氧化還原對Lindqvist型多金屬氧簇復合物自組裝的動態調控
張靜,王麗娜,陳曉飛,王玉峰,牛成艷,吳立新,唐智勇
1山西大學應用化學研究所,太原 030006
2國家納米科學中心納米系統與層次結構制造重點實驗室,北京 100190
3山西大學化學化工學院,太原 030006
4吉林大學超分子結構與材料國家重點實驗室,長春 130012
1 Introduction
Dynamically reversible self-assembly has attracted increasing interest due to their potential applications in many fields of multifunctional materials, switchable devices, drug delivery and so forth1–3. Polyoxometalates (POMs) are a class of well-defined oxide nanoclusters of transition metals (W, Mo or V)4–6. The abundant metal elements and the varied frameworks make POMs possess desired physical and chemical properties,endowing potential applications in catalysis7,8, optics9,10,magnetics11,12, biomedicine13, and energy materials14.Furthermore, as a type of functional inorganic materials, POMs can serve as fascinating building blocks for self-assembly,especially when modified by organic moieties15–18. The synergistic effect of organic components and inorganic clusters has contributed to acquire a wide variety of assembly morphologies19–22. Controllable transformation of organicinorganic hydrid POM complexes in self-assemblies by changing external stimulus have been reported recently. Many assembly parameters inlcuding the structure of organic components23, the size and charge density of inorganic clusters24,the polarity of solvents25, pH and temperature26have been recognized to play the key role on the assembly morphology.
POMs exhibit redox response driven by photo or electric stimulus maintaining the topological structure unchanged,commonly accompanied with obvious colour change, which are considered to be candidates for photo-/electrochromic materials27,28. The reversible photo-/electro-chromism of POMs makes them being the building components for development of dynamic assemblies29,30. The divalent Lindqvist-type hexamolybdate cluster [Mo6O19]2?is one of the least negative-charged POM, which is the ideal building blocks to construct novel assembly structures31. The functionalization is realized by exchange of the counterions through ionic self-assembly procedures32or by covalently modification of organic species33.The intriguing photochromism encourages us to exploit the dynamic assemblies of POM-based complexes.
In this study, a symmetrical linear POM complex comprising single chain cationic surfactants (Scheme 1) was employed for controlled self-assemblies. The dynamically reversible morphology transformation between helical and spherical assemblies was successfully realized through controlling the redox of POM component driven by alternate UV light irradiation and air oxidation. The results obtained here will hold great promise for controlling the self-assembled structure of POM-based functionalized materials and would be regarded as a new strategy to develop smart materials and devices.
2 Experimental
2.1 Materials
Octadecyltrimethylammonium bromide (ODTA.Br) was purchased from Aladdin without further purification.Acetonitrile, isopropanol and other reagents were commercial products from J&K Chemical Technology Company. All the solvents were used without any further purification. The Lindqvist type POM (TBA)2[Mo6O19] was freshly synthesized according to the reported procedures34.
2.2 Sample preparation
The complex (ODTA)2[Mo6O19] was synthesized according to our previous method23. (TBA)2[Mo6O19] (0.54 g) and ODTA.Br (6.28 g) were separately dissolved in 50 mL of acetonitrile. And then, the solution of (TBA)2[Mo6O19] was added dropwise into the solution of ODTA.Br. A greenishyellow flocculate formed immediately. The resulting precipitate was collected by centrifugation, washed with acetonitrile for several times, and dried under vacuum overnight to give the product in yield of 85%.1H NMR (400 MHz, DMSO-d3, ppm):0.854 (triplet,J= 6.8 Hz, 3H), 1.177–1.272 (multiplet, 32H),1.674 (quintuplet, 2H), 3.032 (singlet, 9H), 3.245 (triplet,J= 8.4 Hz, 2H). Elemental analysis calcd (%) for (ODTA)2[Mo6O19](C42H92N2Mo6O19, 1504.82): C 33.52, H 6.16, N 1.86; Found: C 33.49, H 6.12, N 1.83, corresponding to the chemical formula(C21H46N)2Mo6O19, that is (ODTA)2Mo6O19. Assuming that the organic component has decomposed completely and all inorganic residuals are MoO3at 600 °C (MoO3may sublimate after the temperature), the measured residue of 56.9% (w, mass fraction) in total from TGA is in perfect agreement with the calculated value of 57.4% (w) from the given (ODTA)2Mo6O19formula. IR (KBr,ν/cm–1): 3030 (w), 2954 (m), 2920 (s), 2850(s), 1485 (w), 1467 (w), 1388 (w), 962 (s), 906 (s), 802 (s), 605(w), 439 (w).
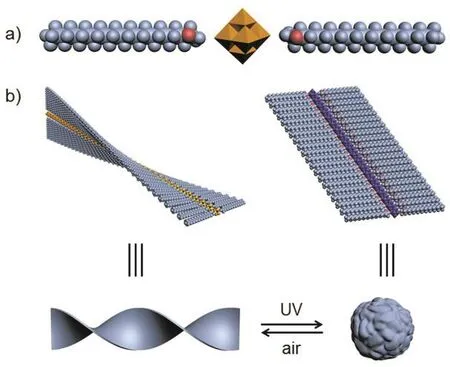
Scheme 1 Scheme of a) structure of (ODTA)2[Mo6O19] and b) dynamic self-assembly between helical strips and spherical assemblies upon UV reduction and air oxidation.
2.3 UV irradiation reduction and air oxidation
Solution samples of (ODTA)2[Mo6O19] in acetonitrile/isopropanol(4 : 1v/v) with a concentration of 1 mg?mL?1was used for the photochromic experiments. For UV irradiation reduction, the solution sealed in a quartz cell was irradiated with a CELHXF300 xenon lamp under stirring. The solution was set at a distance of 10 cm from the light source. For the air oxidation,when the irradiation stopped, the blue solution faded gradually after exposed to air. The decoloration process was completed in about 1 h under stirring.
2.4 Measurements
1H NMR spectra were recorded on a Bruker Avance 400 MHz instrument. FT-IR spectra in pressed KBr pellets were recorded on a Bruker Vertex 80v spectrometer equipped with a deuterated triglycine sulfate detector (32 scans) at a resolution of 4 cm–1.Organic elemental analysis was acquired on a Flash EA1112 from Thermo-Quest Italia S.P.A. UV-Vis absorption measurements were carried out using a Hitachi U-3010 UV-Vis spectrometer. Scanning electron microscope (SEM) images were obtained on a Hitachi S4800 electron microscope. High resolution transmission electron microscope (TEM) imaging was carried out on Tecnai G2 20 S-TWIN at 200 kV. For X-ray diffraction (XRD) measurement, a Bruker AXS D8 ADVANCE X-Ray diffractometer using CuKαradiation of a wavelength of 1.54 ? (1 ? = 0.1 nm) with an mri Physikalische Ger?te GmbH TC-Basic temperature chamber was used. X-ray photoelectron spectroscopy (XPS) was performed on an ES-CALAB Mark(VG Company, UK) 250 spectrometer with a monochromic X-ray source (AlKαline, 1486.6 eV) and the charging shift was corrected by the binding energy of C(1s) at 284.6 eV.
3 Results and discussion
3.1 Photochromism of (ODTA)2[Mo6O19]
The Lindqvist type ionic complex (ODTA)2[Mo6O19] was prepared by counterion exchange of (TBA)2[Mo6O19] cluster with single chain cationic surfactant ODTA.The detailed synthetic procedure of (ODTA)2[Mo6O19] was described in Experimental section. The structure characterizations of the complex were performedvia1H NMR, FT-IR spectra, TGA(Fig. S1–S3, Supporting Information), and elemental analysis.
The photochromic experiment of (ODTA)2[Mo6O19] was carried out in the mixed solvents of acetonitrile and isopropanol with the volume ration of 4 to 1. Upon irradiated with a CELHXF300 xenon lamp for 5 min, the light yellow transparent solution of the complex sealed in quartz cells turned into blue quickly, as displayed in Fig. 1a. A broad absorption band assigned to MoV→ MoVIintervalence charge transfer (IVCT) of molybdenum blue emerged atca.751 nm (Fig. 1b), which originated from the reduction of molybdenum atoms35. After exposed to air for about 1 h, the blue solution was bleached. The bleaching behavior implied that the low valent MoVatoms were oxidized back to the initial high valence. The color change was reversible upon alternate exposure to UV light and air. Such reversible photochromism process of the solution could be conducted for multiple cycles.
3.2 Redox-controlled reversible self-assembly
The Lindqvist type POM complex (ODTA)2[Mo6O19] with symmetric linear topological structure is prone to form helical or twisted self-assemblies, once the symmetry between inorganic and organic moieties is broken by simply adjusting the solvent environment25. Helical strip-like self-assembly was observed in acetonitrile/isopropanol at the concentration of 1 mg?mL?1, as shown in scanning electron microscope (SEM) image (Fig. 2a).Energy-dispersive X-ray analysis (EDX) showed the successful hybridization of ODTA and [Mo6O19]2?, where the elements Mo and O of [Mo6O19]2?as well as C and N of ODTA were uniformly distributed in the helical assemblies (Fig. S4,Supporting Information). The helical assignment was further confirmed by TEM image (Fig. 2b), and the lower electron penetration appeared in the twisted region than in the flat parts.In addition, a fine parallel stripe substructure with a regular layer spacing ofca.2.2 nm in a curved form was discerned from the TEM image as the strong contrast between inorganic cluster and organic moieties (inset in Fig. 2b). The layered structure was further verified by X-ray diffraction (XRD) patterns (Fig. 3) of(ODTA)2[Mo6O19]. A lamellar substructure with a spacing of 2.2 nm as calculated from the three equidistant diffractions at 2θ=4.00°, 8.00°, 11.88°, which was in perfect agreement with the width estimated from TEM image.
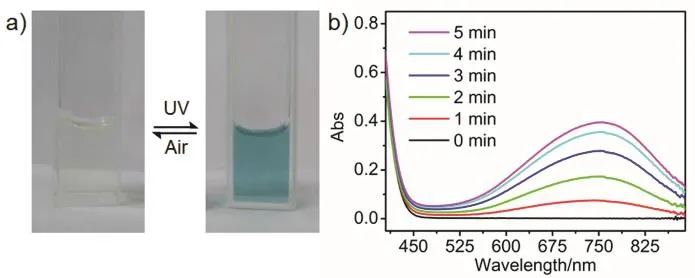
Fig. 1 a) Reversible photochromism of (ODTA)2[Mo6O19] in acetonitrile/isopropanol (4 : 1 v/v) solution with a concentration of 1 mg?mL?1. b) UV-Vis spectra of (ODTA)2[Mo6O19] after UV irradiation for different time.
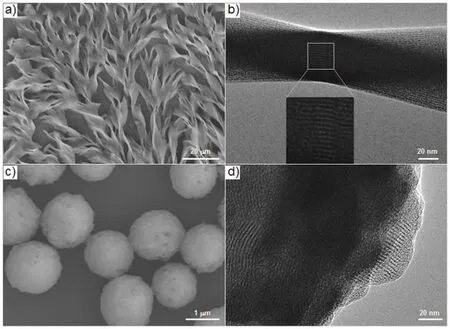
Fig. 2 a) SEM and b) TEM images of helical strips of(ODTA)2[Mo6O19] obtained from acetonitrile/isopropanol(4 : 1 v/v) solution. c) SEM and d) TEM images of spherical aggregates of (ODTA)2[Mo6O19] from acetonitrile/isopropanol(4 : 1 v/v) solution after UV irradiation for 5 min.
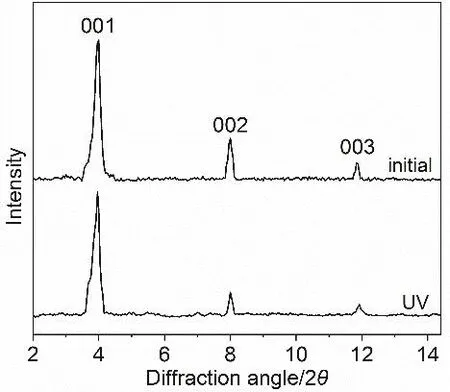
Fig. 3 XRD patterns of (ODTA)2[Mo6O19] assemblies prepared from dichloromethane/propanol (4 : 1 v/v) solution before and after UV irradiation.
More interestingly, the self-assembled morphology of(ODTA)2[Mo6O19] changes evidently with the photochromism.When UV light was applied for 5 min, helical strips evolved into spherical assemblies (Fig. 2c). EDX (Fig. S5, Supporting Information) proved that both elements Mo and O as well as C and N existed in the spherical assemblies homogeneously, which confirmed that the spheres were composed of the organicinorganic ionic hybrid complex. The magnified TEM image indicated the lamellar nature inside the spheres (Fig. 2d).
In order to clarify the morphology transformation process, the intermediate states after different irradiation time were investigated. After a short period of UV light exposure, the fibers tended to shorten their length (Fig. 4a), while the twisted characteristic was still preserved. Further irradiation destroyed the helical structure greatly, making the assemblies reconfigurate into irregular flower-like aggregates with the diameter ofca.4 μm (Fig. 4b). With prolonged irradiation, the sea urchin-like architecture (Fig. 4c, d) was observed at a scale of over 1 μm in diameter. The continuous irradiation made the protuberances shorter and smaller (Fig. 4e, f). After irradiation for 5 min, the protuberances vanished, resulting in formation of spheres (Fig. 4g, h). During the morphology transformation, the layered structure of (ODTA)2[Mo6O19] maintained and the layer spacing did not change as identified by XRD patterns (Fig. 3).

Fig. 4 SEM images of (ODTA)2[Mo6O19] in acetonitrile/isopropanol(4 : 1 v/v) solution after UV light irradiation for a) 0.5, b) 1.0, c) 3.0, e)4.0, and g) 5.0 min, respectively. d), f), and h) the magnification at the position pointed at by arrows in c), e), and g), respectively.
Most significantly, the helical assemblies (Fig. S6,Supporting Information) could be recovered again after air oxidation, implying the reversible morphology transformation driven by redox stimulus. After three cycles of alternate redox,no decomposition was found based on the1H NMR, FT-IR spectra and TGA analysis (Figs. S1–S3).
3.3 Mechanism of redox-responsible self-assembly
The morphology transformation of (ODTA)2[Mo6O19] is triggered by UV irradiation, accompanied by photochromism of the complex, which correlates closely with the change of valent state of molybdenum atoms. X-ray photoelectron spectra (XPS) was employed to investigate the photochromism of (ODTA)2[Mo6O19].At the initial highest oxidation state, the valence of all Mo atoms was +6, as illustrated in Fig. 5a. Upon UV light irradiation, the band of Mo 3d level turned broader obviously, which was attributed to formation of MoV. After light irradiation for 5 min,integral area of MoVwas estimated to beca. 33.79% (Table S1,Supporting Information). Hence, the negative charge of the inorganic cluster becomes ?4.03, based on calculation of the chemical composition, [MoVI6×66.21%MoV6×33.79%O19]. Therefore,one can deduce that the morphology change occurs at the step of more than two-electron reduction. The increased negative charges on polyanionic cluster owing to the reduction of molybdenum would play key role on the electrostatic attraction between organic cations and inorganic anions as well as the electrostatic repulsion between inorganic ionic clusters.
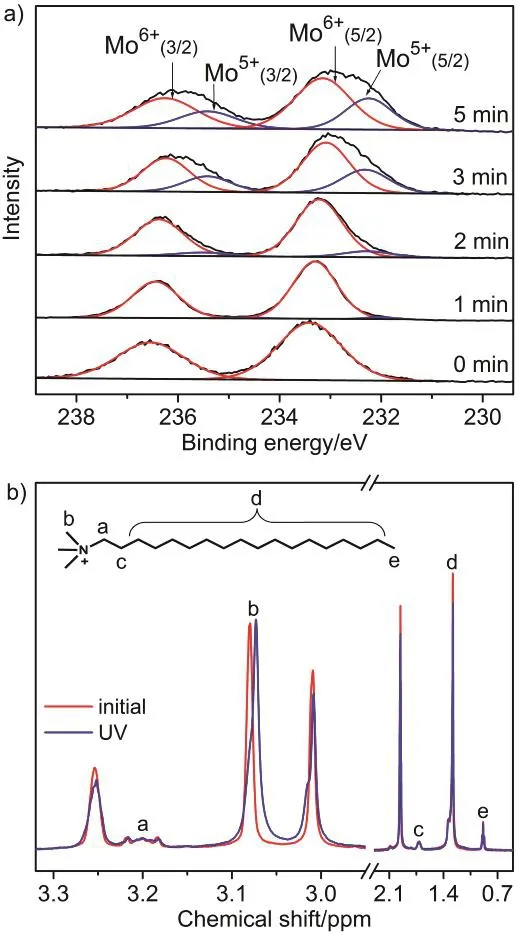
Fig. 5 a) Mo 3d XPS spectra of (ODTA)2[Mo6O19] casting film prepared from acetonitrile/isopropanol (4 : 1 v/v) solution after UV irradiation for different time; and b) 1H NMR spectra of(ODTA)2[Mo6O19] in CD3CN/CD3OD (4 : 1 v/v) before and after UV light irradiation for 5 min.
The variation of electrostatic interactions was identified by1H NMR spectra (Fig. 5b) during the photochromism. Upon UV irradiation, the proton peak Hblocated at N-methyl shifted to high field, which was attributed to the enhancement of shielding effects arising from the increased electron density around36. The increased negative charge on POM led to the enhanced electrostatic interaction, giving rise to the increase of shielding effect on neighboring protons. It was anticipated that the electrostatic repulsions between POM clusters was enhanced simultaneously due to carrying more negative charges after reduction37.
The increased repulsive force between inorganic clusters is unfavourable for them to form compact stacking and thus leads to the increased distance between POMs, which degenerates the orientational arrangement along the polyanion skeleton. As a result, helical strips are shortened. The further increased distance between POMs will expand the space of POMs corresponding to ODTA moieties. Thus, the space mismatch between POMs and ODTA disappears, and helical morphology degenerate into defective flower-like aggregates. In addition, the expansion of POM space increases the flexibility of molecular arrangement,which provides the great possibility to fold into spherical sea urchin-like aggregates with protuberances on the surface. And finally, protuberances shrink and degenerate driven by the minimization of free energy37,38. The morphology evolution during the photochromism changed from helical strips through sea urchin-like aggregates to spherical assemblies is illustrated in Scheme 2.
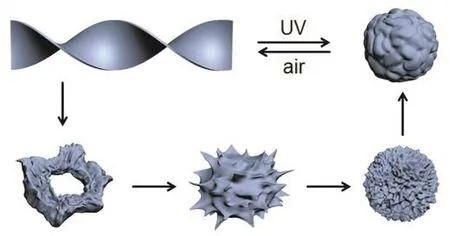
Scheme 2 Scheme of morphology transformation of(ODTA)2[Mo6O19] from helical strips to spherical assemblies under UV irradiation.
4 Conclusions
In summary, a symmetrical linear hexamolybdate complex(ODTA)2[Mo6O19] comprising single chain cationic surfactants was prepared through ion substitution. The complex exhibited reversible photochromism upon alternate UV light irradiation and air exposure. More importantly, the redox exerted by UV photoreduction and air oxidation made dynamically reversible self-assemblies between helical strips and spherical aggregates,which were modulated by change of electrostatic interactions between organic cations and inorganic clusters. We are convinced that this study will be valuable for better understanding the assembly process in supramolecular system and facilitate precise fabrication of smart nanostructured materials.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.

