理性設計核-殼Rh@沸石催化材料用于二烯烴選擇加氫反應
張建,王亮,伍芷毅,王成濤,蘇澤瑞,肖豐收,2,
1北京化工大學北京軟物質科學與工程高精尖創新中心,北京 100029
2浙江大學化學工程與生物工程學院,生物質化工教育部重點實驗室,杭州 310027
3浙江大學化學系,浙江省應用化學重點實驗室,杭州 310028
1 Introduction
Catalytic conversion of unsaturated hydrocarbons such as dienes and alkynes to monoenes is important in the field of pharmacology and organic synthesis1–6. Various strategies have been developed for optimizing the product selectivity. For example, the fast desorption of monoene molecules and the controllable steric adsorption of diene molecules have been regarded as a promising principle for developing the catalysts6–17.The former strategy has been extensively used in the conversion of alkynes to alkenes over various Pd catalysts8–10, which is insufficient for the selective hydrogenation of symmetric dienes.In contrast, the steric adsorption control strategy could work more efficiently, which has been realized through the modification of the metal nanoparticle surface6,7. After inducing of Bi species into Rh nanoparticles, RhBi/SiO2exhibited 90% selectivity to 2-hexene with 95% conversion of 1,4-hexadiene under ambient conditions because of the suppressed adsorption of internal C=C bond6. However, the activity was remarkably decreased. Under the same reaction conditions, the reaction rate of RhBi/SiO2 was about 27 times lower than that of Rh/SiO2.
The controlled steric adsorption of diene molecules could also be achieved by the construction of porous channels around the metal nanoparticles18–20. For example, the metal-organic framework (ZIF-8) or mesoporous silica (MCM-41)encapsulated noble metals exhibited high selectivity for the hydrogenation of terminal C=C bond19,20. However, these catalysts suffer from unsatisfactory in durability under the thermal/hydrothermal reaction/regeneration conditions. In contrast, the zeolite has superior durability under the harsh reaction conditions, but it is rarely used in the semihydrogenation reactions.
On the other hand, we recently found that the metal nanoparticles fixed within zeolite crystals (e.g. ZSM-5 and Beta)are efficient in the selective hydrogenation of various molecules with more than one reducible groups17,21–23. Inspired by these works, we report the synthesis of Rh nanoparticles fixed in CHA zeolite crystals with core-shell structureviaan inter-zeolite transformation method24. As expected, the core-shell Rh@CHA catalyst exhibited high monoene selectivity in the hydrogenation of dienes, which readily outperforms the conventionally supported catalysts.
2 Experimental
2.1 Synthesis of catalysts
2.1.1 Chemicals
KCl (AR),γ-Al2O3(AR), SiO2(AR), TiO2(anatase, AR), and KOH (AR) were purchased from Aladdin Chemical Reagent Co.RuCl3 (AR), NaBH4 (AR), H2SO4 (98%), HCl (35%), NH4NO3(AR), and PVA (MW 9000–10000) were bought from Sinopharm Chemical Reagent Co., LTD. Ultra-stable Y zeolite(Si/Al molar ratio = 2.6) was purchased from Nankai University Catalyst Co., Ltd. All the reagents were directly used without further purification.
2.1.2 Synthesis of Rh@CHA sample
The synthesis of Rh@CHA sample involved two steps:fixation of Rh nanoparticles in Y zeolite (Rh@Y) and subsequent transformation of Rh@Y to Rh@CHA under hydrothermal conditions. The fixation of Rh nanoparticles in Y zeolite was performed as follows: commercial ultra-stable Y(USY) was exchanged with 1 mol·L?1KCl aqueous solution (1 g Y/50 mL solution) at 80 °C for 20 h. After filtrating, washing with large amount of water, drying at 80 °C, 1 g of the solid was dispersed into 40 mL of KCl aqueous solution (0.1 mol·L?1).Then, 50 μL of HCl (0.1 mol·L?1) was added into the above mixture under stirring. After heating the suspension to 90 °C,2.95 mL of RhCl3 aqueous (0.1 mol·L?1) was dropwisely added and stirred for another 6 h. The Rh@Y sample was obtained after filtrating, washing with large amount of water, drying at 80 °C for 4 h, calcining at 350 °C for 3 h in air and reducing at 300 °C for 2 h in 10% H2/Ar. 1 g of Rh@Y zeolite was dispersed in 9 g of KOH aqueous solution (5.4% (mass fraction,w)), followed by hydrothermal treatment at 100 °C for 4 days. The Rh@CHA sample was separated by filtrating, washing with large amount of water, ion-exchanged with NH4NO3aqueous solution (1 g in 50 mL), calcining at 550 °C for 4 h, and reducing at 300 °C for 2 h. The loading amount of Rh in the final product is 0.88% (w)by ICP analysis.
2.1.3 Synthesis of Rh/Al2O3, Rh/SiO2, and Rh/TiO2 samples
The Rh/Al2O3, Rh/SiO2, and Rh/TiO2samples were synthesized from the impregnation method. Typically, the commercialγ-Al2O3, SiO2, and TiO2(anatase) were impregnated with RhCl3aqueous solution. Then water was removed at 60 °C.The solids were calcined at 400 °C for 4 h in air, followed by reduction at 300 °C for 2 h in 10% H2/Ar. The loading amounts of Rh on Rh/Al2O3, Rh/SiO2, and Rh/TiO2samples are ~1.0%(w).
2.1.4 Synthesis of Rh/CHA
The CHA zeolite was synthesized as follows: 1 g of Y zeolite was dispersed in 9 g of KOH aqueous solution (5.4% (w)),followed by hydrothermal treatment at 100 °C for 4 days. After filtrating, washing with large amount of water, ion-exchanging with NH4NO3aqueous solution (1 g/50 mL), calcining at 550 °C for 4 h, and reducing at 300 °C for 2 h, the CHA zeolite was obtained.
For the synthesis of Rh/CHA sample, 20.3 mg of RhCl3and 12 mg of PVA were dissolved in 60 mL of water. After stirring for 30 min at 0 °C, 2 mL of newly-made NaBH4solution (0.1 mol·L?1) was quickly added into the solution under vigorous stirring. After stirring for another 2 h, 1 g of CHA zeolite was added, followed by adjusting the pH value at 3 using H2SO4 (1 mol·L?1). After stirring for another 2 h, filtrating, washing, and drying at 80 °C, the Rh/CHA was finally obtained. The loading amount of Rh in Rh/CHA is 1.0% (w).
2.2 Characterization
X-ray diffraction (XRD) patterns were collected on a Rigaku D/MAX 2550 diffractometer with CuKα radiation (λ= 1.5418 ? (1 ? = 0.1 nm)). Nitrogen sorption isotherms were measured using a Micromeritics ASAP 2020 system. Scanning electron microscopy (SEM) experiments were performed with a Hitachi SU-8010 electron microscope. The samples were degassed for 10 h at 120 °C before each test. Transmission electron microscopy (TEM), scanning transmission electron microscopy(STEM) imaging were performed on a JEOL JEM-2100F electron microscope with an acceleration voltage of 200 kV or on a FEI Tecnai G2F20 microscope. It is difficult to characterize the metal nanoparticles on the surface or within zeolite crystals from the general TEM images. In order to resolve this matter, we have cut the metal@zeolite crystals into thin slices with thickness smaller than 100 nm. The TEM images of these slices are called tomographic TEM images, providing sectional views of the zeolite crystals, providing the evidence of metal nanoparticles fixed inside of the zeolite crystals (Scheme S1, see Supporting Information).
2.3 Catalytic test
The catalytic hydrogenation was performed in a roundbottomed flask equipped with a condenser. Typically, the reactant, catalyst, and solvent were mixed in the flask. The air in the reactor was carefully removed by flowing H2, then the reactor was connected with a gasbag and localized into a preheated oil-bath to initiate the reaction. After reaction, the unreacted substrates and products were analyzed by gas chromatograph equipped with flame ionized detector (FID). In the recyclable tests, the solid catalyst was separated by filtration and washed with large amount of tetrahydrofuran after each run.After drying under ambient conditions, the catalyst was used in the next run, the amount of substrate and solvent were adjusted to make sure that the ratio of substrate to catalysts and solvent to catalyst are constant.
3 Results and discussion

Fig. 1 (a) XRD pattern of Rh@Y and Rh@CHA samples; (b) SEM image, (c) N2-sorption isotherms, and(d) STEM tomographic image of Rh@CHA sample.
The Rh@CHA sample was synthesizedviaan inter-zeolite transformation method24: Rh nanoparticles was loaded on the Y zeolite (Fig. S1, see Supporting Information) by ionexchangement (Rh@Y), followed by transformation of the Rh@Y sample to Rh@CHA sample in KOH aqueous solution under hydrothermal conditions. The successful transformation of Rh@Y into Rh@CHA was confirmed by the XRD patterns (Fig.1a). The Rh@Y sample exhibited typical peaks associated with FAU-type zeolite (Fig. 1a-i). After hydrothermal treatment, the peaks assigned to CHA-type zeolite (Rh@CHA) appeared on the XRD pattern (Fig. 1a-ii). The synthesis procedures are shown in Scheme 1.
Fig. 1b shows the SEM image of the Rh@CHA sample,exhibiting uniform morphology with high crystallinity24,25.After ion-exchange with a NH4NO3aqueous solution and calcination at 550 °C, the Rh@CHA exhibited typical Langmuir sorption curve, confirming the presence of micropores (Fig. 1c).Correspondingly, the BET (Brunauer-Emmett-Teller) surface area is 634.7 m2?g?1, confirming the good crystallinity of the Rh@CHA sample. The STEM tomographic image (Scheme S1,see Supporting Information) of the Rh@CHA sample is shown in Fig. 1d. The uniform Rh nanoparticle distribution with mean size at 1.8 nm (Fig. S2, see Supporting Information) are highly dispersed in the zeolite region. These results demonstrate the successful synthesis of Rh@CHA sample with Rh nanoparticles fixed into the CHA zeolite crystal. In contrast, the Rh/CHA catalyst synthesized from conventional method has only Rh nanoparticles on the external surface of the zeolite crystals (Fig.S3, see Supporting Information).
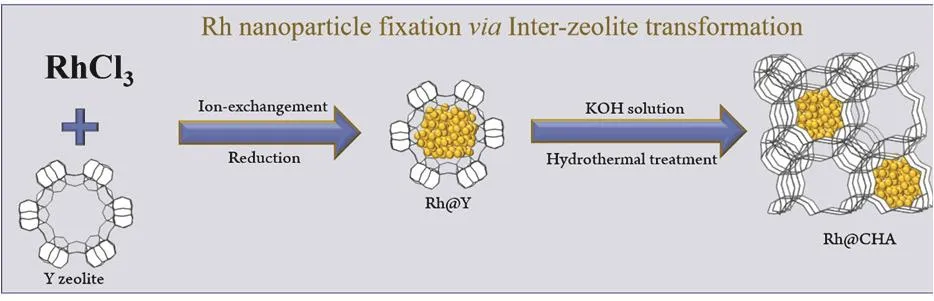
Scheme 1 Proposed synthesis process of Rh@CHA sample.
The catalytic hydrogenation of 1-octene and 2-vinylnaphthalene were performed over Rh@CHA, Rh/CHA,commercial Rh/C (Rh loading at 5% (w)), Rh/Al2O3, Rh/SiO2,and Rh/TiO2catalysts (Figs. S4–S7 (see Supporting Information), Fig. 2a, Table 1). All these catalysts exhibited high activities in the hydrogenation of 1-octene, while the Rh@CHA sample was almost inactive in the catalytic hydrogenation of 2-vinylnaphthalene. This is because that the molecular diameter of 2-vinylnaphthalene is larger than the channels of the CHA zeolite (~3.8 ?) while the 1-octene molecule could facile diffuse inside the CHA zeolite26. The relative ratio of the TOF values in the catalytic hydrogenation of 1-octene (TOF1-octene) and 2-vinylnaphthalene (TOF2-vinylnaphthalene) were calculated as a scale to evaluate the surrounding environment on the Rh nanoparticles. The TOF1-octene/TOF2-vinylnaphthaleneover Rh@CHA,Rh/CHA, commercial Rh/C, Rh/Al2O3, Rh/SiO2, and Rh/TiO2were 5.6, 0.30, 1.2, 1.4, 0.53, and 0.56, respectively (Fig. 2a).The value over Rh@CHA was much higher than those over the unencapsulated Rh nanoparticles, confirming the Rh nanoparticles in the Rh@CHA sample are entirely encapsulated into the CHA zeolite crystals.
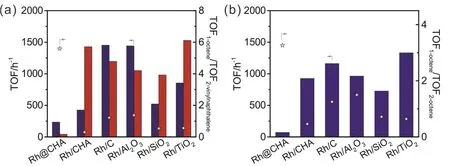
Fig. 2 (a) TOF values in the catalytic hydrogenation of 1-octene (navy column) and 2-vinylnaphthalene (red column) and the ratio of TOF1-octene to TOF2-vinylnaphthalene (TOF1-octene/TOF2-vinylnaphthalene) (star); (b) TOF values in the catalytic hydrogenation of 2-octene (royal column) and the ratio of TOF1-octene to TOF2-octene (TOF1-octene/TOF2-octene) (star) over Rh@CHA, Rh/CHA, commercial Rh/C, Rh/Al2O3,Rh/SiO2, and Rh/TiO2 samples. Reaction conditions: 2 mmol of substrate, 0.05% Rh catalyst, 10 mL of tetrahydrofuran, 30 °C,1 atm (1 atm = 100 kPa) H2. The reaction time are controlled to make sure that the conversions of substrates are lower than 8%.

Table 1 Textual parameters of various Rh-containing samples.
Although the alkene molecules could diffuse into the channel of CHA zeolite, but the internal carbon atoms on the alkene molecules could not access with the Rh nanoparticles within the CHA zeolite because of the steric hindrance of the micropores(See Scheme 2). In other words, the Rh@CHA sample could only catalyze the hydrogenation of terminal C=C bond even in the presence of internal C=C bond. The TOF values in the selective hydrogenation of 1-octene and 2-octene were measured(Fig. 2b). The relative ratios of TOF1-octeneto TOF2-octene(TOF1-octene/TOF2-octene) were calculated to evaluate the activity in the hydrogenation of terminal and internal C=C bonds over various Rh catalysts6. The TOF1-octene/TOF2-octeneover Rh@CHA, Rh/CHA, commercial Rh/C, Rh/Al2O3, Rh/SiO2, and Rh/TiO2were 3.3, 0.46, 1.3, 1.5, 0.72, and 0.64, respectively(Fig. 2b). The ratio over Rh@CHA is higher than those over other supported Rh catalysts, indicating the preferred hydrogenation of terminal C=C bond than the internal C=C for the Rh@CHA sample.
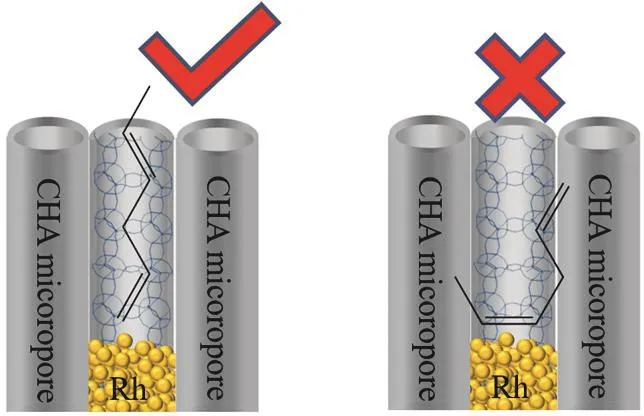
Scheme 2 Proposed model of 1,4-hexadiene adsorbed on Rh@CHA sample.
The results in the catalytic hydrogenation of dienes over Rh@CHA, Rh/CHA, commercial Rh/C, Rh/Al2O3, Rh/SiO2, and Rh/TiO2catalysts are shown in Fig. 3. 1,4-hexadiene and 1,3-hexadiene with both terminal and internal C=C bonds are employed as the substrates. The generally supported Rh catalysts exhibited poor internal alkene selectivity. For example,selectivities to 2-hexene in the catalytic hydrogenation of 1,4-hexadiene over Rh/C, Rh/Al2O3, Rh/SiO2, and Rh/TiO2were 12.1%, 39.1%, 53.3%, and 21.1%, with the conversion of 1,4-hexadiene at > 99.9%, 93.5%, 98.9%, and 94.1%, respectively(Fig. 3a). Interestingly, the Rh@CHA catalyst exhibited high selectivity to 2-hexene in the catalytic hydrogenation of 1,4-hexadiene, achieving 2-hexene selectivity of 86.7% with 91.2% conversion of 1,4-hexadiene (Fig. 3a). In contrast, the generally supported Rh nanoparticle catalyst (Rh/CHA) only gave 37.2% selectivity to 2-hexene although the conversion of 1,4-hexadiene exceeded 99.9% (Fig. 3a).
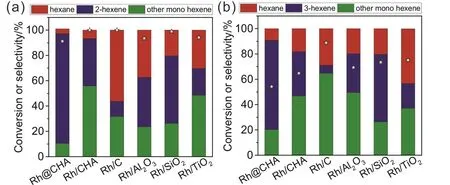
Fig. 3 Catalytic hydrogenation of (a) 1,4-hexadiene and (b) 1,3-hexadiene over Rh@CHA, Rh/CHA, commercial Rh/C, Rh/Al2O3,Rh/SiO2, and Rh/TiO2 catalysts. Reaction conditions: 0.2 mmol of substrate, 0.5% Rh catalyst, 5 mL of tetrahydrofuran, 30 °C;reaction time: 15 min for Rh/CHA, commercial Rh/C, Rh/Al2O3, Rh/SiO2, and Rh/TiO2, 40 min for Rh@CHA.
Moreover, the Rh@CHA catalyst also exhibited higher selectivity to 3-hexene than other Rh nanoparticle catalysts in the catalytic hydrogenation of 1,3-hexadiene (Fig. 3b). The Rh@CHA catalyst showed 3-hexene selectivity of 70.4% with 54.2% conversion of 1,3-hexadiene. The relative lower conversion should be caused by the different properties of the substrates. Similar phenomena are also observed on the Rh/CHA, Rh/C, Rh/Al2O3, Rh/SiO2, and Rh/TiO2catalysts. The conversions of 1,3-hexene over Rh/CHA, Rh/C, Rh/Al2O3,Rh/SiO2, and Rh/TiO2 catalysts were 64.7%, 88.8%, 69.3%,73.4%, 75.1%, which are all lower than those in the catalytic hydrogenation of 1,4-hexadiene. The selectivities to 3-hexene over Rh/CHA, Rh/C, Rh/Al2O3, Rh/SiO2, and Rh/TiO2catalysts were 35.0%, 6.4%, 30.6%, 23.9%, and 19.5%, which are all lower than that of Rh@CHA catalyst (Fig. 3b).
The Rh/CHA sample with Rh nanoparticles on the external surface of the catalyst exhibited similar results with the general Rh/C, Rh/Al2O3, Rh/SiO2, and Rh/TiO2catalysts. However, the Rh@CHA sample with Rh nanoparticles fixed inside of the CHA zeolite crystals displayed much higher internal monoene selectivity. Considering that the Rh@CHA and Rh/CHA have the same CHA zeolite crystal (Fig. 1) and similar Rh nanoparticle sizes (Figs. S1 and S2), the considerable different selectivity of Rh@CHA should be caused by the steric adsorption of dienes on the Rh surface controlled by the micropores of CHA zeolite.
Fig. 4 shows recyclable tests in the hydrogenation of 1,4-hexadiene over the Rh@CHA. After fifth run, the Rh@CHA still gave the 1,4-hexadiene conversion at 88.1% and 2-hexene selectivity at 82.9%, which are only slightly lower than that of the fresh catalyst. The spent Rh@CHA catalyst gives comparable Rh nanoparticle size to the fresh one (Fig. S8). These results suggest the good recyclability of Rh@CHA catalyst.
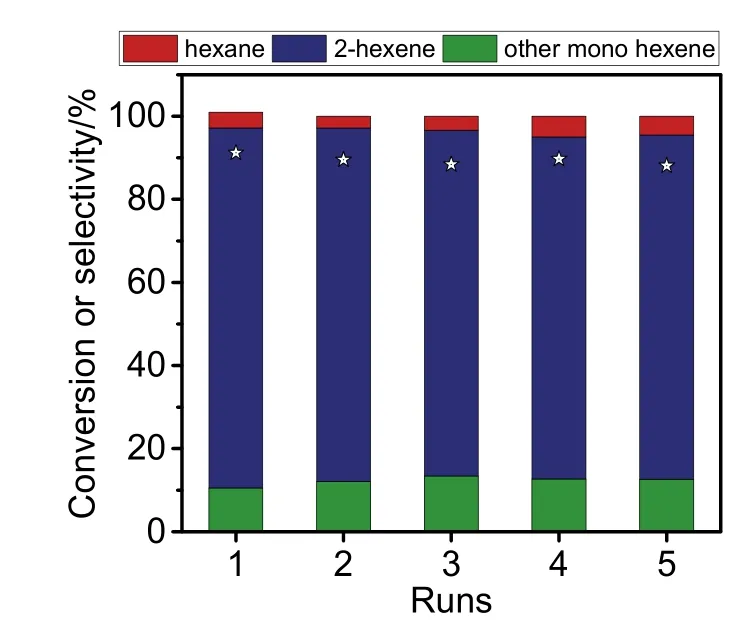
Fig. 4 Recyclable tests in the catalytic hydrogenation of 1,4-hexadiene over Rh@CHA.
4 Conclusions
In summary, we successfully synthesize Rh nanoparticles fixed in CHA zeolite (Rh@CHA) with core-shell structureviaan inter-zeolite transformation method. The Rh@CHA catalyst exhibits high internal monoene selectivity in the catalytic hydrogenation of dienes to outperform the generally supported catalysts. For example, the Rh@CHA exhibited the 2-hexene selectivity of 86.7% with 91.2% conversion of 1,4-hexadiene. In contrast, the generally supported Rh nanoparticle catalyst (Rh/CHA) exhibited the 2-hexene selectivity at only 37.2% under the equivalent reaction conditions. The significant selectivity on Rh@CHA catalyst is reasonably assigned to the steric adsorption of dienes on the Rh surface controlled by the micropores of CHA zeolite. This work demonstrates that the zeolite fixed metal particle with a core-shell structure is powerful for developing efficient catalysts in diene hydrogenation.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.

