烷基鏈工程對兩親有機(jī)半導(dǎo)體熱力學(xué)性能影響的研究
李明亮,李碩,王國治,郭雪峰
1有研工程技術(shù)研究院有限公司,北京 101407
2北京有色金屬研究總院智能傳感功能材料國家重點(diǎn)實(shí)驗(yàn)室,北京 100088
3北京大學(xué)化學(xué)與分子工程學(xué)院,北京分子科學(xué)國家實(shí)驗(yàn)室,分子動態(tài)與穩(wěn)態(tài)結(jié)構(gòu)國家重點(diǎn)實(shí)驗(yàn)室,北京 100871
4北京大學(xué)工學(xué)院先進(jìn)材料與納米技術(shù)系,北京 100871
1 Introduction
With the development of the information industry, demands and requirements of organic semiconductors (OSC) in artificial intelligence and wearable devices are rapidly increasing1-5.Among them, the functional amphiphilic semiconductor (FAS)molecule has great potential in fabrication of biomimetic thin film morphology6-8and functional electronic devices9-11, for the reason that FAS has a special structure, is simple to process,low in cost and easy to form a thin film with good structure and morphology. The amphiphilic semiconductor molecule generally consists of a hydrophilic polar group and a hydrophobic semiconductor group12, but so far, the interaction between the two groups and the correspondence between the molecular property and structure are still blurred. This makes it more difficult to design and optimize semiconductor materials to realize the good performance and target properties.
Thermodynamics is an important material research methodology13. It can get information on the phase, structure and stability of materials through methods based on thermodynamics14-17. This method is fast and efficient,and has gradually attracted more attention by initially applying to amphiphilic semiconductor materials18. Therefore,in this work we designed and synthesized a FAS molecule(benzo[b]benzo[4,5] thieno[2,3-d]thiophene, named CnPABTBT,n= 3-11, the structures shown in Scheme 1). We connected the hydrophilic group and the hydrophobic group through alkyl chains with different lengths to study the effects of the chain length on the thermodynamic property of FAS19,20.The laws summarized from the thermodynamic performance provide an experimental basis for synthesizing and screening functional materials for different purposes.
2 Experimental and computational section
2.1 General methods and information
All reagents and chemicals were analytical reagents obtained from Innochem and Sinopharm, China. They are used without further purification unless otherwise noted. The synthetic route is outlined in Scheme 2. All reactions were performed under an inert atmosphere of argon in dry solvents by using standard Schlenk techniques.1H and13C NMR spectra were recorded on Bruker-400 MHz NMR ARX400, Germany. Chemical shifts of1H and13C NMR signals were quoted to tetramethylsilane (δ=0.00) and CDCl3(δ= 77.00) as internal standards, respectively.Mass spectra (MS) were recorded on a Bruker APEX IV mass spectrometer, Germany. Ultraviolet-visible (UV-Vis) absorption measurement of organic semiconductors in solution and in thin films (on quartz substrates) were determined with a Perkin-Elmer Lambda 950 UV/Vis spectrometer, USA.Thermogravimetric Analysis (TGA) measurements were performed on TA Instruments Q600 SDT thermal analysis system, USA under N2at a heating rate of 10 °C·min-1.Differential Scanning Calorimeter analysis (DSC)measurements were performed by using a TA Instruments Q2000 differential scanning calorimeter, USA under N2. Both heating and cooling speed were 10 °C·min-1. Polarized optical microscopy (POM) with heat stage were obtained on silicon substrates by using Nikon Eclipse LV100 POL, Japan in reflection mode on a home-made heat stage.
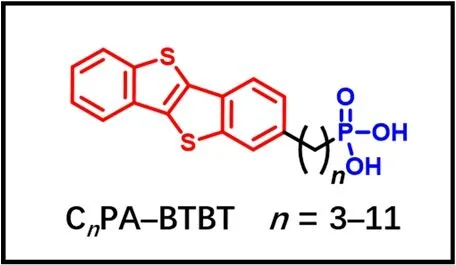
Scheme 1 Molecular Structures of CnPA-BTBT (n = 3-11).The semiconductor backbone and the polar group are red and blue,respectively. Color online.
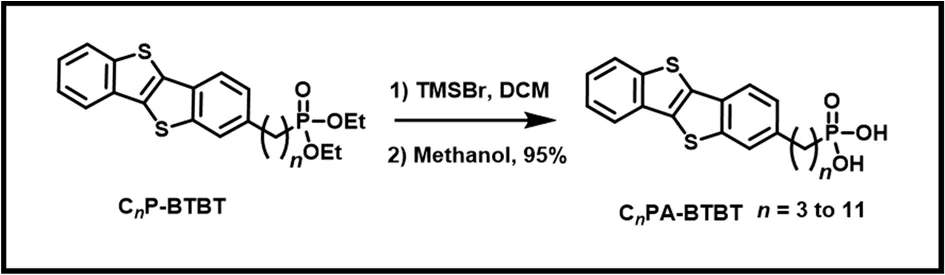
Scheme 2 Synthetic routine of CnPA-BTBT (n = 3-11).
2.2 Synthetics procedures and characterization
Synthetic Routine of CnPA-BTBT (n= 3-11) were synthesized according to the literature21,22. To a solution of CnPBTBT (0.168 mmol) in dry dichloromethane (1 mL) was added bromotrimethylsilane (0.3 mL). The mixture was stirred at the room temperature under argon atmosphere overnight. 2 mL methanol was added to the mixture and the mixture was further stirred for 2 h. All solvent and reagent were removed under reduced pressure to give a white solid.
C3PA-BTBT:1H NMR (d-THF, 500 MHz, 40 °C):δ7.95 (d,1H,J= 8.0 Hz), 7.87 (d, 1H,J= 7.8 Hz), 7.82 (s, 1H), 7.79 (d,1H,J= 8.1 Hz), 7.39 (m, 2H), 7.32 (d, 1H,J= 8.3 Hz), 2.88 (t,2H,J= 7.2 Hz), 2.00 (m, 2H), 1.30 (m, 2H);31P NMR (d-THF,200 MHz):δ31.44. EI-MS: Calcd. for [M - H]-: 361.012747.Found: 361.012749.
C4PA-BTBT:1H NMR (d-THF, 500 MHz, 40 °C):δ7.96 (d,1H,J= 7.8 Hz), 7.87 (d, 1H,J= 7.1 Hz), 7.80 (m, 2H), 7.41 (m,2H), 7.32 (d, 1H,J= 8.4 Hz), 2.80 (t, 2H,J= 7.7 Hz), 1.83 (m,4H), 1.31 (m, 2H);31P NMR (d-THF, 200 MHz):δ31.50. EIMS: Calcd. for [M - H]-: 375.028397. Found: 375.027394.
C5PA-BTBT:1H NMR (d-THF, 500 MHz, 40 °C):δ7.95 (d,1H,J= 7.9 Hz), 7.87 (d, 1H,J= 7.6 Hz), 7.80 (m, 2H), 7.40 (m,2H), 7.30 (d, 1H,J= 9.2 Hz), 2.78 (t, 2H,J= 7.7 Hz), 1.67 (m,6H), 1.50 (m, 2H);31P NMR (d-THF, 200 MHz):δ32.03. EIMS: Calcd. for [M - H]-: 389.044047. Found: 389.044994.
C6PA-BTBT:1H NMR (d-THF, 500 MHz, 40 °C):δ7.95 (d,1H,J= 8.0 Hz), 7.87 (d, 1H,J= 7.2 Hz), 7.80 (m, 2H), 7.41 (m,2H), 7.30 (d, 1H,J= 8.1 Hz), 2.78 (m, 2H), 1.62 (m, 2H), 1.31(m, 8H);31P NMR (d-THF, 200 MHz):δ32.06. EI-MS: Calcd.for [M - H]-: 403.059697. Found: 403.059647.
C7PA-BTBT:1H NMR (d-THF, 500 MHz, 40 °C):δ7.95 (d,1H,J= 8.0 Hz), 7.87 (d, 1H,J= 7.5 Hz), 7.80 (m, 2H), 7.41 (m,2H), 7.31 (d, 1H,J= 8.2 Hz), 2.78 (t, 2H,J= 7.6 Hz), 1.62 (m,4H), 1.31 (m, 8H);31P NMR (d-THF, 200 MHz):δ32.03. EIMS: Calcd. for [M - H]-: 417.075347. Found: 417.076116.
C8PA-BTBT:1H NMR (d-THF, 500 MHz, 40 °C):δ7.95 (d,1H,J= 8.0 Hz), 7.88 (d, 1H,J= 7.6 Hz), 7.80 (m, 2H), 7.41 (m,2H), 7.30 (d, 1H,J= 7.9 Hz), 2.78 (t, 2H,J= 7.6 Hz), 1.61 (m,4H), 1.31 (m, 10H);31P NMR (d-THF, 200 MHz):δ32.05. EIMS: Calcd. for [M - H]-: 431.090989. Found: 431.091066.
C9PA-BTBT:1H NMR (d-THF, 500 MHz, 40 °C):δ7.95 (d,1H,J= 8.0 Hz), 7.88 (d, 1H,J= 7.6 Hz), 7.80 (m, 2H), 7.41 (m,2H), 7.31 (d, 1H,J= 7.9 Hz), 2.78 (t, 2H,J= 7.6 Hz), 1.60 (m,4H), 1.31 (m, 12H);31P NMR (d-THF, 200 MHz):δ32.02. EIMS: Calcd. for [M - H]-: 445.106648. Found: 445.106766.
C10PA-BTBT:1H NMR (d-THF, 500 MHz, 40 °C):δ7.95 (d,1H,J= 8.0 Hz), 7.88 (d, 1H,J= 7.6 Hz), 7.80 (m, 2H), 7.41 (m,2H), 7.30 (d, 1H,J= 8.1 Hz), 2.78 (t, 2H,J= 7.6 Hz), 1.61 (m,4H), 1.31 (m, 14H);31P NMR (d-THF, 200 MHz):δ32.03. EIMS: Calcd. for [M - H]-: 459.122298. Found: 459.122675.
C11PA-BTBT:1H NMR (d-THF, 500 MHz, 40 °C):δ7.95 (d,1H,J= 7.9 Hz), 7.88 (d, 1H,J= 7.5 Hz), 7.80 (m, 2H), 7.41 (m,2H), 7.30 (d, 1H,J= 7.8 Hz), 2.78 (t, 2H,J= 7.7 Hz), 1.60 (m,4H), 1.31 (m, 16H);31P NMR (d-THF, 200 MHz):δ32.04. ESIMS: Calcd. for [M + H]+: 475.15250. Found: 475.15220.
3 Results and discussion
3.1 Molecular design
We designed and synthesized CnPA-BTBT (n= 3-11, Scheme 1) functional molecules with benzo[b]benzo[4,5]thieno[2,3-d]thiophene (BTBT) as the rigidπ-backbone, phosphate groups as polar functional groups, and the two parts were linked through alkyl chains with different lengths (the synthetic procedure in Scheme 2). The molecular design is mainly based on the following three considerations: (i) BTBT is a high-performance semiconductor backbone23,24. The strongπ-πinteraction between BTBT groups is beneficial to ensure the performance of electronic devices; (ii) Phosphate groups with strong polar interaction are low in synthesis cost and simple to process25. (iii)Alkyl chains with different lengths are introduced into the system as substituent, and thus adjust the properties13,14. The conjugate interaction of BTBT groups, the polar interaction of phosphate groups and van der Waals interaction of alkyl chains enrich the diversity of the thermodynamic performance and enhance the ability of self-assembly.
3.2 Fundamental properties.
The fundamental properties of the functional materials CnPABTBT (n= 3-11) were first studied. The UV-Vis spectroscopy is a molecular spectrum formed by valence electron transitions.The band gap of FAS can be calculated from the initial absorption value of UV-Vis. Fig. 1a, b are the UV-Vis curves of the functional molecule solution in chloroform (10-5mol·L-1)and in the solid film, respectively. We found that in the solution,the UV-Vis curves of all functional molecules are substantially coincident, while in the solid phase film, the curves vary greatly.Statistical calculation of the energy gap is shown in Fig. 1c.Compared with the band gaps of solution system atca.4.4 eV,the ones of solid film fluctuate and areca.1 eV lower on average.This is due to the better arrangement of FAS molecules in the solid film, which makes the transition of valence electrons easier.The curves show a trough and a peak whenn= 3 andn= 7,respectively, which may be due to the comprehensive effect of the alkyl chain on the intermolecular arrangement and the conjugation of intramolecular groups. On the other hand, in the case of short alkyl chains (n= 3, 4), the polar group and the semiconductor group are significantly coupled. As shown in Fig.1d,31P NMR of phosphate group was conducted. Whenn= 3 and 4, the phosphorus atom in the phosphate group is chemically shielded from the conjugated BTBT group, which makes a chemical shift up to 0.4 to the low field in comparison with those whennis more than 5. It indicates that whenn= 3 and 4, the coupling of the phosphate group and BTBT is much stronger than when alkyl chains are longer (n> 5).

Fig. 1 Fundamental properties of CnPA-BTBT (n = 3-11).(a) and (b) are initial absorption range of UV-Vis in solution (10-5 mol·L-1) and film, respectively. (c) band gaps calculated from (a) and (b); (d) 31P NMR spectrum.
3.3 Thermodynamic studies
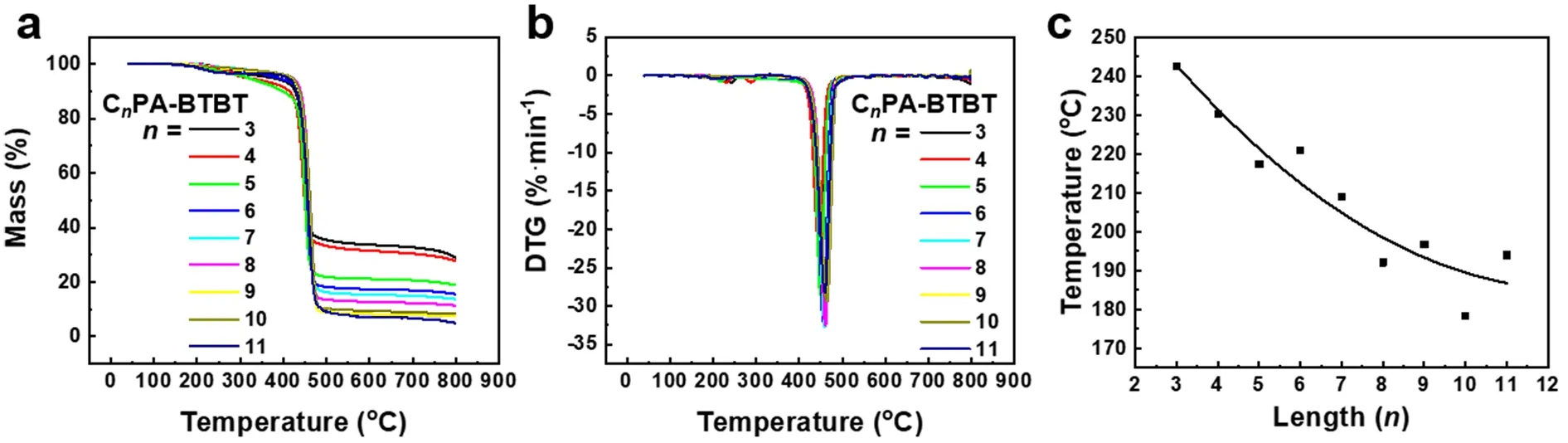
Fig. 2 (a) TGA plot and (b) DTG plot of CnPA-BTBT (n = 3-11); (c) fitting curve of the temperatures of the first peak in DTG in (b).The heating rate is 10 °C·min-1.

Fig. 3 DSC spectra of CnPA-BTBT (n = 3-11).The heating rate are 10 °C·min-1.
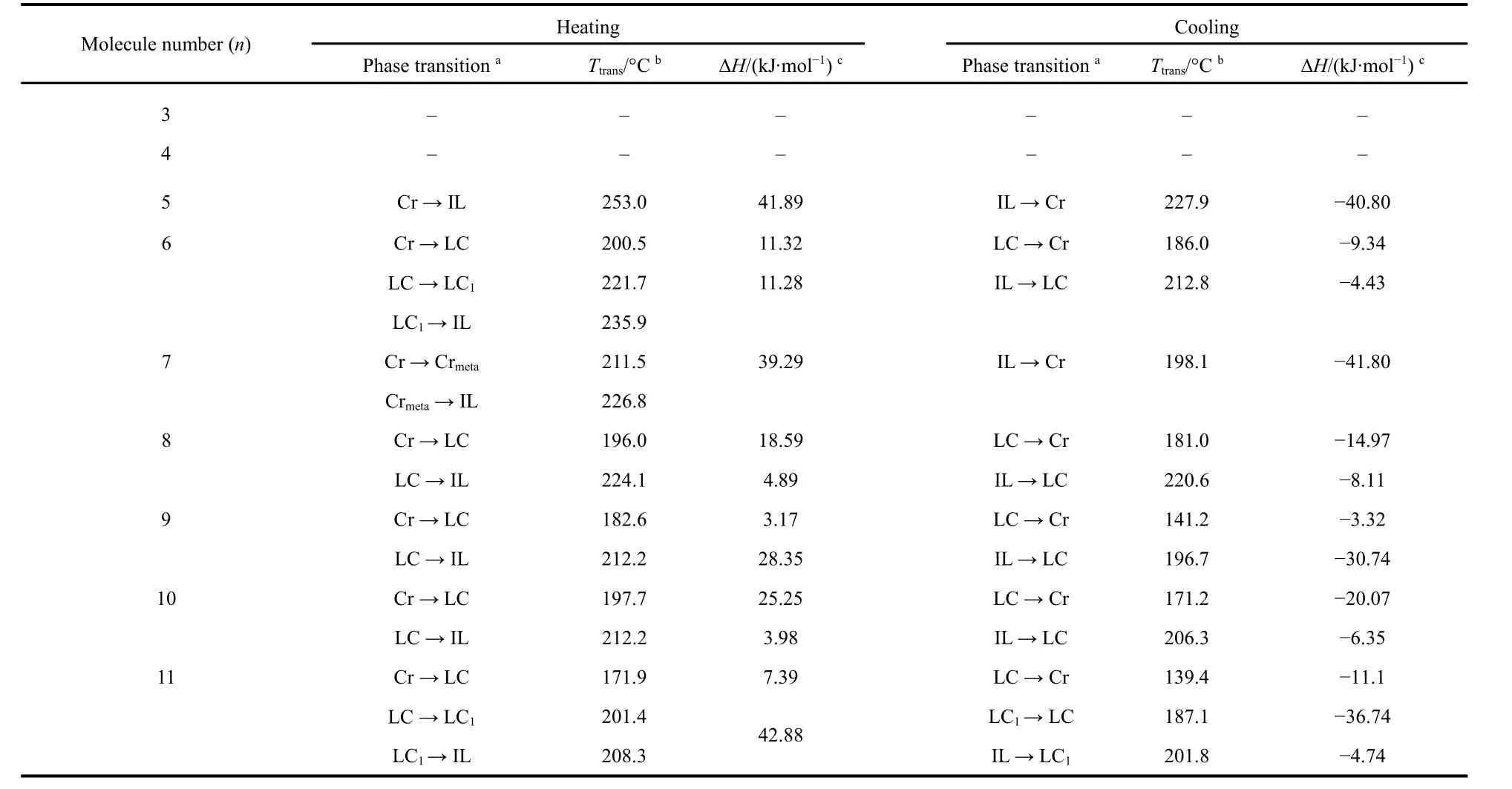
Table 1 Summaries of DSC thermodynamic parameters of CnPA-BTBT (n = 3-11).
Thermodynamic studies of functional materials are based on TGA and DSC. The TGA curves are shown in Fig. 2a, and the functional molecules rapidly decompose atca.450 °C. The derivatives of the TGA curve are then processed to obtain a differential thermal gravity (DTG) curve, as shown in Fig. 2b. It is found that the FAS molecules show a weak decomposition peak at 180-250 °C, at which time the materials lose the crystal water bound with phosphate groups. The temperature value of the first weight loss peak was extracted and shown in Fig. 2c. It was found that the temperature of the water loss peak fluctuated and fell with the increase of the alkyl chain, indicating that the binding ability of the FAS molecule to water molecules was regulated by the odd-even alternating effect of the alkyl chain and the intramolecular coupling with BTBT. Based on the TGA test, we performed a detailed DSC characterization of the FAS molecules CnPA-BTBT (n= 3-11), as shown in Fig. 3. The temperature is heating and cooling at a rate of 10 °C·min-1. The first cycle (black solid line) aims to eliminate the effects of thermal history left during processing and remove crystal water to study the intrinsic properties of the FAS molecule. Therefore,the analysis subject is the second cycle (red solid line), in which the thermodynamic temperatures and enthalpy values are listed in Table 1. According to the thermodynamic performance of the FAS molecules, it is found that when the alkyl chain length is 3 or 4, the molecules do not show any thermodynamic peak below the thermal decomposition temperature, so there is no thermodynamic transition and molecular rearrangement during the heating process. It indicates that the molecules have good stability and rigidity for BTBT is strongly coupled with the phosphate group, and the influence of alkyl chain can be neglected, which further confirms the conclusions in UV-Vis and31P NMR tests. However, at this time, due to excessive rigidity,the material may not be suitable for a simple solution processing method such as spin-coating, and it may increase the difficulty of post-processing of device fabrication. As the length of the alkyl chain increases (n= 5, 7), the distance between BTBT and phosphoric acid increases and the coupling degree decreases, the alkyl chain acts in equilibrium with the conjugation of BTBT and phosphate groups, and a pair of classical fusion and solidification peaks appear in the DSC plots. Furthermore, when the alkyl chain lengthnis more than 8, the DSC plots show two pairs of thermodynamic peaks and a liquid crystal region between the thermodynamic peaks in the heating or cooling processes. At this time, the length of the alkyl chain in the FAS molecule is sufficient to block the coupling of BTBT and phosphate groups, and dominate the performance of the alkyl chain. In order to demonstrate this, FAS molecules with alkyl chain length of 10 and 11 were selected and incubated at 203.2 °C and 185.3 °C, respectively, according to the liquid crystal temperature region in DSC. As shown in Fig. 4a, b, by the POM, Maltese cross of spherulites, which is the mark of a liquid crystal, was observed in both FAS molecules, which confirmed the liquid crystal properties of the designed FAS materials. It is worth noting that unlike other molecules, the molecular chain length of molecule 6 is moderate, but it has a standard liquid crystal curve of two thermodynamic transitions,which may be caused by fluctuations of the odd-even alternating effect. Moreover, the molecular peak of C11PA-BTBT appeared at the position of 208.3 °C after the main melting peak of 201.4 °C, indicating that the alkyl chain was incompletely free during the melting process due to the excessive length of the alkyl chain, and further structure adjustments need to be carried out. The thermodynamic peak temperatures and enthalpy values were extracted and summarized in Fig. 4d, e. It was found that both the clearing point and the freezing point temperatures and the enthalpy values showed strong odd-even alternating effects.the phase transition temperature was negatively correlated with the chain length, which means the increase in alkyl chain gives the material a degree of freedom, allowing longer alkyl chain molecules to act the same phase transition at lower temperatures.For enthalpy, whennis less than 7, the temperatures of the freezing point and the clearing point go synchronously. But when the alkyl chain length is greater than 7, the peak value of the two peaks in the same process alternates, indicating that the main thermodynamic phase transition is switching. The performance exhibited by the material is a combination of odd-even alternating effects, intramolecular coupling, intermolecular interactions and other related effects. According to the law of material properties, it is the ultimate goal to select and synthesize the most suitable functional semiconductor molecules to meet actual needs.
3.4 Molecular models
Accordingly, it can be divided into three models as the alkyl chain grows as shown in Fig. 5. When the alkyl chain is short (n=3, 4, Fig. 5a), the BTBT is strongly coupled with the phosphate group, molecular rigidity predominates and alkyl chain interaction is negligible. Molecules do not exhibit the thermodynamic phase transition, which can be described by brick-like model. As the length of the alkyl chain increases (n=5, 7, Fig. 5b), BTBT and the phosphate group go separately,which leads to a comparatively weaken coupling. The functional molecule is equivalent in rigidity and flexibility, showing a certain degree of freedom by a simple thermodynamic phase transition. However, as the alkyl chain is further lengthened (n=6, 8-11, Fig. 5c), BTBT and the phosphate group are completely independent by volume effect. The role of the alkyl chain is dominant at this time, and the material can show prominent liquid crystal properties.
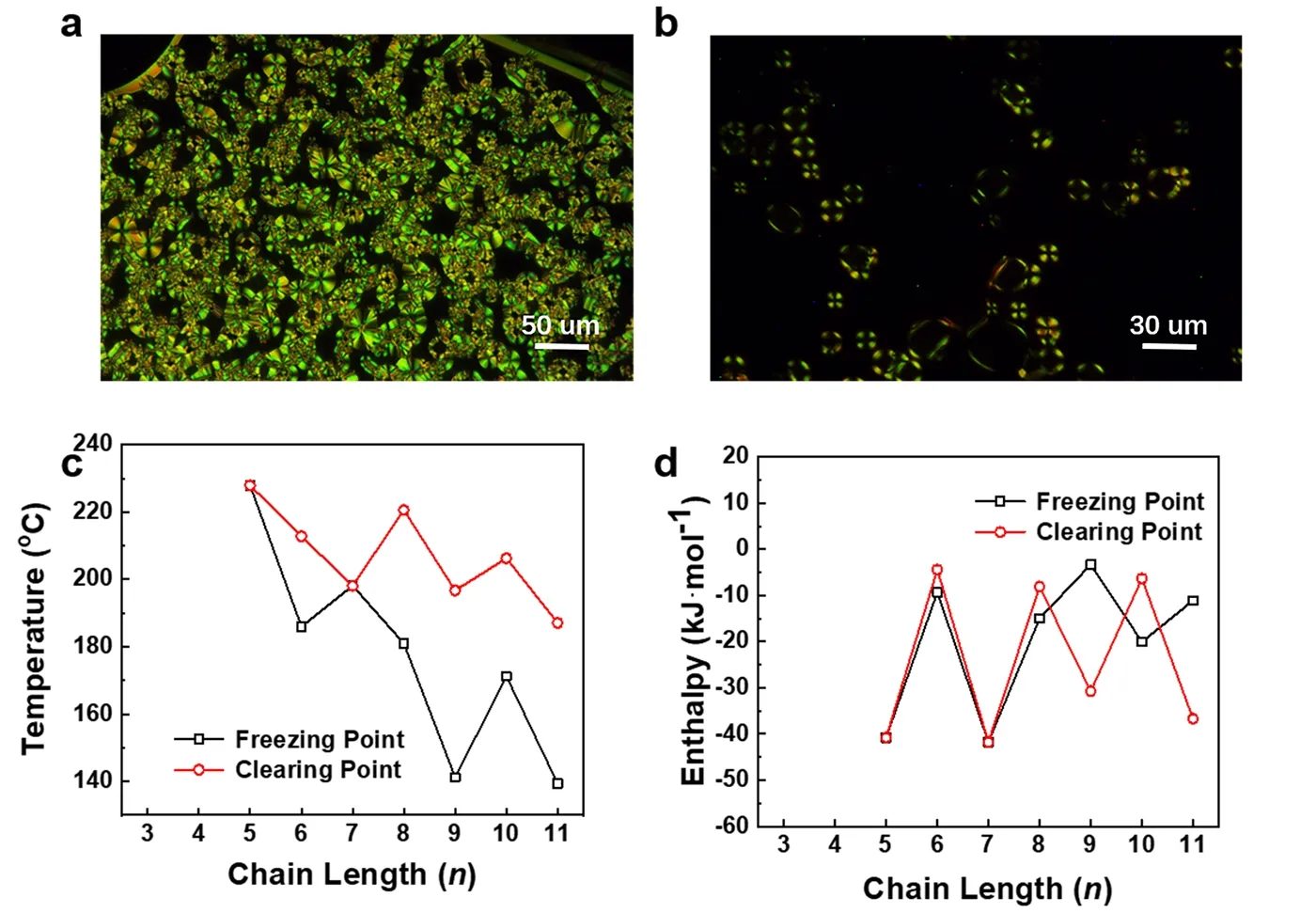
Fig. 4 (a) and (b) are POM images showing Maltese cross of C10PA-BTBT preserved at 203.2 °C and C11PA-BTBT preserved at 185.3 °C,respectively. (c) and (d) are temperatures and enthalpy curves of phase transitions from the cooling process. The molecule has only one thermodynamic transition, when the freezing point meets the clearing point.
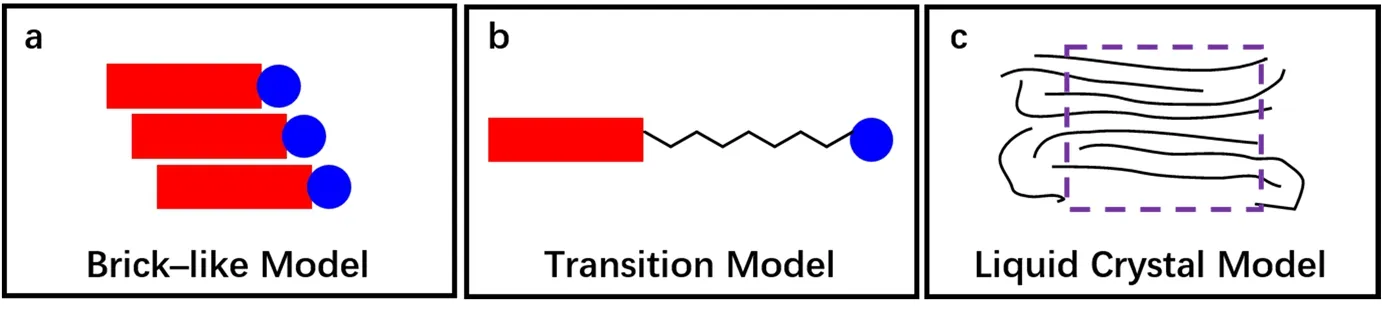
Fig. 5 Molecular models for CnPA-BTBT (n = 3-11).Left (n = 3, 4): brick-like model, the coupling between functional groups and semiconductors is so strong that the molecule shows no flexibility; Middle (n = 5, 7):Functional molecule model with medium chain length; Right (n = 6, 8-11): the liquid crystal model, long alkyl chains predominate with phosphate group and BTBT ignored.The purple dashed line indicates liquid crystal region. Red rectangle, blue circle and black line are the BTBT backbone, phosphate group and alkyl chain, respectively. Color online.
4 Conclusions
In summary, this project designed and synthesized a class of FAS molecules CnPA-BTBT (n= 3-11). Based on alkyl-chain engineering, the molecule links the semiconductor backbone and the polar group through alkyl chains of different lengths, thereby utilizing the volume effect, the odd-even alternating effect and the flexibility of the alkyl chain to adjust the intermolecular and intramolecular coupling. The project analyzes and summarizes the material properties through TGA and DSC-based thermodynamics studies, and proposes a molecular model,which provides experimental basis for material screening and organic synthesis methodology with target properties.

