重要的應用前景[6-9]。在石墨烯的宏觀塊體中, 兩個相鄰片層的面與面之間容易發生p-p堆疊, 損失表面積; 在面內方向, 兩個相似片層之間通常依靠范德華力聯結, 接觸電阻、熱阻較大; 此外, 片片之間的堆積孔道曲折無序, 不利于外來物質的擴散。這三點限制了基于石墨烯的電化學電極等應用性能的提升[10]。
三維石墨烯材料是一種石墨烯塊體, 與石墨烯粉體、石墨烯薄膜并列, 如圖1所示。在概念上, 三維石墨烯是將石墨烯單元連接形成sp2雜化的三維網絡。在理論上三維石墨烯可以繼承二維納米材料的本征高比表面積、電導率、熱導率等優點。三維網絡結構提供的雙聯通通道為固相網絡通道和內部孔道聯通形成的空腔通道, 前者用于輸運電子、聲子和力; 后者用于傳質。三維石墨烯在界面相關應用場景中具有重要意義, 包括電極、吸附等[11]。
學者們報導了數種制備三維石墨烯的方法: 基于氧化還原石墨烯(RGO)的凝膠化方法(包括體相凝膠化[12-15]、界面凝膠化法[16]、冰模板法[17]、模板/交聯劑輔助法[18-19])、基于泡沫鎳的化學氣相沉積法(CVD)[11,20-21]、基于鋅分層效應的熱裂解法[22]、生物質熱裂解法[23]、發泡法[24]和3D打印[25]等。凝膠化方法主要依靠非共價鍵組裝, 內部聯結較弱; 泡沫鎳等多孔模板較難循環利用。發泡法工藝簡單、成本低、易擴大生產, 其產品的固相網絡內部聯結強、表面積大, 是一種極具產業價值的制備方法。除了三維石墨烯, 硫化物、雙氫氧化物等二維材料多孔塊體也是二維材料三維化設計的熱門領域[26-28]。
回溯歷史, 發泡法起源于傳統泡沫塑料加工, 但在近年開始用于制備二維材料泡沫體。2011~2013年, 王學斌等受吹泡啟發, 將發泡過程首次引入到二維材料領域, 發展了化學發泡法制備石墨烯泡沫體[24]、氮化硼(BN)納米片泡沫體[29]。隨后, LEI[30]和WANG[31]等利用硝酸鹽輔助法制備了石墨烯泡沫體; WANG[32]、ZHAO[33]和DONG[34]等制備了氮摻雜石墨烯泡沫; DONG[35]、ZHU[36]、WU[37]、CAI[38]和TAN[39-41]等制備了擔載各種功能物質的石墨烯泡沫; LU等[42]使用銨鹽發泡劑制備了氮化碳泡沫; ZHAO等[43]制備了BN泡沫。
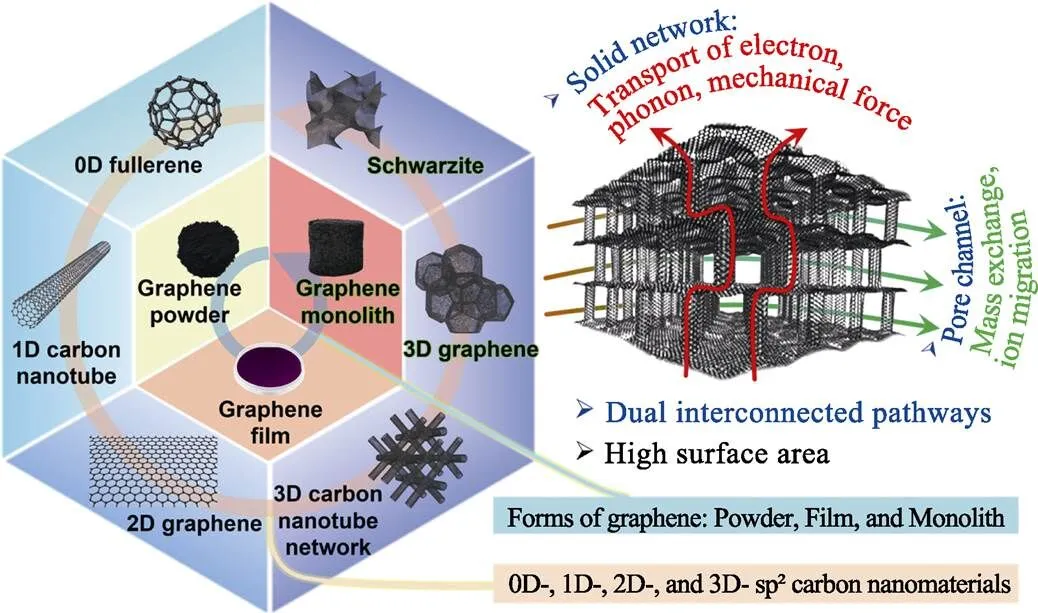
圖1 多種維度的sp2雜化碳納米材料, 石墨烯粉體、薄膜和塊體三種形態, 以及設計三維石墨烯的理念
本文總結了采用發泡法制備二維材料泡沫體的發泡原理、不同類型泡沫體及其應用, 以期闡明未來的發展前景。
1 發泡原理
1.1 發泡流程
發泡過程包括三步(圖2): ①前驅體與發泡劑混合; ②在內/外部作用下產生氣體源, 使氣泡成核、長大; ③流體泡沫經歷穩定化過程最終變成固體泡沫。根據氣源不同, 發泡法分為化學發泡和物理發泡, 前者通過化學反應產生氣體, 后者則利用沸騰或減壓膨脹產生氣體。流體泡沫目前基本沒有工程用途, 它需經歷固化、硬化和結晶等穩定化過程。
泡沫的基本單元為氣泡, 在平衡態時呈多面體形狀。氣泡由泡壁、筋和交點構成, 分別定義為2、3、4個氣泡的結合部, 亦即幾何學中的面、棱和點, 如圖2所示。按照Plateau定律, 3個泡壁相交于1條筋(Plateau通道), 且傾向對稱分布; 4條筋相交于1個交點, 也傾向對稱分布。
1.2 發泡幾何學與靜力學
泡沫幾何學主要研究“干泡沫”的多面體堆砌問題。歐拉定律認為氣泡多面體的大多數面應為五邊形[44]; Aboav-Weaire定律認為一個多面體擁有的面數越多, 則其相鄰多面體的面數就越少[45]。Lewis定律認為多面體的體積隨面數的增加而增加[46]。因此, 一個大氣泡通常被小氣泡所包圍。此外, 表面張力等靜力學因素也影響泡沫的結構。表面能與表面積成正比, 泡沫表面能最小的為密堆多面體, 因為它的表面積最小, 即熟知的Kelvin問題。1887年, Thomson提出了Kelvin結構; 1993年, Weaire和Phelan構建了Weaire-Phelan結構[47], 其表面積比Kelvin結構的表面積減少了0.3%, 如圖3(a,b)所示。堆砌問題的最優方案迄今仍無確切結論。
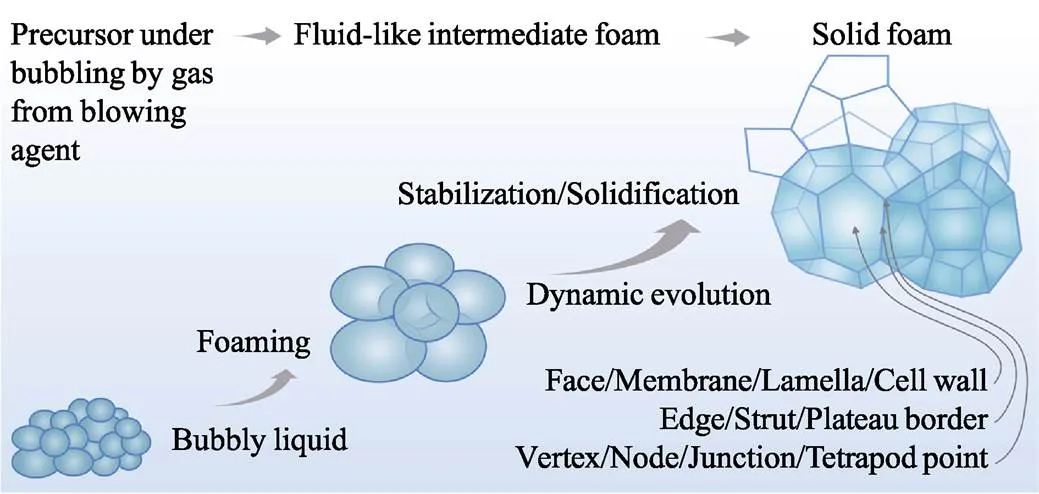
圖2 發泡過程示意圖
1.3 發泡動力學與動態學
發泡動力學主要包括: ①氣泡的均/異相成核; ②氣泡的長大; ③氣泡結構的動態演變; ④穩定化過程, 如圖3(c)所示。氣泡成核與晶體成核有共通之處, 一旦初生氣泡大于臨界尺寸, 則傾向于長大; 反之則趨于消失。進一步, 內/外部驅動力驅使氣源持續擴散進入氣泡, 使氣泡長大。
在形核和長大的過程中, 流體泡沫也進行著動態學演化, 包括滲流、熟化、破裂、重排等行為。
(1)滲流是流體泡沫中的流體在表面張力或重力驅動下, 在泡壁和筋內部流動的過程[48]。前者由于不同位置的曲率半徑不同, 導致Lapalce壓力差, 驅使流體自泡壁流向Plateau通道。后者由于流體泡沫的質心要降低, 則流體沿著Plateau通道自上而下流動。
(2)熟化/粗化是小氣泡內的氣體向相鄰大氣泡中擴散的過程, 這是由于小氣泡的半徑小, 內壓大, 與大氣泡之間有壓差。
(3)液膜破裂。伴隨著滲流或流體的揮發, 液膜不斷減薄, 當其厚度減薄至黑膜狀態時, 楔壓會削弱表面張力, 造成液膜破裂, 使兩個氣泡發生合并。
(4)流體泡沫具有流變特性, 例如典型的T1重排。
氣泡的成核和生長在很大程度上決定氣泡的直徑分布, 動態演變亦影響了氣泡尺寸、泡壁厚度等。實踐發現, 發泡的關鍵在于發泡劑產氣過程與聚合物固化過程之間的匹配。兩者的匹配影響了發泡動力學與動態學、流體泡沫的結構, 最終形成固體泡沫。
2 采用發泡法制備二維材料泡沫體
傳統發泡工業制造了泡沫塑料[49]、泡沫金屬[50]、泡沫陶瓷[51]和泡沫炭[52-54]等民用和特種泡沫。近年來, 學者們拓展了用發泡法制備二維材料泡沫體[24,29]。二維材料泡沫體不僅具有固體泡沫的性質, 又可以呈現二維材料的本征優異特性[28]。基于B、C、N和O的聚合物種類豐富, 易發泡, 而且在發泡過程中易進行摻雜、包裹/擔載金屬元素, 發展了多種多樣的B–C–N–O體系二維材料泡沫體, 包括石墨烯、摻雜和擔載型石墨烯、氮化碳和氮化硼等, 如圖4所示。
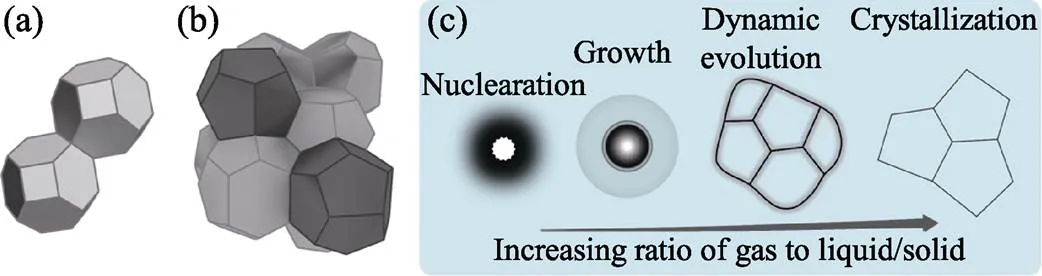
圖3 堆砌的(a)Kelvin結構和(b)Weaire-Phelan結構; (c)發泡動力學示意圖
2.1 石墨烯類泡沫體
2.1.1 石墨烯泡沫體
2013年, 王學斌等[24]開拓了化學發泡法制備一種新型三維石墨烯——筋撐石墨烯(strutted graphene, SG), 如圖5(a~d)所示。化學發泡法以糖為碳源, NH4Cl為發泡劑, 在加熱過程中誘發美拉德反應, 同時NH4Cl分解釋放出NH3和HCl, 使類黑精聚合物發泡。隨著氣泡膨脹、表面張力滲流和熱解反應, 類黑精發泡體的泡壁逐漸減薄, 最終其聚合物泡壁厚度可薄至約20 nm[55], 如圖5(e~f)所示。類黑精泡沫體經1350 ℃退火, 轉變為SG。SG的泡壁為單層或寡層石墨烯薄膜, 如圖5(g~i), 它們附著在石墨筋上。這種筋耦合膜的結構提升了機械彈性, 經80%的壓應變后依然能恢復原狀; 同時消除了石墨烯堆疊問題, 實現了1005 m2/g的比表面積。
為了匹配發泡劑產氣與聚合物固化兩過程, 選擇適當發泡劑是控制泡沫結構的重要手段。無殘留的發泡劑除了NH4Cl (產氣溫度可持續在200~270 ℃, 下同), 還可以選擇(NH4)2CO3(50~80 ℃)、NH4NO3(150~200 ℃)、(NH4)2SO4(330~380 ℃)、尿素(170~ 220 ℃)、草酸(190~220 ℃)和三聚氰胺(270~320 ℃) 等。若產氣溫度高于或低于聚合物固化溫度, 則不易發泡。加熱速度可以影響產氣速度和持續時間, 高速加熱時氣泡成核多, 則最終泡孔較小; 反之則泡孔較大, 如圖5(j~m)。使用銨鹽等無殘留發泡劑的報道歸納于表1中。
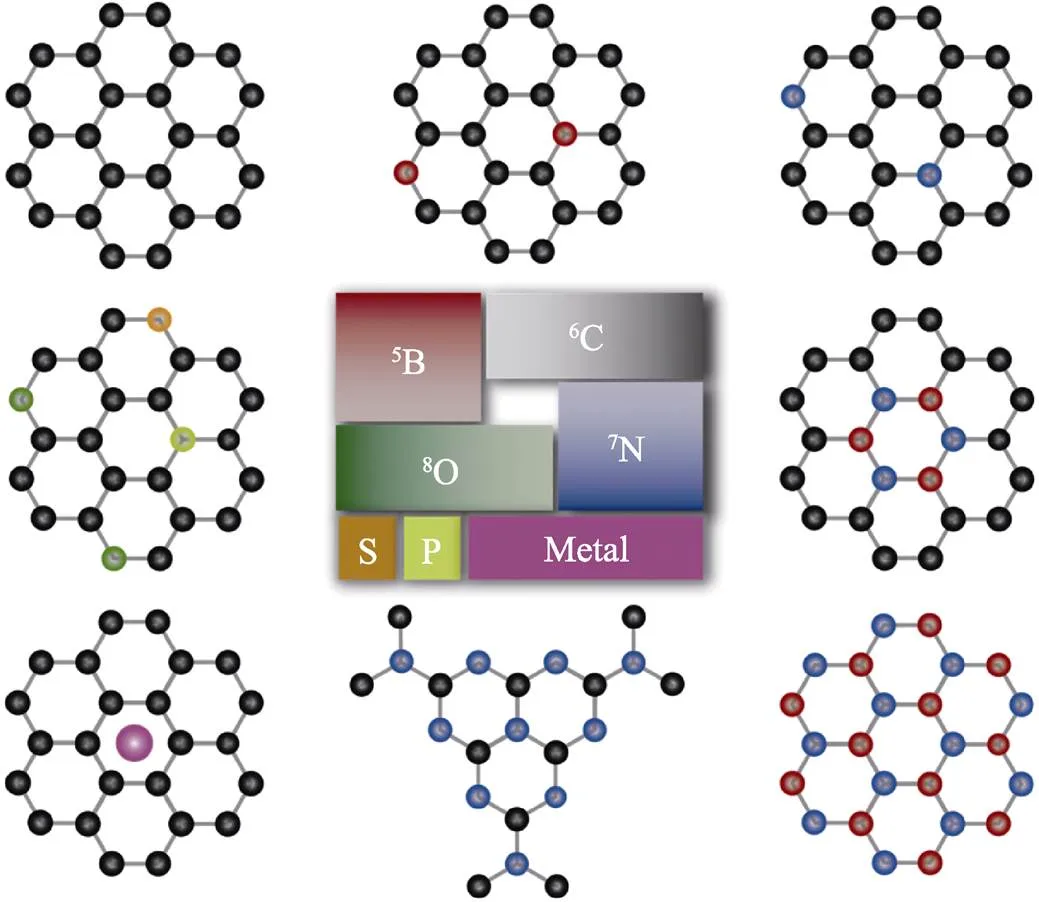
圖4 適合發泡的輕元素B-C-N-O體系
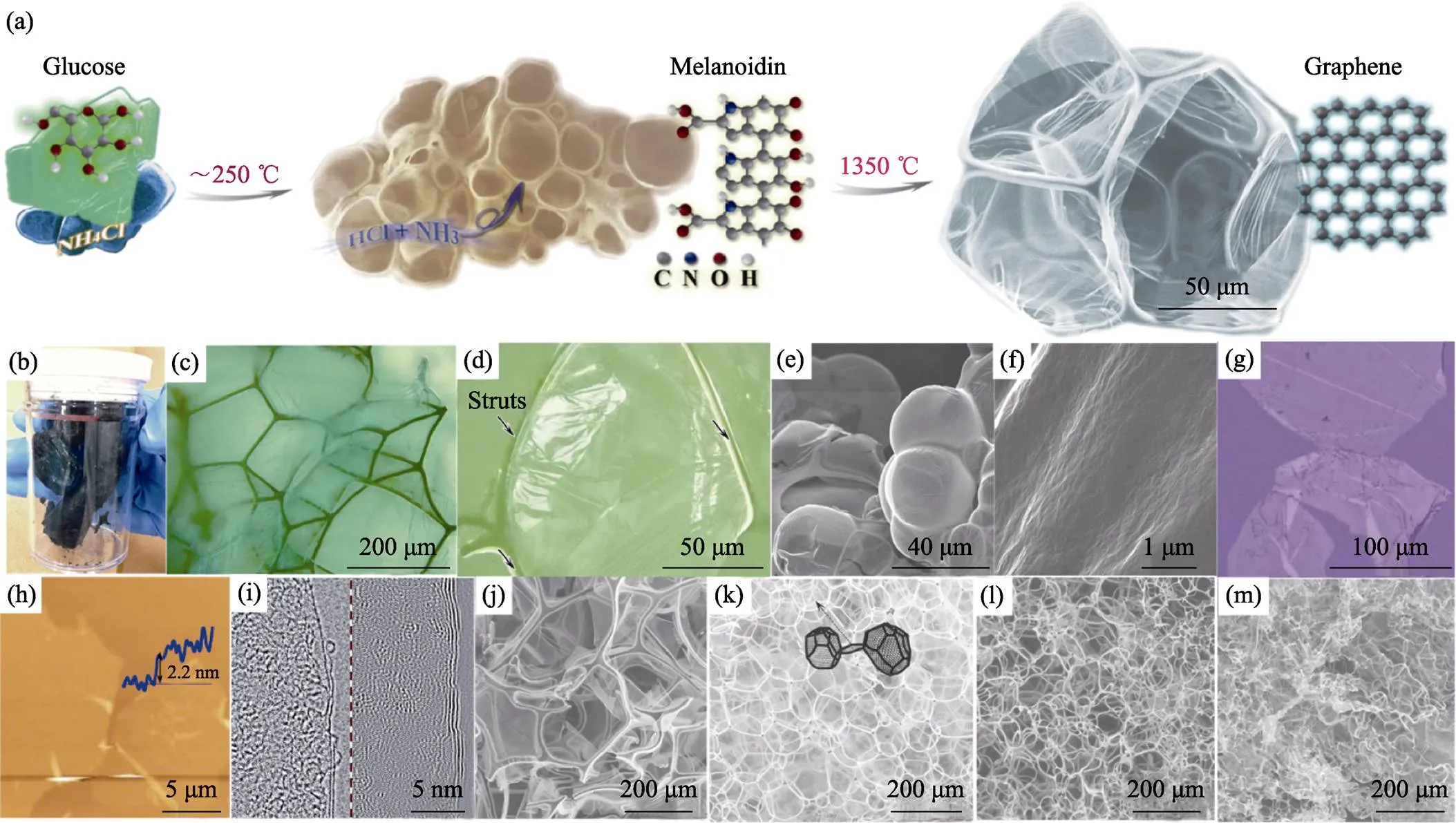
圖5 (a)化學發泡法示意圖; (b, c)SG實物和光學圖像; (d)SG中石墨烯壁和石墨筋的光學圖像; (e, f)中間體聚合物泡沫及其泡壁的SEM照片; (g)純化后的石墨烯片層的光學照片; (h)石墨烯片層的AFM圖像; (i)SG的HRTEM照片; (j~m)分別以1、4、20、100 ℃/min加熱發泡得到的不同SG的SEM照片[24,55]
含金屬的鹽類也可以作為發泡劑。例如, Fe(NO3)3[30]、ZnCl2[56]、KHCO3[57]和Ni(NO3)2[58]分別使葡萄糖、水解淀粉、纖維素、麥芽糖發泡, 用來制備石墨烯泡沫體。從金屬元素的角度而言, Na、K和Zn等低沸點金屬在發泡過程中對碳有插層/刻蝕作用, 產生活化[59]、分層[22]等效應, 有利于提高表面積。過渡金屬元素Fe、Ni等, 在加熱時可以提高碳的石墨化程度[30,58,60-62], 但需要用強酸洗去這些高沸點金屬以獲得純碳泡沫體。
2.1.2 摻雜石墨烯泡沫體
發泡過程要經歷聚合物流體狀態, 其聚合反應步驟中易于摻入雜原子。N是常用摻雜元素, 例如吡啶氮可以激活其相鄰碳原子成為氧還原催化活性中心[63]。前述銨鹽發泡劑就可以在SG中摻入N元素, N含量在300 ℃時為18at%, 在1400 ℃時為0.4at%[55]。由于雜原子在高溫下容易流失, 需要提供額外的雜原子源以補償此損失[64-66], 提高摻雜量。在葡萄糖-NH4Cl發泡體系中加入三聚氰胺, 其發泡體在1100 ℃時含N為8.36at%[65]。此外, 也可進行多元素共摻雜[67-70], 如三聚氰胺分解使季戊四醇三聚氰胺磷酸鹽(PMP)發泡, 得到N–P–O共摻雜石墨烯泡沫體, 如圖6所示[67]。
2.1.3 擔載型石墨烯泡沫體
在發泡過程中, 若采用含過渡金屬鹽類作為發泡劑, 則其金屬元素可以保留在最終產品中, 構成擔載型石墨烯泡沫體。聚乙烯吡咯烷酮(PVP)-Fe(NO3)3體系經歷發泡, 可以制得擔載Fe2O3的碳泡沫體[35], 如圖7(a,b)。在葡萄糖-NH4Cl體系中分別加入CuCl2、CrCl3和Co(NO3)2, 則最終得到擔載Cu[71]、CrN[72]和Co2P[36]的碳泡沫體, 如圖7(c,d)。此外, 也可以利用水熱法等手段在石墨烯泡沫體上擔載功能物質, 例如SG擔載NiFeP超薄納米片[73], 如圖7(e,g)。使用含金屬鹽發泡劑的報道匯總于表2中。
2.2 類石墨氮化碳泡沫體
LU[42]、GUO[87]、WANG[88]和TALAPANENI[89]等利用發泡法制備了類石墨氮化碳g-C3N4納米片的泡沫體。將三聚氰胺或雙氰胺與NH4Cl混合[42,87-88], 在550~600 ℃下共熱, 則NH4Cl分解產氣可使Melem等中間體發泡, 最終得到g-C3N4泡沫體, 如圖8所示。g-C3N4泡沫體可以增強光催化產氫[42,88]和降解有機污染物[87-88]。
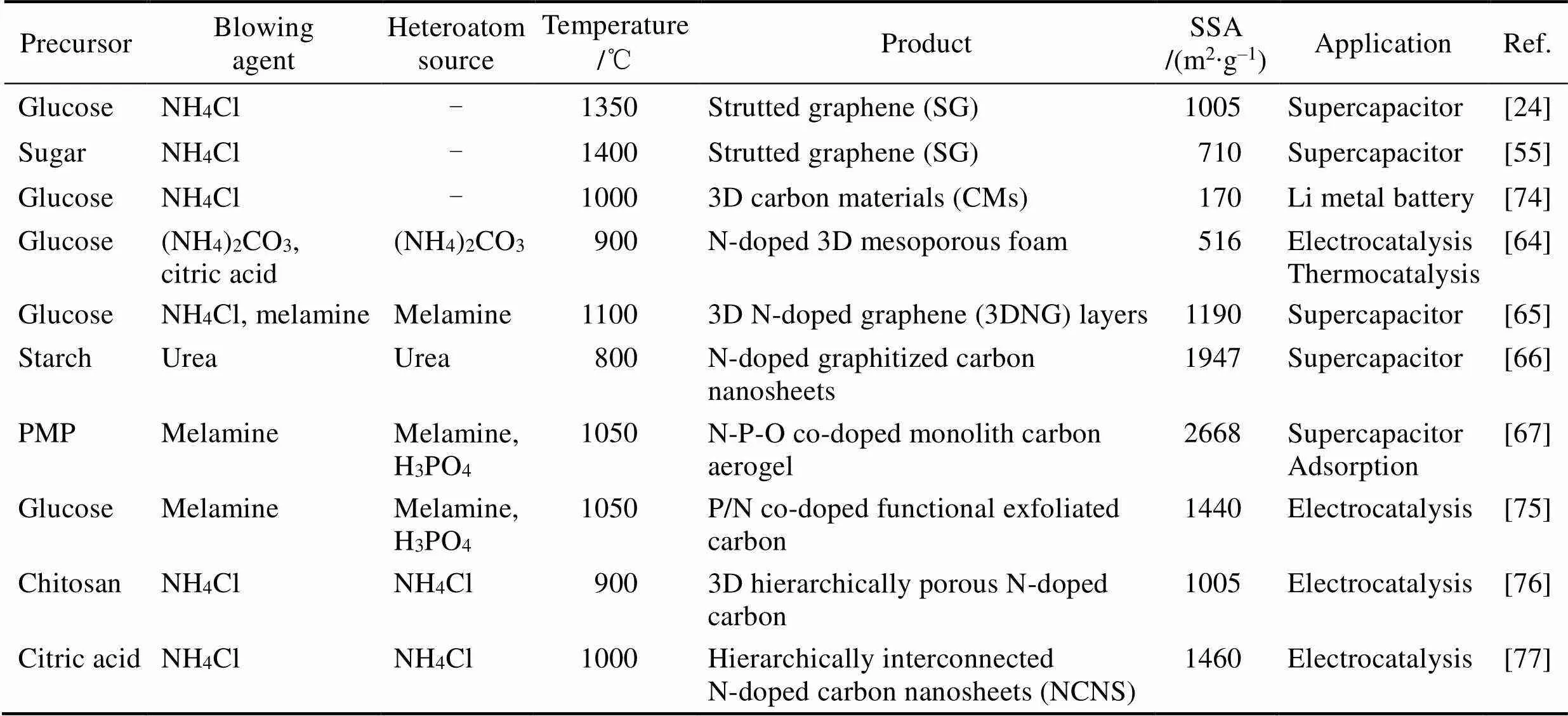
表1 采用發泡法制備的石墨烯泡沫體和摻雜石墨烯泡沫體(使用無殘留的銨鹽發泡劑)
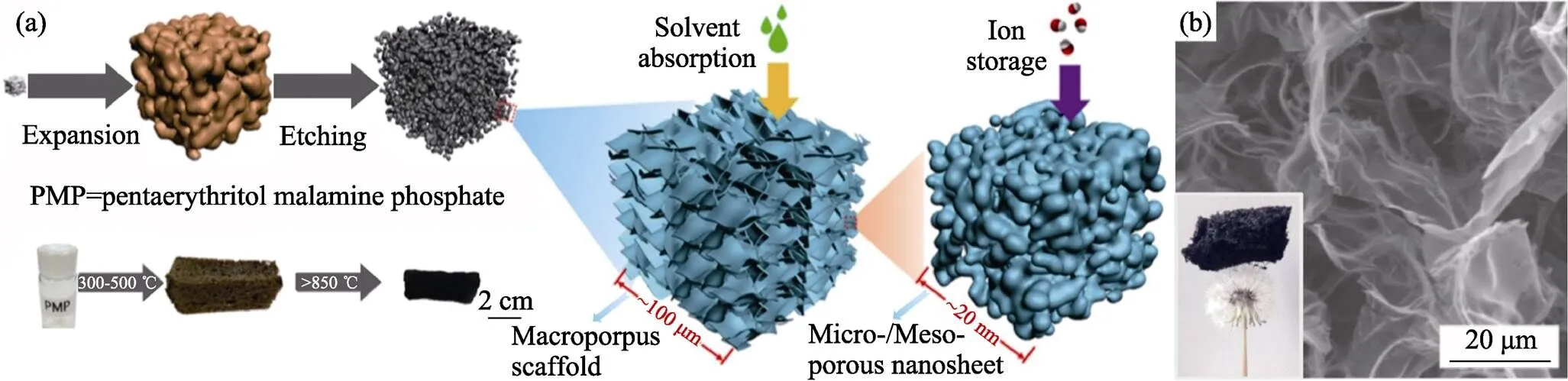
圖6 (a)以三聚氰胺發泡PMP過程示意圖; (b)N-P-O共摻雜石墨烯泡沫體的SEM照片[67]
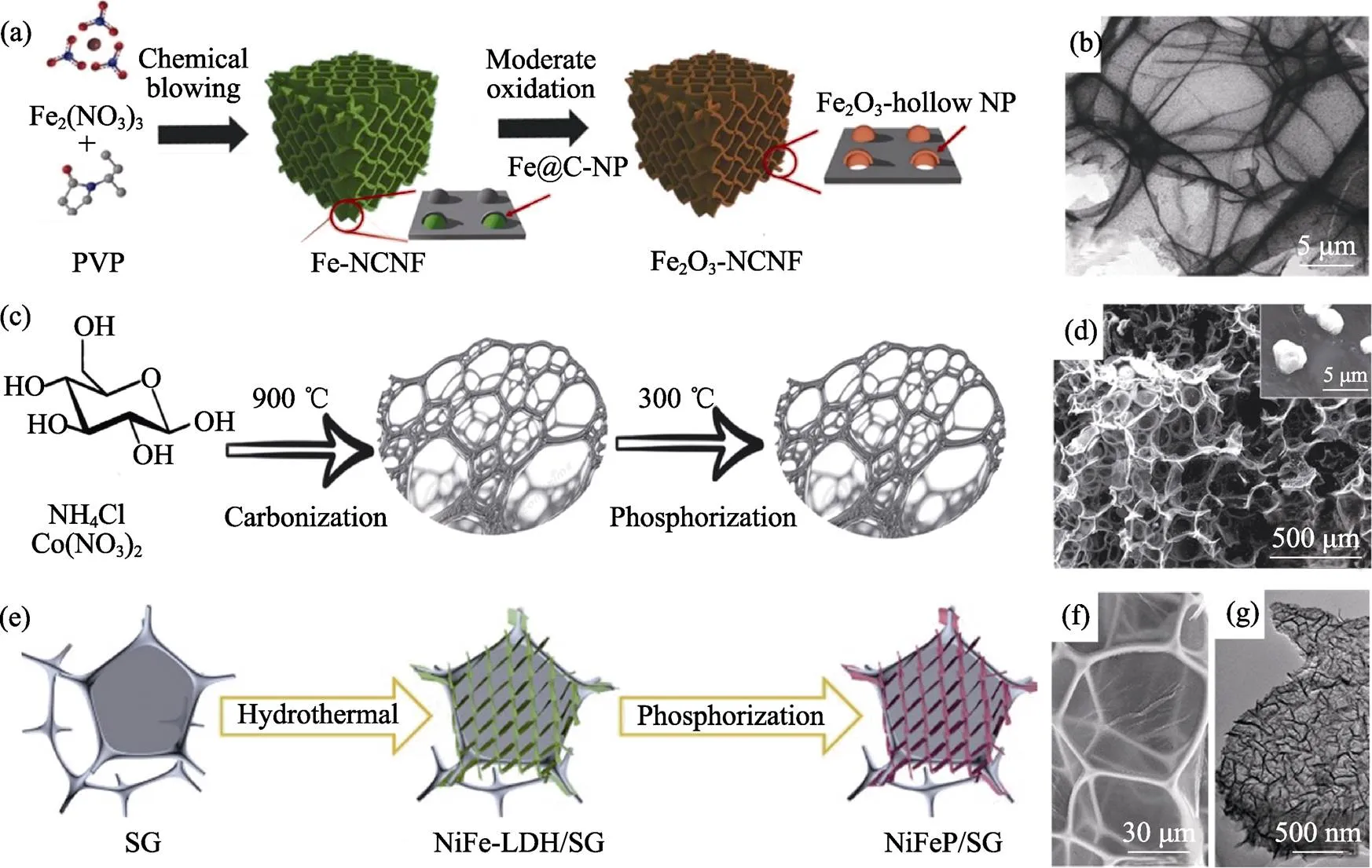
圖7 擔載型石墨烯泡沫體制備示意圖及其SEM/HRTEM照片
(a, b) Fe2O3[35]; (c, d) Co2P[36]; (e, g) NiFeP[73]
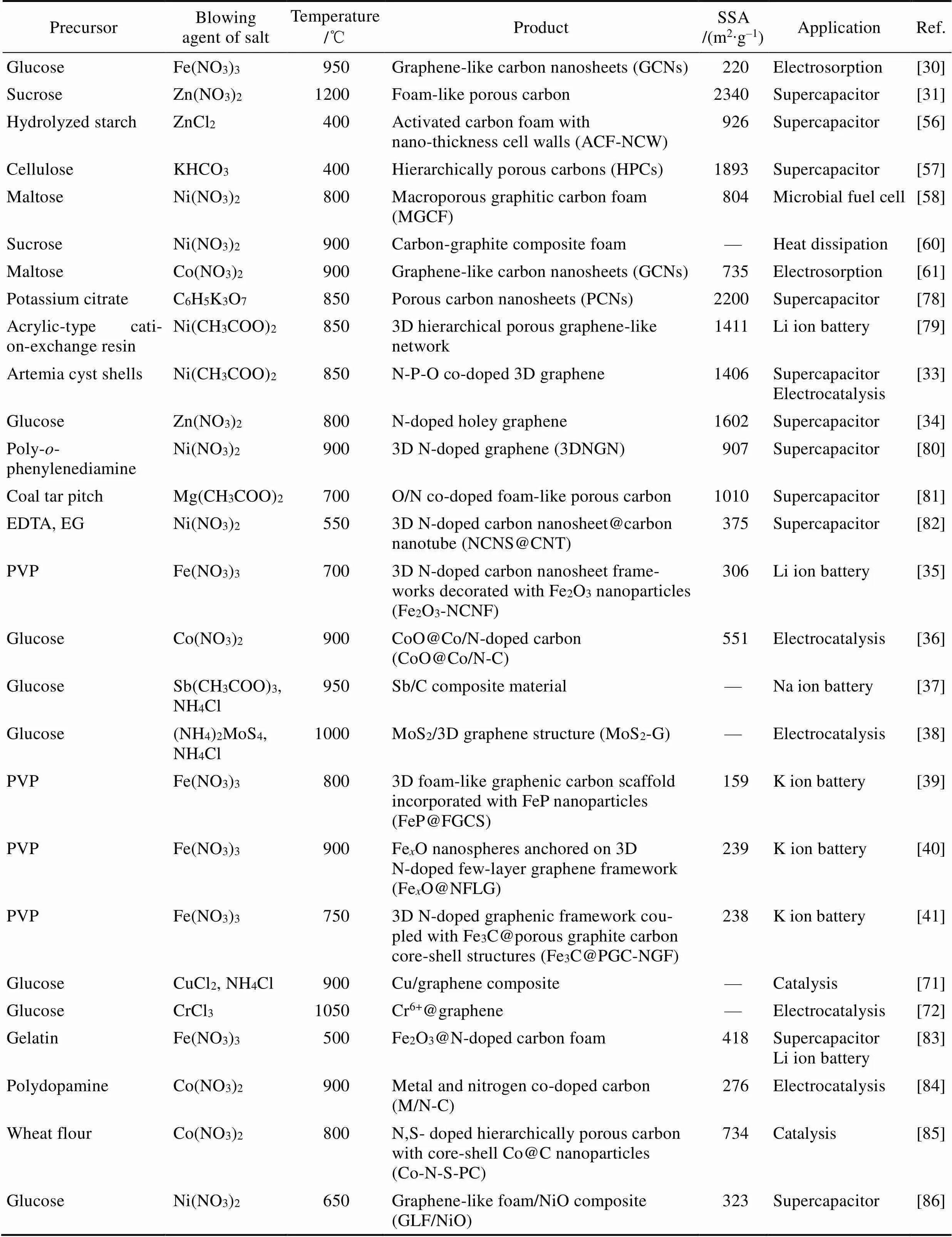
表2 采用發泡法制備石墨烯泡沫體和擔載型石墨烯泡沫體(使用含金屬元素的鹽類作為發泡劑)
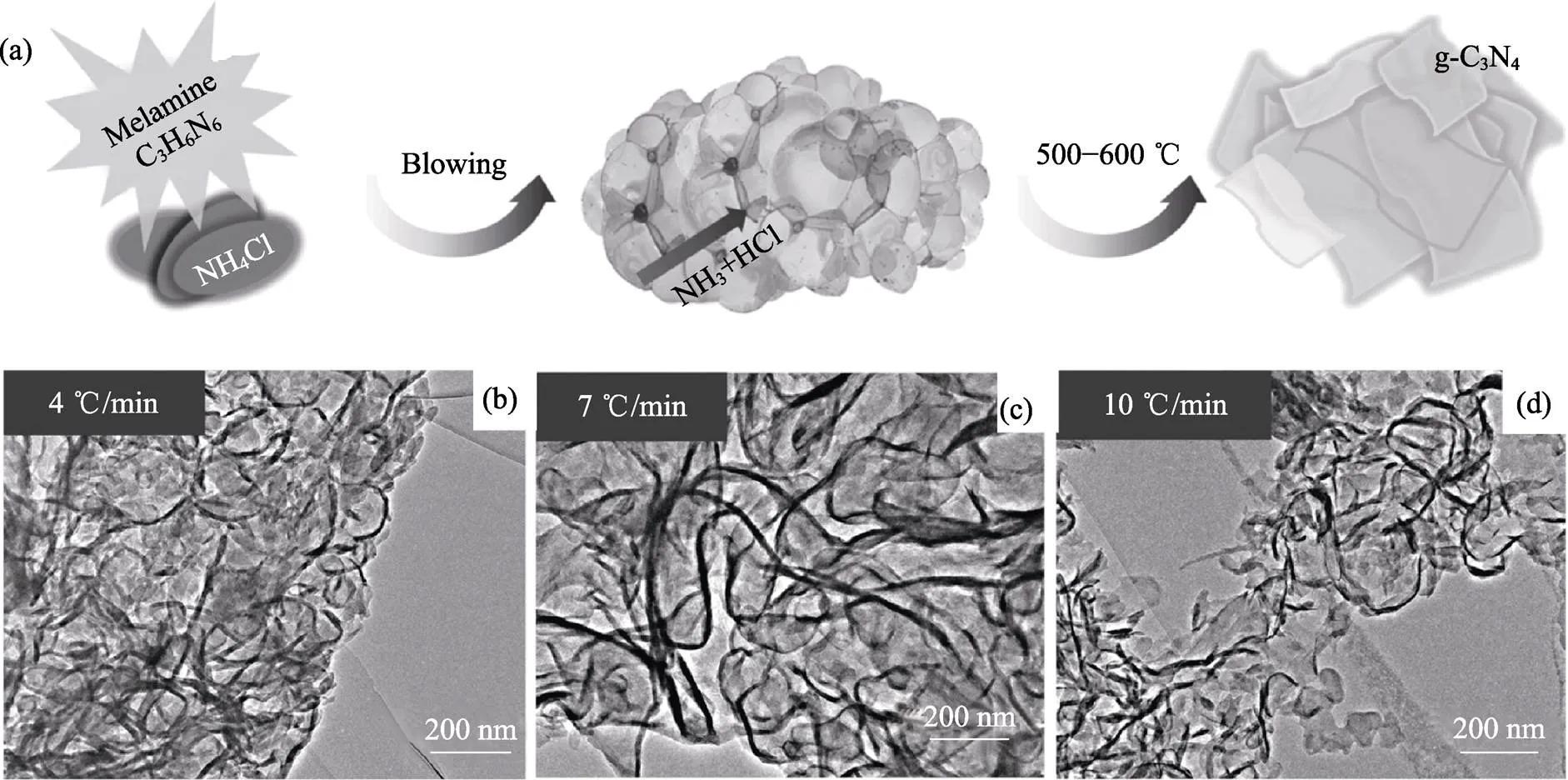
圖8 g-C3N4泡沫體的(a)合成示意圖及(b~d)不同加熱速率下發泡所獲得樣品的TEM照片[87]
2.3 氮化硼泡沫體及硼碳氮雜化泡沫體
六方氮化硼(BN)具有與石墨類似的強層內σ鍵和弱層間范德華力, 與石墨有類似的熱學和力學性質。由于異原子成鍵, BN具有部分離子性, 其光學、電學性質與石墨截然相反, 如寬帶隙、絕緣。BN有獨特的用途, 例如導熱、潤滑、絕緣、深紫外發光、中子吸收和抗癌等[90-94]。
王學斌等[29]通過加熱氨硼烷(AB)使之自發泡, 制備了BN泡沫體, 如圖9(a~e)所示。在加熱過程中, AB聚合為聚氨硼烷(PAB)、聚亞氨硼烷(PIB), 并釋放出H2, H2恰能使PAB和PIB發泡, 最終在1200 ℃下得到BN泡沫體, 經進一步純化可以獲得BN納米片。作為改良, 對AB進行預處理[95-96], 可以提高產品表面積。外加發泡劑可以使發泡過程更加可控, 例如在AB中添加硫脲或氨基硫脲發泡劑[43]。考慮到AB成本較高, WENG等[97]探索了更低成本的前驅體。將硼酸–聚環氧乙烷(PEO)在NH3氣氛下加熱, PEO為發泡劑, 最終得到多孔BN, 如圖9(f~h)。其它相關體系為氧化硼–鹽酸胍[98]、氟硼酸銨–疊氮化鈉[99]、硼酸–氰胺類物質[100-101]、硼酸–甲醛–雙氰胺[102]和硼酸–尿素[103-104]等。
鑒于BN和石墨互補的電學和光學性質, BCN有望實現帶隙調變[105]。在上述AB自發泡過程中, 通入乙醇氣氛, 可以獲得C-BN雜化納米片的發泡體,可在0.3~0.7之間調變, 具有半導體特性[29]。
2.4 氧化物納米片泡沫體
利用金屬鹽類發泡劑進行發泡時, 如果將惰性氣氛調整為氧化性氣氛, 則在合適條件下可得到氧化物納米片泡沫體[32,35,106]。在陶瓷漿料的傳統物理發泡過程中, 往往要加入表面活性劑降低發泡難度[107]。使用金屬鹽類發泡劑使碳基聚合物發泡, 則聚合物泡沫起到模板的作用, 引導金屬元素的分布, 最后經氧化轉化為氧化物的筋、膜, 構成氧化物泡沫體, 例如Mn3O4[32]、Fe2O3[32,35]、MgO[106]、MnO2[107]、V2O5[107]和MoO3[107]等。
3 二維材料泡沫體的應用
二維材料泡沫體兼備二維材料和泡沫結構的優點, 是一種有潛力的新材料, 不僅可以用作結構支撐材料, 也可用作多領域功能材料, 如圖10所示。
在力學方面, 泡沫體是一種輕質支撐材料, 如同自然界演化出的骨頭和樹木, 可用作包裝、填充和漂浮等[29,96,108-109]。石墨烯泡沫體可以支撐自身重量50000倍的物體[17]。三維網絡結構可以經99%應變后表現出良好的回彈性, 可循環上千次[110]。石墨烯泡沫體還可以吸收沖擊能量[17,110]。低楊氏模量的泡沫體可用于壓敏傳感[111]。
在熱學方面, 石墨烯泡沫體和BN泡沫體在熱控領域應用廣泛。它們可以作為三維化填料用于導熱增強復合材料, 以減小填料-填料的界面熱阻。石墨烯泡沫體還可以用作相變蓄熱材料的填料[112]。BN泡沫體可以用于增強聚合物基復合材料的熱導率[113-118], 用于電子封裝等方面。
在吸附相關方面, 石墨烯泡沫體和BN泡沫體可以捕獲CO2[119,120]、儲氫[120,121]、儲甲烷[120]、吸附有機污染物[122-125]、油水分離[126-128]、吸附重金屬離子[99,102]和氣敏傳感[21]。BN泡沫體吸附能力高, 而且化學穩定性強, 循環性能優異。

圖9 氨硼烷(AB)自發泡法制備BN泡沫體: (a)示意圖, (b)SEM照片, (c)光學照片, (d)AFM和HRTEM圖像[29]; 硼酸-PEO體系發泡制備BN泡沫體: (f)示意圖, (g, h)SEM照片[97]
在電化學方面, 石墨烯泡沫體可用作先進的電化學多孔電極。石墨烯泡沫體適用于超級電容器, 其高表面積有助于實現高容量; 同時高電導、內部孔腔網絡有助于實現高功率[129-132]。摻雜和擔載型石墨烯泡沫體可以用于電化學儲能和催化, 包括贗電容器[133]、鋰離子電池[35,79,83]、鈉離子電池[37]、鉀離子電池[39-41],、燃料電池[33,64,75,84]、微生物燃料電池[58]和電催化分解水[36,73,84]等方面。
在電學方面, 石墨烯泡沫體可用作多孔集流體, 代替泡沫鎳等, 以改善電流分布[134]。它具有憎水性, 可以用作防水透氣氣體電極。石墨烯基泡沫體還可以吸聲、吸波、屏蔽電磁波和吸收散亂電子[135]。
4 結束語
二維材料的三維化設計是二維材料宏觀塊體材料發展的必由之路。二維材料泡沫體具有優良的機械特性, 同時提供了雙聯通(固相、空腔)的網絡結構、高比表面積, 對于界面相關的應用意義重大。發泡法是一種低成本、可工業化的工藝, 可以制備先進的二維材料泡沫體, 但本領域仍有一些亟需研究的難點和發展前沿:
1)可控性是發泡制備工藝的難點。由于發泡體系的復雜性, 目前尚難以精確控制泡孔的尺寸和均勻性。此外, 開發混合發泡劑也是一個新的研究方向。

圖10 基于二維材料泡沫體的應用
2)發泡法目前集中于B–C–N–O輕元素體系, 極少用于其它二維材料泡沫體。有待大力發展過渡金屬硫化物、層狀雙氫氧化物和過渡金屬碳氮化物的泡沫體。
3)設計制備新型結構的泡沫體是新材料的發展重點, 例如, 負泊松比的擠縮泡沫體、分等級多級孔泡沫體和負曲率曲面的泡沫體等。
期待通過完善發泡理論, 探索發泡體系并提高工藝可控性, 發展多種二維材料泡沫體新材料; 解析二維材料泡沫體的構效關系, 開發其在多學科多領域的豐富應用。
[1] LIAO L, PENG H, LIU Z. Chemistry makes graphene beyond graphene., 2014, 136(35): 12194–12200.
[2] HUANG X, QI X, BOEY F,Graphene-based composites., 2012, 41(2): 666–686.
[3] NAIR R R, BLAKE P, GRIGORENKO A N,Fine structure constant defines visual transparency of graphene., 2008, 320(5881): 1308.
[4] BALANDIN A A, GHOSH S, BAO W,Superior thermal conductivity of single-layer graphene., 2008, 8(3): 902–907.
[5] BOLOTIN K I, SIKES K J, JIANG Z,Ultrahigh electron mobility in suspended graphene., 2008, 146(9/10): 351–355.
[6] LENG K, ZHANG F, ZHANG L,Graphene-based Li-ion hybrid supercapacitors with ultrahigh performance., 2013, 6(8): 581–592.
[7] XIE G, ZHANG K, GUO B,Graphene-based materials for hydrogen generation from light-driven water splitting., 2013, 25(28): 3820–3839.
[8] HOU J, SHAO Y, ELLIS M W,Graphene-based electrochemical energy conversion and storage: fuel cells, supercapacitors and lithium ion batteries., 2011, 13(34): 15384–15402.
[9] BROWNSON D A C, KAMPOURIS D K, BANKS C E. An overview of graphene in energy production and storage applications., 2011, 196(11): 4873–4885.
[10] LU B, LI T, ZHAO H,. Graphene-based composite materials beneficial to wound healing., 2012, 4(9): 2978–2982.
[11] CHEN Z, REN W, GAO L,Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition., 2011, 10(6): 424–428.
[12] LI D, MULLER M B, GILJE S,. Processable aqueous dispersions of graphene nanosheets., 2008, 3(2): 101–105.
[13] XU Y, SHENG K, LI C,. Self-assembled graphene hydrogela one-step hydrothermal process., 2010, 4(7): 4324–4330.
[14] XU Y, LIN Z, HUANG X,Functionalized graphene hydrogel- based high-performance supercapacitors., 2013, 25(40): 5779–5784.
[15] MAO S, LU G, CHEN J. Three-dimensional graphene-based composites for energy applications., 2015, 7(16): 6924–6943.
[16] YANG X, QIU L, CHENG C,. Ordered gelation of chemically converted graphene for next-generation electroconductive hydrogel films., 2011, 50(32): 7325–7328.
[17] QIU L, LIU J, CHANG S,. Biomimetic superelastic graphene- based cellular monoliths., 2012, 3: 1241.
[18] TANG Z, SHEN S, ZHUANG J,. Noble-metal-promoted three-dimensional macroassembly of single-layered graphene oxide., 2010, 122(27): 4707–4711.
[19] XU Y, WU Q, SUN Y,Three-dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels., 2010, 4(12): 7358–7362.
[20] CAO X, SHI Y, SHI W,Preparation of novel 3D graphene networks for supercapacitor applications., 2011, 7(22): 3163–3168.
[21] YAVARI F, CHEN Z, THOMAS A V,High sensitivity gas detection using a macroscopic three-dimensional graphene foam network., 2011, 1:166.
[22] JIANG X, LI R, HU M.. Zinc-tiered synthesis of 3D graphene for monolithic electrodes., 2019, 31(25): e1901186.
[23] GAO T, XU C, LI R,. Biomass-derived carbon paper to sandwich magnetite anode for long-life Li-ion battery., 2019, 13(10): 11901–11911.
[24] WANG X, ZHANG Y, ZHI C,Three-dimensional strutted graphene grown by substrate-free sugar blowing for high-power- density supercapacitors.,2013, 4(4): 2905.
[25] JIANG Y, XU Z, HUANG T,. Direct 3D printing of ultralight graphene oxide aerogel microlattices., 2018, 28(16): 1707024.
[26] SHEHZAD K, XU Y, GAO C,Three-dimensional macro-structures of two-dimensional nanomaterials.,2016, 45(20): 5541–5588.
[27] ITO Y, TANABE Y, QIU H J,High-quality three-dimensionalnanoporous graphene.,2014, 126(19):4922–4926.
[28] QIU L, HE Z, LI D. Multifunctional cellular materials based on 2D nanomaterials: prospects and challenges.,2017, 30(4): 1704850.
[29] WANG X, ZHI C, LI L,“Chemical blowing” of thin-walled bubbles: high-throughput fabrication of large-area, few-layered BN and C()-BN nanosheets.,2011, 23(35): 4072–4076.
[30] LEI H, YAN T, WANG H,Graphene-like carbon nanosheets prepared by a Fe-catalyzed glucose-blowing method for capacitive deionization.,2015, 3(11): 5934–5941.
[31] WANG C, O’CONNELL M J, CHAN C K. Facile one-pot synthesis of highly porous carbon foams for high-performance supercapacitors using template-free direct pyrolysis., 2015, 7(16): 8952–8960.
[32] WANG D, ZHOU W, ZHANG R,Mass production of large-sized, nonlayered 2D nanosheets: their directed synthesis by a rapid “gel-blowing” strategy, and applications in Li/Na storage and catalysis., 2018, 30(43): 1803569.
[33] ZHAO Y, HUANG S, XIA M,N-P-O co-doped high performance 3D graphene prepared through red phosphorous-assisted“cutting-thin” technique: a universal synthesis and multifunctional applications.,2016, 28: 346–355.
[34] DONG X, HU N, WEI L,A new strategy to prepare N-doped holey graphene for high-volumetric supercapacitors.,2016, 4(25): 9739–9743.
[35] DONG Y, YU M, WANG Z,A top-down strategy toward 3D carbon nanosheet frameworks decorated with hollow nanostructures for superior lithium storage.,2016, 26(42): 7590–7598.
[36] ZHU C, FU S, XU B,Sugar blowing-induced porous cobalt phosphide/nitrogen-doped carbon nanostructures with enhanced electrochemical oxidation performance toward water and other small molecules.,2017, 13(33): 1700796.
[37] WU Z, JOHANNESSEN B, ZHANG W,incorporation of nanostructured antimony in an N-doped carbon matrix for advanced sodium-ion batteries., 2019, 7(20): 12842–12850.
[38] CAI L, LIN Z, WANG M,. Improved interfacial H2O supply by surface hydroxyl groups for enhanced alkaline hydrogen evolution., 2017, 5(46): 24091–24097.
[39] TAN Q, ZHAO W, HAN K,. The multi-yolk/shell structure of FeP@foam-like graphenic scaffolds: strong P-C bonds and electrolyte- and binder-optimization boost potassium storage., 2019, 7(26): 15673–15682.
[40] TAN Q, LI P, HAN K,. Chemically bubbled hollow FexO nanospheres anchored on 3D N-doped few-layer graphene architecture as a performance-enhanced anode material for potassium- ion batteries., 2019, 7(2): 744–754.
[41] HAN K, LIU Z, LI P,High-throughput fabrication of 3D N-doped graphenic framework coupled with Fe3C@porous graphite carbon for ultrastable potassium ion storage., 2019, 22: 185–193.
[42] LU X, XU K, CHEN P,Facile one step method realizing scalable production of g-C3N4nanosheets and study of their photocatalytic H2evolution activity.,2014, 2(44): 18924–18928.
[43] ZHAO H, SONG X, ZENG H. 3D white graphene foam scavengers: vesicant-assisted foaming boosts the gram-level yield and forms hierarchical pores for superstrong pollutant removal applications.,2015, 7(3): e168.
[44] LAKATOS I. Proofs and refutations: the logic of mathematical discovery, 4th ed. Cambridge: Cambridge University Press, 2015, 1–183.
[45] WEAIRE D. Some remarks on the arrangement of grains in a polycrystal.,1974, 7(2): 157–160.
[46] RIVIER N. Recent results on the ideal structure of glasses.,1982, 43(C9): 91–95.
[47] WEAIRE D, PHELAN R. A counter-example to Kelvin's conjecture on minimal surfaces.,1994, 69(2): 107–110.
[48] 黃晉, 孫其誠. 液態泡沫滲流的機理研究進展. 力學進展, 2007, 37(2): 269–278.
[49] ZHOU C, YANG K, WANG K,Combination of fused deposition modeling and gas foaming technique to fabricated hierarchical macro/microporous polymer scaffolds.,2016, 109: 415–424.
[50] BANHART J. Light-metal foams-history of innovation and technological challenges.,2013, 15(3): 82–111.
[51] STUDART A R, GONZENBACH U T, TERVOORT E,Processing routes to macroporous ceramics: a review.,2006, 89(6): 1771–1789.
[52] INAGAKI M, QIU J, GUO Q. Carbon foam: preparation and application.,2015, 87: 128–152.
[53] LIU M, GAN L, ZHAO F,Carbon foams with high compressive strength derived from polyarylacetylene resin.,2007, 45(15): 3055–3057.
[54] CHEN S, HE G, HU H,Elastic carbon foamdirect carbonization of polymer foam for flexible electrodes and organic chemical absorption.,2013, 6(8): 2435–2439.
[55] JIANG X, WANG X, DAI P,High-throughput fabrication of strutted graphene by ammonium-assisted chemical blowing for high-performance supercapacitors.,2015, 16: 81–90.
[56] LEI H, CHEN D, HUO J. Blowing andactivation of carbonaceous “lather” from starch: preparation and potential application.,2016, 92: 362–370.
[57] DENG J, XIONG T, XU F,Inspired by bread leavening: one-pot synthesis of hierarchically porous carbon for superca-pacitors.,2015, 17(7): 4053–4060.
[58] JIANG H, YANG L, DENG W,Macroporous graphitic carbon foam decorated with polydopamine as a high-performance anode for microbial fuel cell.,2017, 363: 27–33.
[59] ZHU Y, MURALI S, STOLLER M D,Carbon-based supercapacitors produced by activation of graphene., 2011, 332(6037): 1537–1541.
[60] JANA P, BARRIO E P D, FIERRO V,Design of carbon foams for seasonal solar thermal energy storage.,2016, 109: 771–787.
[61] JIANG H, WANG S, DENG W,Graphene-like carbon nanosheets as a new electrode material for electrochemical determination of hydroquinone and catechol.,2017, 164: 300–306.
[62] LI Y, LI Z, SHEN P. Simultaneous formation of ultrahigh surface area and three-dimensional hierarchical porous graphene-like networks for fast and highly stable supercapacitors.,2013, 25(17): 2474–2480.
[63] GUO D, SHIBUYA R, AKIBA C,Active sites of nitrogen- doped carbon materials for oxygen reduction reaction clarified using model catalysts.,2016, 351(6271): 361–365.
[64] BA H, LIU Y, TRUONG-PHUOC L,N-doped food-grade- derived 3D mesoporous foams as metal-free systems for catalysis.,2016, 6(3): 1408–1419.
[65] HAO J, SHU D, GUO S,Preparation of three-dimensional nitrogen-doped graphene layers by gas foaming method and its electrochemical capactive behavior., 2016, 193: 293–301.
[66] CHANG B, YIN H, ZHANG X,Chemical blowing strategy synthesis of nitrogen-rich porous graphitized carbon nanosheets: morphology, pore structure and supercapacitor application., 2017, 312: 191–203.
[67] QI F, XIA Z, JIN J,Chemical foaming coupled self-etching: a multiscale processing strategy for ultrahigh-surface-area carbon aerogels.,2018, 10(3): 2819–2827.
[68] ZHENG Y, JIAO Y, GE L,Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis., 2013, 52(11): 3110–3116.
[69] ZHAO Y, YANG L, CHEN S,. Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes?, 2013, 135(4): 1201–1204.
[70] JIAO Y, ZHENG Y, DAVEY K,Activity origin and catalyst design principles for electrocatalytic hydrogen evolution on heteroatom- doped graphene., 2016, 1(10): 16130.
[71] JIN L, HE G, XUE J,Cu/graphene with high catalytic activity prepared by glucose blowing for reduction of-nitrophenol., 2017, 161: 655–662.
[72] YAO Y, XU Z, CHENG F,Unlocking the potential of graphene for water oxidation using an orbital hybridization strategy.,2018, 11(2): 407–416.
[73] LI R, WANG B, GAO T,. Monolithic electrode integrated of ultrathin NiFeP on 3D strutted graphene for bifunctionally efficient overall water splitting., 2019, 58: 870–876.
[74] XU Y, WANG L, JIA W,. Three-dimensional carbon material as stable host for dendrite-free lithium metal anodes., 2019, 301: 251–257.
[75] JIN J, GU L, JIANG L,A direct phase separation approach synthesis of hierarchically porous functional carbon as an advanced electrocatalyst for oxygen reduction reaction.,2016, 109: 306–313.
[76] WANG Y, HAO J, YU J,. Hierarchically porous N-doped carbon derived from biomass as oxygen reduction electrocatalyst for high-performance Al-air battery., 2020, 45: 119–125.
[77] WANG H, YI Q, GAO L,Hierarchically interconnected nitrogen- doped carbon nanosheets for an efficient hydrogen evolution reaction., 2017, 9(42): 16342–16348.
[78] SEVILLA M, FUERTES A B. Direct synthesis of highly porous interconnected carbon nanosheets and their application as high- performance supercapacitors.,2014, 8(5): 5069–5078.
[79] LI Y, ZHANG H, SHEN P. Ultrasmall metal oxide nanoparticles anchored on three-dimensional hierarchical porous gaphene-like networks as anode for high-performance lithium ion batteries.,2015, 13: 563–572.
[80] DENG W, ZHANG Y, TAN Y,Three-dimensional nitrogen- doped graphene derived from poly-o-phenylenediamine for high- performance supercapacitors., 2017, 787: 103–109.
[81] SHAO J, MA F, WU G,Facile preparation of 3D nanostructured O/N co-doped porous carbon constructed by interconnected carbon nanosheets for excellent-performance supercapacitors.,2016, 222: 793–805.
[82] ZHOU W, DU Y, ZENG J,A modified “gel-blowing” strategy toward the one-step mass production of a 3D N-doped carbon nanosheet@carbon nanotube hybrid network for supercapacitors., 2019, 11(16): 7624–7633.
[83] LI J, WANG N, DENG J,Flexible metal-templated fabrication of mesoporous onion-like carbon and Fe2O3@N-doped carbon foam for electrochemical energy storage.,2018, 6(27): 13012–13020.
[84] LI B, CHEN Y, GE X,Mussel-inspired one-pot synthesis of transition metal and nitrogen co-doped carbon (M/N-C) as efficient oxygen catalysts for Zn-air batteries.,2016, 8(9): 5067–5075.
[85] TIAN W, ZHANG H, QIAN Z,Bread-making synthesis of hierarchically Co@C nanoarchitecture in heteroatom doped porous carbons for oxidative degradation of emerging contaminants.,2018, 225: 76–83.
[86] HE C, JIANG Y, ZHANG X,. A simple glucose-blowing approach to graphene-like foam/NiO composites for asymmetric supercapacitors., 2019: 1900923.
[87] GUO Q, ZHANG Y, ZHANG H,3D foam strutted graphene carbon nitride with highly stable optoelectronic properties.,2017, 27(42): 1703711.
[88] WANG H, WU Y, FENG M,Visible-light-driven removal of tetracycline antibiotics and reclamation of hydrogen energy from natural water matrices and wastewater by polymeric carbon nitride foam.,2018, 144: 215–225.
[89] TALAPANENI S N, LEE J H, JE S H,. Chemical blowing approach for ultramicroporous carbon nitride frameworks and their applications in gas and energy storage., 2017, 27(1): 1604658.
[90] WENG Q, WANG X, WANG X,Functionalized hexagonal boron nitride nanomaterials: emerging properties and applications.,2016, 45(14): 3989–4012.
[91] JIANG X, WENG Q, WANG X,Recent progress on fabrications and applications of boron nitride nanomaterials: a review.,2015, 31(6): 589–598.
[92] WANG X, ZHI C, WENG Q,. Boron nitride nanosheets: novel syntheses and applications in polymeric composites., 2013, 471: 012003.
[93] LI X, WANG X, ZHANG J,Hollow boron nitride nanospheres as boron reservoir for prostate cancer treatment.,2017, 8: 13936.
[94] WENG Q, WANG B, WANG X,Highly water-soluble, porous, and biocompatible boron nitrides for anticancer drug delivery.,2014, 8(6): 6123–6130.
[95] MALEKI M, BEITOLLAHI A, SHOKOUHIMEHR M. Template- free synthesis of porous boron nitride using a single source precursor.,2015, 5(58): 46823–46828.
[96] WANG X, PAKDEL A, ZHI C,High-yield boron nitride nanosheets from “chemical blowing”: towards practical applications in polymer composites.,2012, 24(31): 314205.
[97] WENG Q, IDE Y, WANG X,Design of BN porous sheets with richly exposed (002) plane edges and their application as TiO2visible light sensitizer.,2015, 16: 19–27.
[98] LEI W, PORTEHAULT D, LIU D,Porous boron nitride nanosheets for effective water cleaning.,2013, 4(2): 1777.
[99] LIAN G, ZHANG X, ZHANG S,Controlled fabrication of ultrathin-shell BN hollow spheres with excellent performance in hydrogen storage and wastewater treatment.,2012, 5(5): 7072–7080.
[100] WENG Q, WANG X, BANDO Y,One-step template-free synthesis of highly porous boron nitride microsponges for hydrogen storage.,2014, 4(7): 1301525.
[101] WENG Q, WANG X, ZHI C,Boron nitride porous microbelts for hydrogen storage., 2013, 7(2): 1558–1565.
[102] XUE Y, DAI P, JIANG X,Template-free synthesis of boron nitride foam-like porous monoliths and their high-end applications in water purification.,2016, 4(4): 1469–1478.
[103] NAG A, RAIDONGIA K, HEMBRAM K P S S,Graphene analogues of BN: novel synthesis and properties.,2010, 4(3): 1539–1544.
[104] WU P, ZHU W, CHAO Y,A template-free solvent-mediated synthesis of high surface area boron nitride nanosheets for aerobic oxidative desulfurization.,2016, 52(1): 144–147.
[105] CI L, SONG L, JIN C,Atomic layers of hybridized boron nitride and graphene domains.,2010, 9(5): 430–435.
[106] LIU S, WANG Z, HAN T,. Mesoporous magnesium oxide nanosheet electrocatalysts for the detection of lead (II)., 2019, 2(5): 2606–2611.
[107] LU K, XU J, ZHANG J,. General preparation of three- dimensional porous metal oxide foams coated with nitrogen- doped carbon for enhanced lithium storage., 2016, 8(27): 17402–17408.
[108] MEZA L R, DAS S, GREER J R. Strong, lightweight, and recoverable three-dimensional ceramic nanolattices., 2014, 345(6202): 1322–1326.
[109] SCHAEDLER T A, JACOBSEN A J, TORRENTS A,. Ultralight metallic microlattices., 2011, 334(6058): 962–965.
[110] WU Y, YI N, HUANG L,Three-dimensionally bonded spongy graphene material with super compressive elasticity and near-zero Poisson’s ratio., 2015, 6: 6141.
[111] QIU L, COSKUN M B, TANG Y,Ultrafast dynamic piezoresistive response of graphene-based cellular elastomers., 2016, 28(1): 194–200.
[112] JI H, SELLAN D P, PETTES M T,Enhanced thermal conductivity of phase change materials with ultrathin-graphite foams for thermal energy storage., 2014, 7(3): 1185–1192.
[113] WANG X, WENG Q, WANG X,Biomass-directed synthesis of 20 g high-quality boron nitride nanosheets for thermoconductive polymeric composites.,2013, 8(9): 9081–9088.
[114] WANG X, PAKDEL A, ZHANG J,Large-surface-area BN nanosheets and their utilization in polymeric composites with improved thermal and dielectric properties.,2012,7(1): 662.
[115] XU C, MIAO M, JIANG X,Thermal conductive composites reinforcedadvanced boron nitride nanomaterials.,2018, 10: 103–109.
[116] ZENG X, YAO Y, GONG Z,. Ice-templated assembly strategy to construct 3D boron nitride nanosheet networks in polymer composites for thermal conductivity improvement., 2015, 11(46): 6205–6213.
[117] XUE Y, DAI P, ZHOU M,. Multifunctional superelastic foam-like boron nitride nanotubular cellular-network architectures., 2017, 11(1): 558–568.
[118] TIAN Z, SUN J, WANG S,A thermal interface material based on foam-templated three-dimensional hierarchical porous boron nitride.,2018, 6(36): 17540–17547.
[119] NARASIMMAN R, VIJAYAN S, PRABHAKARAN K. Carbon particle induced foaming of molten sucrose for the preparation of carbon foams.,2014, 189: 82–89.
[120] SUN Q, LI Z, SEARLES D J,. Charge-controlled switchable CO2capture on boron nitride nanomaterials., 2013, 135(22): 8246–8253.
[121] WENG Q, WANG X, WANG X,Preparation and hydrogen sorption performances of BCNO porous microbelts with ultra- narrow and tunable pore widths.,2013, 8(12): 2936–2939.
[122] JIA H, LI J, LIU Z,Three-dimensional carbon boron nitrides with a broken, hollow, spherical shell for water treatment.,2016, 6(82): 78252–78256.
[123] LI J, HUANG Y, LIU Z,Chemical activation of boron nitride fibers for improved cationic dye removal performance.,2015, 3(15): 8185–8193.
[124] LIN J, XU L, HUANG Y,Ultrafine porous boron nitride nanofibers synthesizeda freeze-drying and pyrolysis process and their adsorption properties.,2016, 6(2): 1253–1259.
[125] ZHANG X, LIAN G, ZHANG S,Boron nitride nanocarpets: controllable synthesis and their adsorption performance to organic pollutants., 2012, 14: 4670–4676.
[126] CHANDKIRAM G CHANDRA S T, SUJIN J. Synthesis of low- density, carbon-doped, porous hexagonal boron nitride solids.,2015, 9(12): 12088–12095.
[127] LIN J, YUAN X, LI GSelf-assembly of porous boron nitride microfibers into ultralight multifunctional foams of large sizes.,2017, 9(51): 44732–44739.
[128] WU C, WANG B, WANG Y. One-step fabrication of boron nitride fibers networks.,2018, 44(5): 5385–5391.
[129] CHOI B G, YANG M H, HONG W H,3D macroporous graphene frameworks for supercapacitors with high energy and power densities.,2012, 6(5): 4020–4028.
[130] CHEN P, YANG J, LI S,Hydrothermal synthesis of macroscopic nitrogen-doped graphene hydrogels for ultrafast supercapacitor.,2013, 2(2): 249–256.
[131] YANG X, ZHU J, QIU L,Bioinspired effective prevention of restacking in multilayered graphene films: towards the next generation of high-performance supercapacitors.,2011, 23(25): 2833–2838.
[132] YANG X, CHENG C, WANG Y,Liquid-mediated dense integration of graphene materials for compact capacitive energy storage.,2013, 341(6145): 534–537.
[133] DONG X, XU H, WANG X,3D Graphene-cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection., 2012, 6(4): 3206–3213.
[134] KIM B, YANG G, PARK M,Three-dimensional graphene foam-based transparent conductive electrodes in GaN-based blue light-emitting diodes., 2013, 102(16): 161902.
[135] CHEN Z, XU C, MA C,Lightweight and flexible graphene foam composites for high-performance electromagnetic interference shielding., 2013, 25(9): 1296–1300.
Blowing Route to Fabricate Foams of 2D Materials
GAO Tian, XIAO Qinglin, XU Chenyang, WANG Xuebin
(National Laboratory of Solid State Microstructures, Collaborative Innovation Center of Advanced Microstructures, Jiangsu Key Laboratory of Artificial Functional Materials, College of Engineering and Applied Sciences, Nanjing University, Nanjing 210093, China)
Graphene, as a representative of two-dimensional (2D) materials, has excellent intrinsic properties such as high specific surface area and conductivity, but its macroscopic bulk behaves poorly owing to severe face-to-face restacking and hand-in-hand contact resistance. Three-dimensional (3D) design of 2D materials can deliver the excellent nanoscaled properties to the macroscopic world, to realize the high surface area, conductivity, interconnected pores, and good mechanics of the bulks. It is necessary and highlighted to develop the porous monolith of 2D materials for applications as electrodes, adsorbents, elastomers,. The blowing route has the advantages of low cost and simple processing, which has been accentually developed to produce the foams of 2D materials for several years. This article introduces the principle of the blowing method, summarizing the recent examples of blown foams of graphene, boron nitride nanosheet, and others. The scientific front about foams of 2D materials is discussed, and the broad applications of the new materials are prospected in energy, environment,.
2D material; blowing method; graphene; boron nitride; foam; review
TB321
A
1000-324X(2020)12-1315-12
10.15541/jim20200096
2020-02-29;
2020-04-09
青年千人計劃; 國家自然科學基金(51972168, 51672124, 21603096); 江蘇雙創計劃
Thousand Talents Plan for Youth; National Natural Science Foundation of China (51972168, 51672124, 21603096); Program for Innovative Talents and Entrepreneur in Jiangsu
高天(1992–), 男, 博士. E-mail: 1217387634@qq.com
GAO Tian(1992–), male, PhD. E-mail: 1217387634@qq.com
王學斌, 教授. E-mail: wangxb@nju.edu.cn
WANG Xuebin, professor.E-mail: wangxb@nju.edu.cn
- 無機材料學報的其它文章
- An Injectable Composite Bone Cement Based on Mesoporous Borosilicate Bioactive Glass Spheres
- A Layered Uranyl Coordination Polymer with UV Detection Sensitivity, Stability, and Reusability
- 原位聚合三維陶瓷骨架增強全固態鋰電池電解質
- WO3納米花的熱處理晶格調控及WO3/CdS/α-S異質結的構筑
- 共擠出法制備雙層中空纖維陶瓷復合膜
- Stable Zirconium Carbide Fibers Fabricated by Centrifugal Spinning Technique

