由醚氧橋聯四羧酸配體構筑的兩個一維鎘(Ⅱ)配位聚合物的合成、晶體結構及熒光性質
黎 彧 趙振宇 鄒訓重 馮安生 邱文達*,
(1廣東輕工職業技術學院,廣東省特種建筑材料及其綠色制備工程技術研究中心/佛山市特種功能性建筑材料及其綠色制備技術工程中心,廣州 510300)
(2深圳信息職業技術學院智能制造與裝備學院,深圳 518172)
0 Introduction
The design and synthesis of functional coordination polymers have become the subjects of extensive investigation owing to their intriguing architectures and functional properties,such as magnetism,catalysis,luminescence,and gas storage[1-5].However,there still remains a great challenge for directional construction of functional coordination polymers with predictable molecular structures and expected properties,because a lot of factors influence the construction of complexes,such as the structural features of organic ligands,the coordination requirements of metal ions,solvent systems,temperatures,and pH values[6-10].
In this context,various types of aromatic polycarboxylic acids have been extensively utilized to synthesize various coordination polymers owing to their strong coordination ability in diverse modes and the fact that they are able to satisfy the geometric requirement of the metal centers[2-3,6,10-14].
As a combination of the aforementioned aspects and our previous research work,we have selected a new asymmetric tetracarboxylate ligand,2,3,3′,4′-diphenyl ether tetracarboxylic acid(H4L,Scheme 1)and explored it for the construction of novel coordination polymers.The selection of H4L has been governed by the following reasons.(1)This ligand contains two phenyl rings that are interconnected by a rotatableO-ether group providing a subtle conformational adaptation.(2)H4L contains two different types of functionalities(i.e.,-COOH andO-ether)and has nine potential coordination sites,which can result in diverse coordination patterns and high dimensionalities,especially when acting as a multiply bridging spacer.(3)This tetracarboxylic acid block remains poorly used for the generation of coordination polymers.
In this work,we report the syntheses,crystal structures,and luminescent properties of two Cd(Ⅱ)coordination polymers constructed from the tetracarboxylate ligand.

Scheme 1 Structures of H4L and the auxiliary ligands
1 Experimental
1.1 Reagents and physical measurements
All chemicals and solvents were of AR grade and used without further purification.H4L was acquired from Jinan Henghua Sci.&Technol.Co.,Ltd.Carbon,hydrogen and nitrogen were determined using an Elementar Vario EL elemental analyzer.IR spectra were recorded using KBr pellets and a Bruker EQUINOX 55 spectrometer.Thermogravimetric analysis(TGA)data were collected on a LINSEIS STA PT1600 thermal analyzer with a heating rate of 10℃·min-1.Excitation and emission spectra were recorded on an Edinburgh FLS920 fluorescence spectrometer using the solid samples at room temperature.
1.2 Synthesis of[Cd2(μ5-L)(phen)2]n(1)
A mixture of CdCl2·H2O(0.040 g,0.20 mmol),H4L(0.035 g,0.10 mmol),phen(0.040 g,0.20 mmol),NaOH(0.016 g,0.40 mmol),and H2O(10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10℃·h-1.Colourless blockshaped crystals of 1 were isolated manually,and washed with distilled water.Yield:43%(based on H4L).Anal.Calcd.for C40H22Cd2N4O9(%):C 51.80,H 2.39,N 6.04;Found(%):C 51.93,H 2.38,N 6.01.IR(KBr,cm-1):1 588s,1 515w,1 431w,1 397s,1 363m,1 256w,1 240w,1 144w,1 099w,1 060w,980w,924w,851m,783w,727m,699w,637w.
1.3 Synthesis of{[Cd2(μ4-L)(2,2′-bipy)2(H2O)2]·2H2O}n(2)
The preparation of 2 was similar to that of 1 except 2,2′-bipy was used instead of phen.After cool-ing the reaction mixture to room temperature,colourless block-shaped crystals of 2 were isolated manually,washed with distilled water,and dried.Yield:48%(based on H4L).Anal.Calcd.for C36H30Cd2N4O13(%):C 45.44,H 3.18,N 5.89;Found(%):C 45.21,H 3.16,N 5.93.IR(KBr,cm-1):3 390w,3 074w,1 567s,1 470w,1 436m,1 391s,1 313w,1 256w,1 234m,1 154w,1 060w,1 015w,980w,856w,823w,806w,766m,733w,699w,654w.
The compounds are insoluble in water and common organic solvents,such as methanol,ethanol,acetone,and DMF.
1.4 Structure determinations
Two single crystals with dimensions of 0.22 mm×0.21 mm×0.19 mm(1)and 0.21 mm×0.20 mm×0.18 mm(2)were collected at 293(2)K on a Bruker SMART APEX Ⅱ CCD diffractometer with MoKαradiation(λ=0.071 073 nm).The structures were solved by direct methods and refined by full matrix least-square onF2using the SHELXTL-2014 program[15].All non-hydrogen atoms were refined anisotropically.All the hydrogen atoms were positioned geometrically and refined using a riding model.A summary of the crystallography data and structure refinements for 1 and 2 is given in Table 1.The selected bond lengths and angles for compounds 1 and 2 are listed in Table 2.Hydrogen bond parameters of compound 2 are given in Table 3.
CCDC:1971327,1;1971328,2.

Table 1 Crystal data for compounds 1 and 2

Table 2 Selected bond distances(nm)and bond angles(°)for compounds 1 and 2

Continued Table 1

Table 3 Hydrogen bond parameters of compound 2
2 Results and discussion
2.1 Description of the structures
2.1.1 [Cd2(μ5-L)(phen)2]n(1)
The molecular structure of compound 1 is shown in Fig.1.The asymmetric unit contains two Cd atoms(Cd1 and Cd2),aμ5-L4-ligand,and two phen moieties.The six-coordinated Cd1 center displays a distorted octahedral{CdN2O4}environment filled by four carboxylate O atoms from three individualμ5-L4-blocks and two N atoms from the phen moiety.The Cd2 center is also six-coordinated and features a distorted octahedral{CdN2O4}geometry that is taken by four carboxylate O atoms from two differentμ5-L4-blocks and two N atoms from the phen moiety.The lengths of the Cd-O and Cd-N bonds are 0.218 9(6)~0.253 3(5)and 0.228 6(6)~0.238 1(6)nm,respectively,which are within the normal values for related Cd(Ⅱ)derivatives[6,10,16].In 1,the L4-block acts as aμ5-ligand,wherein the COO-groups are monodentate,bidentate or tridentate(modeⅠ,Scheme 2).The carboxylate groups of fourμ5-L4-blocks link the adjacent Cd(Ⅱ)centers into a Cd4subunit(Fig.2).Such subunits are further assembled via the remaining COO-groups ofμ5-L4-blocks to a 1D chain(Fig.2).
2.1.2 {[Cd2(μ4-L)(2,2′-bipy)2(H2O)2]·2H2O}n(2)

Fig.1 Drawing of asymmetric unit of compound 1 with 30% probability thermal ellipsoids
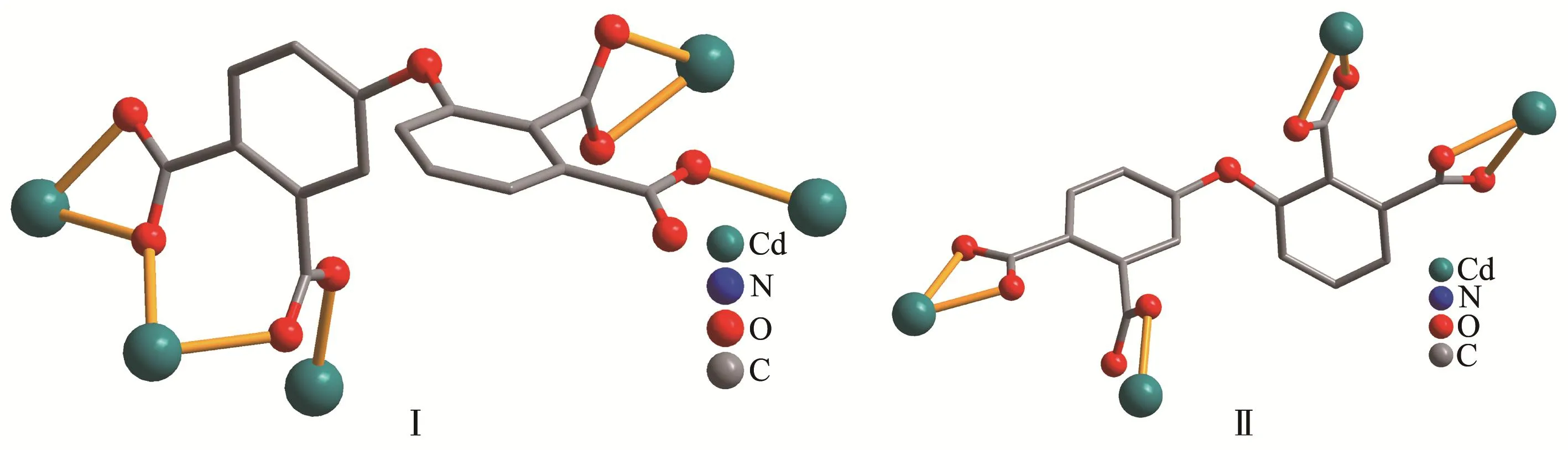
Scheme 2 Coordination modes of L4-ligands in compounds 1 and 2
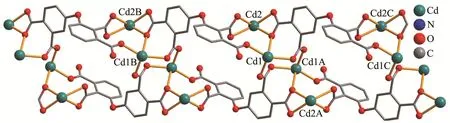
Fig.2 View of 1D chain in compound 1 along b axis
The asymmetric unit of 2 bears two Cd(Ⅱ)centers(Cd1 and Cd2),oneμ4-L4-linker,two 2,2′-bipy moieties,two terminal water ligands and two lattice water molecules(Fig.3).The seven-coordinated Cd1 center adopts a distorted pentagonal bipyramid{CdN2O5}environment taken by four carboxylate O atoms from two independentμ4-L4-blocks,one O atom from the H2O li-gand,and a pair of N atoms from the 2,2′-bipy moiety.The Cd2 center is six-coordinated and reveals a distorted octahedral{CdN2O4}geometry.It is completed by three carboxylate O donors from twoμ4-L4-blocks,one O atom from the H2O ligand,and two N donors from the 2,2′-bipy moiety.The Cd-O(0.227 9(6)~0.256 2(6)nm)and Cd-N(0.229 7(8)~0.233 3(7)nm)bonds are within standard values[14,16-17].The L4-block behaves as a heptadentateμ4-linker(Scheme 2,mode Ⅱ).Twoμ4-L4-blocks link adjacent Cd(Ⅱ)centers to form a Cd2unit(Fig.4).Furthermore,these Cd2units are further extended byμ4-L4-blocks into a 1D chain(Fig.4).Compounds 1 and 2 were obtained under similar reaction conditions except using different auxiliary ligands(phen for 1 and 2,2′-bipy for 2),which result in different structures.

Fig.3 Drawing of asymmetric unit of compound 2 with 30% probability thermal ellipsoids

Fig.4 View of 1D chain in compound 2 along a axis
2.2 TGA analysis
To determine the thermal stability of compounds 1 and 2,their thermal behaviors were investigated under nitrogen atmosphere by thermogravimetric analysis(TGA).As shown in Fig.5,TGA curve of compound 1 shows that the sample remained stable until 327℃.Compound 2 lost its two lattice water molecules and two H2O ligands in a range of 45~157℃ (Obsd.7.2%;Calcd.7.6%),followed by the decomposition at 254℃.

Fig.5 TGA curves of compounds 1 and 2
2.3 Luminescent properties
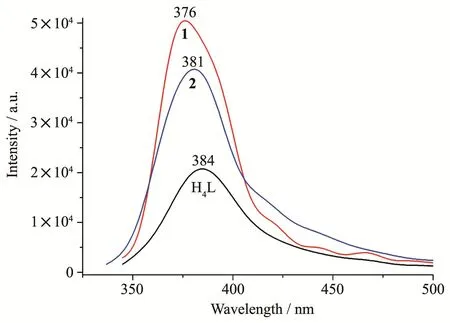
Fig.6 Solid-state emission spectra of H4L and compounds 1 and 2 at room temperature
Solid-stateemissionspectraofH4Landcadmium(Ⅱ)compounds 1 and 2 were measured at room temperature(Fig.6).The spectrum of H4L revealed a weak emis-sion with a maximum at 384 nm(λex=320 nm).In comparison with H4L,compounds 1 and 2 exhibited more extensive emission with maximum at 376 and 381 nm(λex=320 nm).These emissions correspond to intraligandπ-π*orn-π*transition of H2L[6,10,17].Enhancement of the luminescence in 1 and 2 compared to H4L can be explained by the coordination of ligands to Cd(Ⅱ);the coordination can augment a rigidity of ligands and reduce an energy loss due to radiationless decay[10,16-17].
3 Conclusions
In summary,we have successfully synthesized and characterized two new cadmium coordination polymers by using a tetracarboxylate acid as a ligand under hydrothermal condition.Compounds 1 and 2 show 1D chains composed of Cd4and Cd2units,respectively.Besides,the luminescent properties were also investigated and discussed.The results show that such tetracarboxylic acid can be used as a versatile multifunctional building block toward the generation of new coordination polymers.

