添加膳食纖維對大鼠腦缺血后炎性反應的影響
李海濤 曲波 楊事達
[摘要] 目的 探討添加膳食纖維對大鼠腦缺血后炎性反應的影響。 方法 按照隨機分配的原則,將34只成年雄性大鼠分為假手術組(n = 10),缺血組(n = 12),缺血+膳食纖維組(n = 12)。缺血組和缺血+膳食纖維組顱內注射內皮素制作腦缺血模型,有4只大鼠死亡;假手術組顱內注射等體積的生理鹽水,所有大鼠均存活;最終假手術組10只、缺血組10只、缺血+膳食纖維組10只。假手術組與缺血組正常飲食,缺血+膳食纖維組添加膳食纖維。測量梗死體積,免疫熒光染色觀測各組大鼠梗死周邊皮層Iba-1/BrdU與GFAP/BrdU雙染陽性細胞數量。 結果 缺血組與缺血+膳食纖維組梗死體積比較,差異無統計學意義(P > 0.05)。缺血組Iba-1/BrdU雙染陽性細胞數、GFAP/BrdU雙染陽性細胞數多于假手術組,缺血+膳食纖維組Iba-1/BrdU雙染陽性細胞數、GFAP/BrdU雙染陽性細胞數少于缺血組,差異均有高度統計學意義(均P < 0.01)。 結論 腦梗死恢復期給予膳食纖維可抑制梗死周邊新生的小膠質細胞與星形膠質細胞激活,從而抑制腦梗死后的炎性反應。
[關鍵詞] 腦梗死;膳食纖維;炎性反應;腸道菌群
[中圖分類號] R743.31 ? ? ? ? ?[文獻標識碼] A ? ? ? ? ?[文章編號] 1673-7210(2020)07(b)-0010-03
[Abstract] Objective To investigate the effect of dietary fiber on inflammatory response after cerebral ischemia in rats. Methods Thirty-four adult male rats were divided into sham group (n = 10), ischemic group (n = 12), ischemic+dietary fiber group (n = 12) according to the principle of random distribution. Endothelin was injected into the brain of the ischemia group and ischemic+dietary fiber group to make the cerebral ischemia model, 4 rats died; while sham operation group was injected with isovolume normal saline, all the rats survived. Finally, 10 rats in sham operation group, 10 rats in ischemia group, and 10 rats in ischemia+dietary fiber group. Normal diet was added to the sham group and ischemia group, dietary fiber was added to the ischemia+dietary fiber group. The infarct volume was measured and the number of Iba-1/BrdU and GFAP/BrdU double staining positive cells in the infarct peripheral cortex of each group was observed by immunofluorescence staining. Results There was no significant difference in infarct volume between ischemia group and ischemia+dietary fiber group (P > 0.05). The number of Iba-1/BrdU double staining positive cells, GFAP/BrdU double staining positive cells in ischemia group were more than those in sham operation group, while the number of Iba-1/BrdU double staining positive cells, GFAP/BrdU double staining positive cells in ischemia+dietary fiber group were less than those in ischemia group, and the differences were all highly statistically significant (all P < 0.05). Conclusion Dietary fiber can inhibit the activation of new microglia and astrocytes around the infarct in the recovery period of cerebral infarction, and thus inhibit the inflammatory response after cerebral infarction.
[Key words] Cerebral infarction; Dietary fiber; Inflammatory response; Gut flora
近年來的研究發現,腦與胃腸道可以通過由中樞神經系統、自主神經系統、下丘腦-垂體-腎上腺軸以及腸神經系統等結構組成的神經-免疫-內分泌網絡相互作用[1-3]。此外,有研究[4]顯示,腸道菌群與腦血管疾病密切相關。長期的腸道營養可以短暫或永久地改變宿主腸道菌群的穩態[5]。高膳食纖維飲食可以被腸道菌群代謝發酵產生短鏈脂肪酸,主要包括乙酸、丙酸和丁酸[6]。本研究探討添加膳食纖維對腦梗死后大鼠腦內炎癥細胞的影響,旨在為腦梗死的臨床康復提供新的理論基礎及治療靶點。
1 對象與方法
1.1 實驗動物
34只成年雄性Wistar大鼠,體重200~250 g,由中國醫科大學實驗動物中心提供,實驗動物合格證號:211002300052024,動物許可證號:SCXK[遼]2015-0001。所有動物實驗操作程序符合中國醫科大學倫理委員會管理條例要求。動物分組按隨機分配的原則,將其隨機分為三組:假手術組(n = 10),缺血組(n = 12),缺血+膳食纖維組(n = 12)。
1.2 儀器與試劑
BrdU(生產批號:B3023)、ET-1(生產批號:E6877)均購于美國Sigma-Aldrich公司;Rabbit anti-Iba-1(生產批號:ab178846)、Rabbit anti-glial fibrillary acidic protein(GFAP)(生產批號:ab7260)均購于美國Abcam公司;Alexa fluor 488 goat anti-rabbit IgG(生產批號:A-11034)、Alexa fluor 594 donkey anti-sheep IgG(生產批號:A-11016)均購于美國Invitrogen公司。微量注射器(型號:87943)購于美國 Hamilton 公司;微量注藥系統(型號:62000)購于深圳瑞沃德生命科技有限公司;共聚焦顯微鏡購(型號:Leica TCS SP2)購于德國Leica SP2公司;搖床(型號:6173000)購于德國Heidolph公司。膳食纖維飼料來源于廣州市賽柏諾生物科技有限公司。
1.3 方法
1.3.1 顱內注射內皮素(ET-1)制作腦缺血模型 ?將ET-1用生理鹽水稀釋成0.500 μg/μL,根據大鼠腦解剖圖譜注射到以下3點(AP示以前囟為中心向前,ML示以前囟為中心向側方,DV示以前囟為中心向下方[7-8]):①AP+0.700 mm,ML+2.200 mm,DV-2.000 mm;②AP+2.300 mm,ML+2.500 mm,DV-2.300 mm;③AP+0.700 mm,ML+3.800 mm,DV-5.800 mm。ET-1用微量注射器以0.500 μL/min注射。注射ET-1后約3 h(此時大鼠麻醉已清醒)若大鼠出現提尾倒懸時右上肢向胸前屈曲或行走時向右側傾倒或者向右側轉圈,且癥狀持續超過24 h,可判定為缺血模型制作成功。
缺血組與缺血+膳食纖維組大鼠制作腦缺血模型,有4只大鼠死亡。10只大鼠顱內注射等體積的生理鹽水,所有的假手術組大鼠均存活。最終分組為假手術組(n = 10)、缺血組(n = 10)和缺血+膳食纖維組(n = 10)。
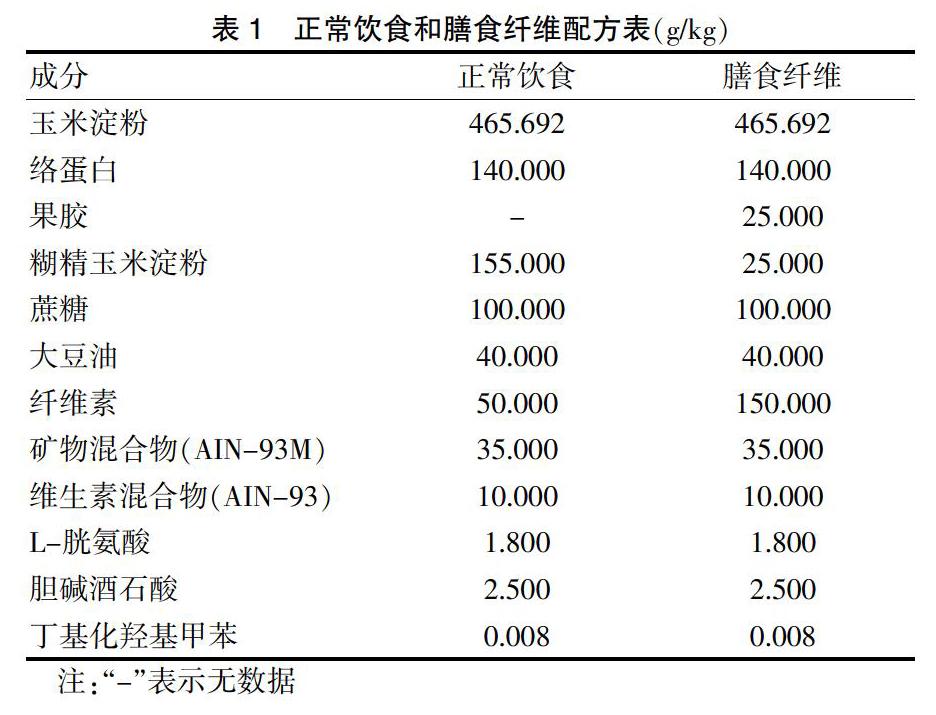
1.3.2 膳食纖維的添加 ?在大鼠大群飼養中,假手術組與缺血組正常飲食,缺血+膳食纖維組在7~35 d添加膳食纖維,經口喂入。大鼠富含膳食纖維的飼料成分配比見表1。
1.3.3 梗死體積的測量 ?第40天時,使用尼氏染色測量缺血組、缺血+膳食纖維組腦梗死的體積。腦片對側及同側半球區域使用NIH Image J 進行測量,每張切片完整半球區域減去梗死半球區域再乘以間隔得到總梗死體積。
1.3.4 免疫熒光染色 ?免疫熒光染色觀測大鼠梗死區域周邊GFAP/BrdU及Iba-1/BrdU陽性細胞及GFAP/BrdU、Iba-1/BrdU染色情況:腦片漂洗,血清孵育后,在rabbit anti-Iba-1(1∶500)或GFAP(1∶1000)中4℃孵育過夜,漂洗,在二抗Alexa fluor 488 goat anti-guinea pig IgG或Alexa fluor 488 goat anti-rabbit IgG(1:500)中室溫避光孵育2 h,漂洗;然后在2N HCl 37℃水浴箱中孵育45 min,漂洗,血清孵育;隨后在抗BrdU抗體(1:500)中4℃孵育過夜,PBS漂洗后,在二抗Alexa fluor 594 donkey anti-sheep IgG(1∶500)中室溫避光孵育2 h,漂洗后貼片、封片。其中Iba-1為小膠質細胞標志物,GFAP為星形膠質細胞標志物,通過Iba-1和GFAP的數量觀察膳食纖維對腦缺血后小膠質細胞及星形膠質細胞的影響。
1.4 統計學方法
采用SPSS 16.0統計學軟件進行數據分析,計量資料用均數±標準差(x±s)表示,多組間比較采用單因素方差分析,兩兩比較采用LSD-t檢驗。以P < 0.05為差異有統計學意義。
2 結果
2.1 缺血組與缺血+膳食纖維組梗死體積比較
缺血組梗死體積為(132.823±10.571)mm3,缺血+膳食纖維組梗死體積為(128.214±11.283)mm3,兩組梗死體積比較,差異無統計學意義(P > 0.05)。
2.2 三組炎性反應情況比較
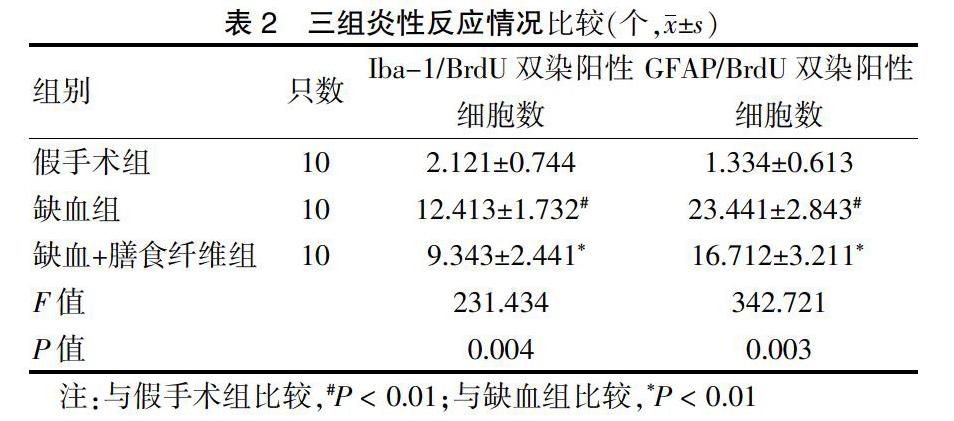
缺血組Iba-1/BrdU雙染陽性細胞數、GFAP/BrdU雙染陽性細胞數多于假手術組,缺血+膳食纖維組Iba-1/BrdU雙染陽性細胞數、GFAP/BrdU雙染陽性細胞數少于缺血組,差異均有高度統計學意義(均P < 0.01)。見圖1(封四),表2。
3 討論
腦缺血后梗死區域的小膠質細胞及星形膠質細胞會被激活[9],形成的惡性循環促進繼發性腦損傷的發生,更加重神經功能的缺失[10]。本研究發現,腦缺血后大鼠梗死區域GFAP與Iba-1細胞明顯被激活,與以往研究一致[11]。在大鼠腦缺血后的早期,小膠質細胞及星形膠質細胞就被激活,過早的形成膠質瘢痕[12]。因此,如果能對腦梗死后的炎性反應加以抑制,有可能有效促進神經功能的恢復。
近期有研究[5]顯示,長期腸道營養可以短暫或者永久地改變宿主腸道菌群的穩態。膳食纖維可以被腸道菌群代謝發酵而產生短鏈脂肪酸。短鏈脂肪酸可以改變腸道菌群的組成[13-15],還可以參與神經膠質細胞的穩態調節,維持中樞神經系統內環境穩定[16-19]。更為重要的是,通過調控中樞神經系統的免疫反應,短鏈脂肪酸被證實能夠減輕多發性硬化動物模型中的神經元軸突損傷[5]。提示應用膳食纖維能夠促進神經元的恢復。但其中的確切機制尚不清楚,值得進一步深入探討。本研究首次探討添加膳食纖維對大鼠梗死周邊炎癥的影響。結果顯示,腦梗死后應用膳食纖維并不能夠減小梗死體積,但能夠抑制梗死周邊的炎性反應,促進腦梗死后的神經功能修復。
綜上所述,本研究推測在腦梗死恢復期給予膳食纖維,可能通過抑制梗死周邊新生的小膠質細胞與星形膠質細胞激活,從而抑制腦梗死后的炎性反應。可以更好地了解膳食纖維發揮作用的可能機制并為腦梗死的臨床康復提供了新的理論基礎及治療靶點。
[參考文獻]
[1] ?Bercik P,Collins SM,Verdu EF. Microbes and the gut-brain axis [J]. Neurogastroenterol Motil,2012,24(5):405-413.
[2] ?Cryan JF,O′Mahony SM. The microbiome-gut-brain axis:from bowel to behavior [J]. Neurogastroenterol Motil,2011, 23(3):187-192.
[3] ?Geschwind DH,Rakic P. Cortical evolution:judge the brain by its cover [J]. Neuron,2013,80(3):633-647.
[4] ?Karlsson FH,F?覽k F,Nookaew I,et al. Symptomatic atherosclerosis is associated with an altered gut metagenome [J]. Nat Commun,2012,3(4):1245.
[5] ?Haghikia A,J?觟rg S,Duscha A,et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine [J]. Immunity,2015,43(4):817-829.
[6] ?Ganapathy V,Thangaraju M,Prasad PD,et al. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host [J]. Curr Opin Pharmacol,2013,13(6):869-874.
[7] ?Soleman S,Yip P,Leasure JL,et al. Sustained ?sensorimotor ?impairments ?after ?endothelin-1 induced focal cerebral ischemia (stroke) in aged rats [J]. Exp Neurol,2010, 222(1):13-24.
[8] ?Biernaskie J,Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury [J]. J Neurosci,2001,21(14):5272-5280.
[9] ?Stoll G,Jander S,Schroeter M. Inflammation and glial responses in ischemic brain lesions [J]. Prog Neurobiol,1998, 56(2):149-171.
[10] ?Ekdahl CT,Claasen JH,Bonde S,et al. Inflammation is detrimental for neurogenesis in adult brain [J]. Proc Natl Acad Sci U S A,2003,100(23):13632-13637.
[11] ?Takano K,Ogura M,Nakamura Y,et al. Neuronal and glial responses to polyamines in the ischemic brain [J]. Curr Neurovasc Res,2005,2(3):213-223.
[12] ?Badan I,Buchhold B,Hamm A,et al. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery [J]. J Cereb Blood Flow Metab,2003,23(7):845-854.
[13] ?Cryan JF,Dinan TG. More than a gut feeling:the microbiota regulates neurodevelopment and behavior [J]. Neuropsychopharmacology,2015,40(1):241-242.
[14] ?Lu Y,Fan C,Li P,et al. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota [J]. Sci Rep,2016,6:37589.
[15] ?Smith PM,Howitt MR,Panikov N,et al. The microbial metabolites,short-chain fatty acids,regulate colonic Treg cell homeostasis [J]. Science,2013,341(6145):569-573.
[16] ?Braniste V,Al-Asmakh M,Kowal C,et al. The gut microbiota influences blood-brain barrier permeability in mice [J]. Sci Transl Med,2014,6(263):263ra158.
[17] ?Sharon G,Sampson TR,Geschwind DH,et al. The Central Nervous System and the Gut Microbiome [J]. Cell,2016,167(4):915-932.
[18] ?Lin HV,Frassetto A,Kowalik EJ Jr,et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms [J]. PLoS One,2012,7(4):e35240.
[19] ?Wong JM,De Souza R,Kendall CW,et al. Colonic Health:Fermentation and Short Chain Fatty Acids [J]. J Clin Gastroenterol,2006,40(3):235-243.
(收稿日期:2020-01-06)

