Serum vitamin D in Indian children with vernal keratoconjunctivitis
Abstract
?KEYWORDS:vernal keratoconjunctivitis; vitamin D deficiency; children
INTRODUCTION
Vernal keratoconjunctivitis (VKC) is a chronic, sight threatening, allergic disease of the eye, affecting mainly young males. It is characterized by a seasonally recurring, bilateral inflammation of the conjunctiva and the cornea[1-5]. Vitamin D deficiency has been implicated in the pathophysiology of VKC along with endocrine, neurogenic, genetic, environmental and socioeconomic risk factors[6-7].
There are 4 types of allergic conjunctivitis which have different types of pathological reactions: simple allergic conjunctivitis (seasonal, acute and perennial) is type I hypersensitivity reaction. Atopic and giant papillary conjunctivitis is combined type I and IV hypersensitivity reaction. Vernal Keratoconjunctivitis resembles Type I (Ig E dependent) and IV (Ig E independent) hypersensitivity reaction[8]. It shows the presence of eosinophils, mast cells, fibroblasts, T and B cells[9]which plays an active role in its pathogenesis.
VKC commonly affects young boys, but both sexes can be involved. Onset is usually around 5 years of age, around 95% of the cases show regression during or soon after puberty[3]. Though the usual presentation is during spring and summer seasons, perennial cases are also seen. Symptoms consist of intense itching, blurred vision, lacrimation, photophobia, blepharospasm and mucoid discharge. The disease may be classified as tarsal (primarily involving the upper tarsal conjunctiva), limbal, or mixed phenotype. Tarsal disease presents with conjunctival hyperaemia and flat-topped macro-papillae (<1 mm) known as cobblestone papillae, or giant papillae (>1 mm) on the superior tarsal conjunctiva, whereas the limbal disease presents with gelatinous aggregates of epithelial cells and eosinophils (Horner-Trantas dots) at the limbus; corneal involvement may result in punctate epithelial keratitis, epithelial macro-erosions, shield ulcer and plaque formation[6].
Vitamin D is an important fat-soluble prohormone, having numerous functions in the body, including the regulation of calcium and phosphate meatabolism[10-11]. The effects are mediated via a receptor called as VDR. VDR has also been discovered in tissues and cells which are not involved in calcium homeostasis, such as on T cells, B cells, neutrophils, macrophages, and dendritic cells. It has been shown to have a role in the regulation of immunity, cells proliferation and differentiation, angiogenesis and apoptosis[12-16]. Vitamin D has been shown to suppress the allergic pathways by inhibiting the dendritic cells maturation, migration and reprogramming as tolerogenic phenotype, decreasing the interleukin-12 (IL-12), IL-23 and cytokine production, inducing IL-10 secreting regulatory T cells, and inhibiting Th1 and Th17 cells[15-19].
Other effects such as prevention of cardiovascular, immune-related and infectious diseases have also been proposed[20]and many studies have suggested a correlation between vitamin D levels and immuno-mediated and atopic diseases[21-23].
Although many researchers evaluated the relationship between vitamin D and allergic disease providing conflicting results, its levels in patients with VKC have been assessed in only two studies: Zicarietal[8]conducted a randomised prospective study on 110 children in Italy demonstrated that children affected by VKC have lower vitamin D levels when compared to healthy controls and highlights a significant correlation between its levels and disease severity.
Another study conducted in Turkey by Bozkurtetal[6]on 62 non-atopic healthy children and 29 VKC children showed that children with VKC in the studied population had lower serum vitamin D levels compared with age and sex matched healthy children and half of these patients had severe vitamin D deficiency, which seems to develop secondary to decreased sun exposure.
A recent study in Italy published in 2019 with 242 children with active VKC showed that ocular treatment with immunomodulator eye drops (cyclosporine or tacrolimus) could allow for improvement in serum vitamin D levels[24]. Few other studies have mentioned vitamin D deficiency as risk for developing seasonal allergic conjunctivitis, childhood asthma and systemic allergies[25-26].
In India, no such study has been done so far which correlates the association between VKC and serum vitamin D levels. The present study is intended to investigate the serum vitamin D levels in children affected by vernal keratoconjunctivitis.
SUBJECTS AND METHODS
A total of 30 VKC children and 30 healthy children were included in this case control study from April 2108 to November 2018. The study was approved by the Ethical Committee (Institute Ethics Committee, V.M.M.C. and Safdarjung hospital, New Delhi, India, 110029) and informed consent was taken from the parents.
Newly diagnosed VKC patients with a VKC score ≥7, in the age group of 5-15 years, willing to follow up were included in the study. Individuals having any prior ocular surgery, history of asthma or systemic allergy, any other ocular disease except refractive errors, history of rheumatoid arthritis, cystic fibrosis, sarcoidosis, thyroid dysfunction, obesity and on any drugs like barbiturates, bisphosphonate, sulphasalazine, omega 3 fatty acids, calcium, and vitamin D, were excluded from the study. Control subjects were chosen from healthy children with no history of atopy or any ocular diseases other than refractive errors.
A meticulous history taking was done to rule out the exclusion criteria. Patients were asked about the symptoms like itching, photophobia, tearing, foreign body and burning sensation. Patients were also asked about how many hours they spend outdoors on an average per day. Clinical examination was done by evaluation of anterior segment using slit lamp examination to look for conjunctival hyperaemia, tarsal and/or limbal papillae, giant papillae, punctate epitheliopathy, or shield ulcer in patients with VKC. Best corrected visual acuity determination, refraction, fundus examination, corneal topography was also done for all the patients.
Determination of VKC score: 1) Conjunctival hyperaemia; 2) Tarsal and/or limbal papillae; 3) Giant papillae; 4) Itching; 5) Photophobia; 6) Tearing; 7) Foreign body sensation; 8) Burning sensation. Each variable was graded as follows: 0: absent, 1: mild, 2: moderate, 3: severe.
In the score other two parameters were included: Duration of symptoms (0 if < 1 years; 1 if > 1 years and 2 if > 2 years); Corneal involvement (0: absent, 1: de-epithelialization, 2: keratitis, 3; ulcer). Children with a total score ≥ 7 were included in the study[27].
After the diagnosis, patients were divided into four grades of VKC[28]according to presenting symptoms and signs,i.e. mild, moderate, severe and blinding.
Fasting venous blood samples from the antecubital vein was obtained in the morning after overnight fasting and put into EDTA anticoagulated tubes. Serum vitamin D level was analysed by chemiluminescence assay in the department of pathology.
In this study serum 25 (OH) D3 levels were measured, as it has a significantly more stable hormone-receptor complex than 25 (OH) D2, and has been shown to provide levels of vitamin D in circulation more effectively. Detection limit for 25(OH) D3 is 5 ng/mL. The Endocrine Society Clinical Practice Guidelines suggested considering deficiency if 25 (OH) D is below 20 ng/mL (50 nmol/L), while levels <10 ng/mL are accepted as severe vitamin D deficiency[29-30]. The children having vitamin D deficiency were referred to the department of paediatrics for treatment and vitamin D supplementation.
StatisticalAnalysisStatistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 21.0. Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean±SD and median. Normality of data was tested by Kolmogorov-Smirnov test. If the normality was rejected then non-parametric test was used.
Statistical tests were applied as follows: Quantitative variables were compared using Independent T test/Mann-Whitney Test (when the data sets were not normally distributed) between the two groups and ANOVA test for correlation of vitamin D level with severity. Qualitative variables were correlated using Chi-Square test.
Pearson correlation coefficient was used to assess the association of severity of VKC with vitamin D deficiency severity. APvalue of <0.05 was considered statistically significant.
RESULTS
There were 30 patients in the VKC group (25 males, 83.33%; 5 females, 16.67%) and 30 patients in the control group (21 males, 70%; 9 females, 30%), with no statistical differences (P=0.22).
The mean ages of the VKC group was 8.27±2.79 years and the control group was 8.9±2.52 years, with no statistical difference (P=0.922).
The best corrected visual acuity (BCVA) ranged from 6/6-6/24, with 53.33% having 6/6, 40% having 6/9, 3.33% having 6/12 and 3.33% having 6/24 in VKC group. All the controls had a BCVA of 6/6.
The mean spherical refraction in the VKC group was-0.69±1.01, and in the control group was -0.68±1.02. The mean maximum (K1) and minimum (K2) topographic keratometric values in the VKC group were 43.83±0.78 D and 43.42±0.79 D respectively, and in the control group were 43.9±0.82 D (K1) and 43.57±0.87 D (K2). VKC was tarsal in 13 subjects (43.33%), limbal in 3 subjects (10%) and mixed in 14 subjects (46.67%). The severity of VKC was mild in 1 (3.33%), moderate in 19 (63.33%), severe in 9 (30.0%) and blinding in 1 (3.33%) patient.
The mean serum 25 (OH) D3 level in the VKC group was significantly lower (mean 19.17±10.76 ng/mL, median 17.05 ng/mL, range 5-53.4 ng/mL) as compared to the control group (mean 31.19± 9.09 ng/mL, median 32.15 ng/mL, range 12-49.2 ng/mL) (P=0.0003).
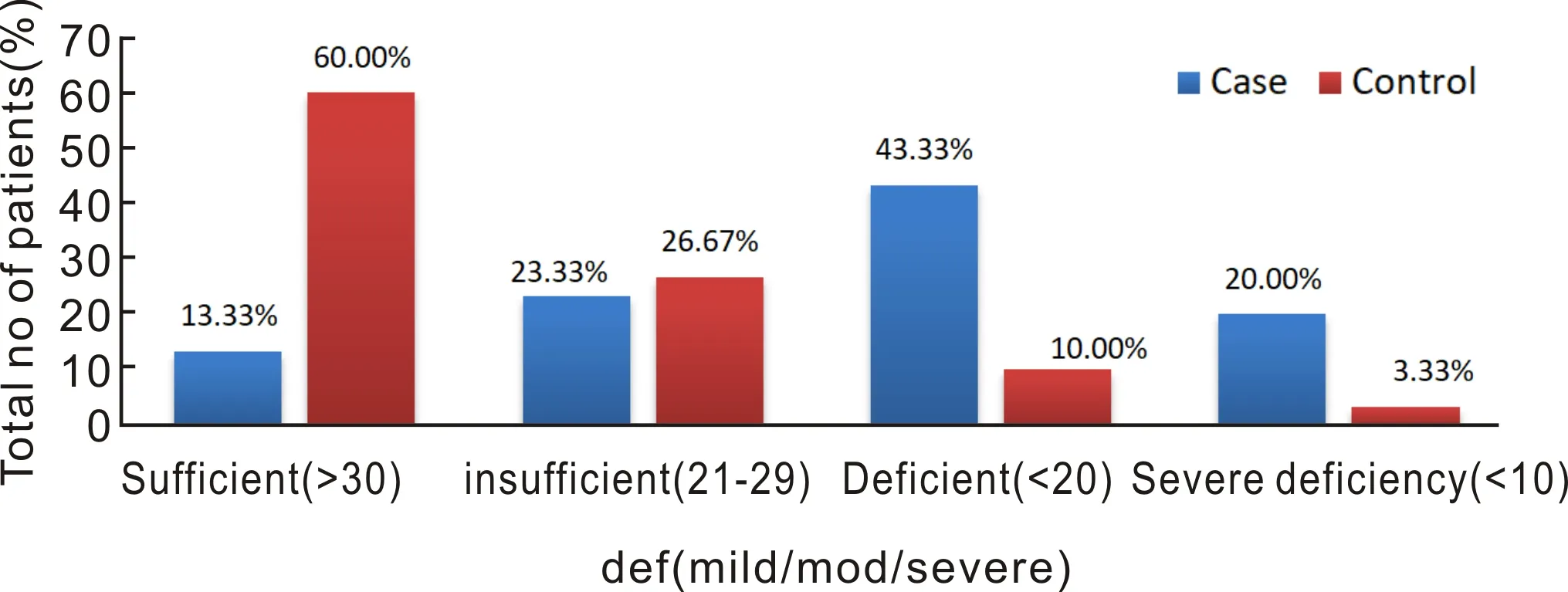
The vitamin D levels were found to be sufficient (>30 ng/mL) in 13.33%, in-sufficient (21-29 ng/mL) in 23.33%, deficient (10-20 ng/mL) in 43.33% and severe deficiency (<10 ng/mL) was found in 20% of the VKC patients. In the control group, the vitamin D levels were sufficient (>30 ng/mL) in 60%, in-sufficient (21-29 ng/mL) in 26.67%, deficient (10-20 ng/mL) in 10% and severe deficiency(<10 ng/mL) was found in 3.33% of controls (Figure 1).
Figure 1 Chart depicting the distribution of serum vitamin D levels in cases and controls.
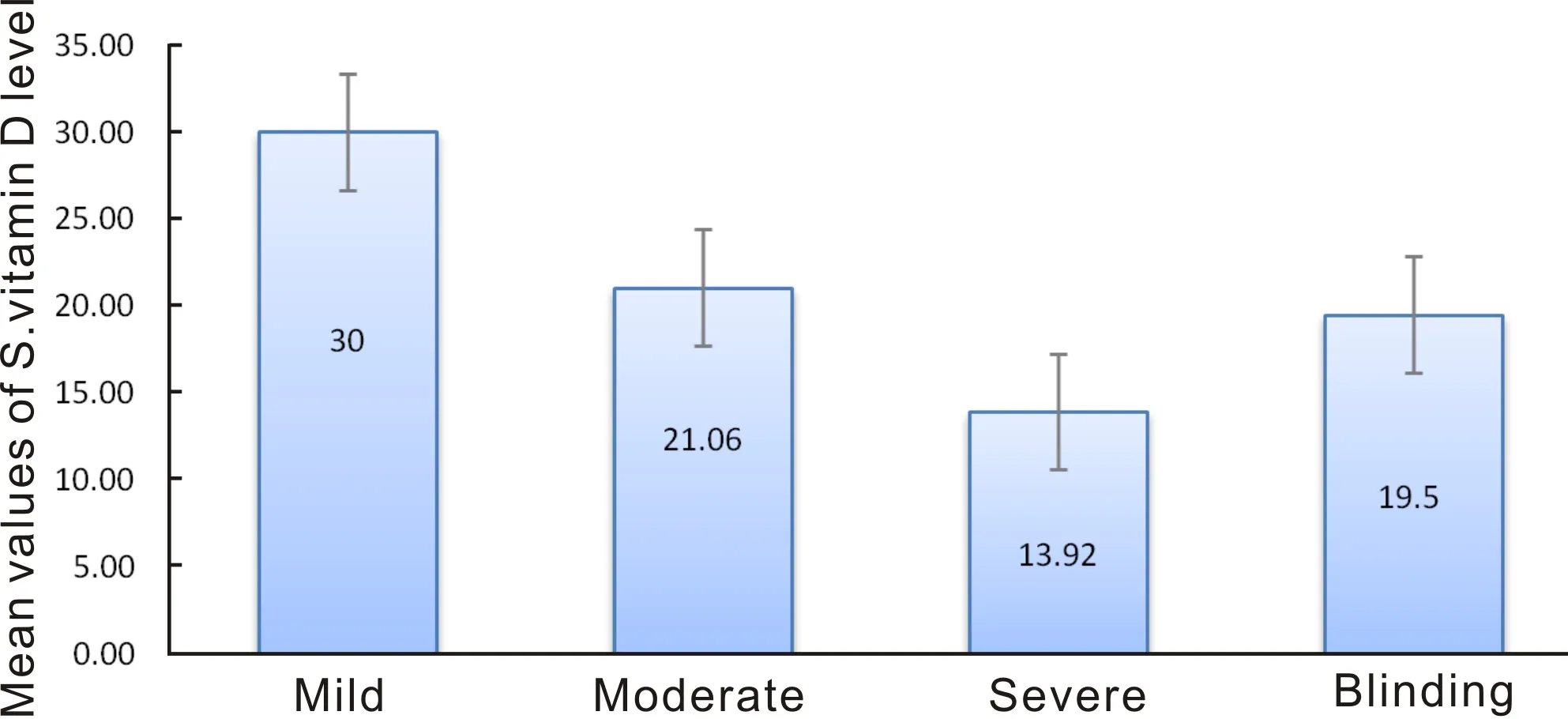
Figure 2 Chart depicting the mean vitamin D levels according to severity of VKC.
According to severity of the disease, the mean vitamin D level in mild VKC was 30 ng/mL, moderate: 21.06±11.61 ng/mL, severe: 13.92±7.87 ng/mL and in blinding: 19.5 ng/mL (P=0.157) (Figure 2). The correlation of deficiency of vitamin D with the increased levels of severity of VKC was statistically significant (P<0.02).
The VKC score and the serum vitamin D levels did not show a statistically significant correlation (correlation coefficient -0.342,P=0.0646) but was clinically significant.
The time spent outside in the VKC group was 1.07±0.76h, with a median of 1h and range 0-3h, whereas in the healthy subjects it was 2.08±0.72h, median 2h and range 1-4h, which was statistically significant (P<0.0001).
The time spent outside correlated with the vitamin D levels, with higher Vitamin D levels in children who spent more time outside (r=0.478,P<0.001).
DISCUSSION
Vernal keratoconjunctivitis is one of the disorders in the vast spectrum of allergic eye diseases. Various studies have been done evaluating the risk factors for seasonal allergic conjunctivitis. It has been suggested that Vitamin D deficiency may play a role in the onset of allergic diseases[31]. In this study, a total of 60 subjects were taken, 30 in the VKC group and 30 in the control group (non-VKC group). There was no statistical difference in the age and sex distribution between the two groups.
Of the 30 patients, majority had mixed VKC (46.47%), followed by tarsal (43.33%) and limbal (10%). 19 patients (63.3%) had a moderate (intermittent) disease and 9 (30%) had a severe disease. One patient had a mild disease and one had blinding disease. The child with mild disease was included in the study due to the severity of his presenting symptoms, which resulted in his severity score being 7. We encountered 2 patients with chronic moderate VKC but as they were already on topical medications, they were not included in the study. The mean VKC score was 10.33±2.62 (median 10, range 7-15). Each patient was treated based on the severity of VKC with mild patients receiving topical lubricants, mast cell stabilizers/ antihistamines, moderate severity patients receiving low dose steroids in addition, severe patients receiving potent topical steroids with tacrolimus/cyclosporine and those with blinding disease receiving management of shield ulcer, in addition to the above[28]. In a study by Bozkurtetal[6], 51.7% patients had tarsal VKC and 48.3% had mixed VKC.
The best corrected visual acuity ranged from 6/6-6/24 (median: 6/6). The visual acuity of 6/24 was found in the child having blinding disease, which was attributed to the corneal ulceration that was present. The patients having BCVA 6/9 (40%) and 6/12 (3.33%) had varying degrees of superficial punctate erosions and macro-erosions of the cornea, along with intense photophobia. All the children in the control group had a BCVA of 6/6. The mean spherical refraction in the VKC group was -0.69±1.01, and in the control group was 0.68±1.02.
As per the Endocrine Society Clinical Practice Guidelines, vitamin D deficiency is considered if serum 25 (OH) D is below 20 ng/mL (50 nmol/L), while levels <10 ng/mL are accepted as severe vitamin D deficiency[29-30]. Insufficiency is considered at levels 21-29 ng/mL. In our study there was a significant difference in the mean serum 25 (OH) D3 levels between the two groups, with the VKC group having a mean level of 19.17±10.76 ng/mL and controls having a mean of 31.19±9.09 ng/mL. The vitamin D levels were found to be deficient (43.33%) in majority of the VKC patients. The vitamin D deficiency (sufficient, insufficient, deficiency and severe deficiency) correlated with the severity of VKC (mild, moderate, severe and blinding), with patients having increased severity of VKC having lower vitamin D levels(P=0.0216, correlation coefficient: 0.418).
At least two studies have reported an association between VKC and vitamin D levels[6,32]. In a study by Zicarietal[32], lower levels of vitamin D were found in the VKC group when compared to controls (20.4±6.7 ng/mLvs25.2±6.5 ng/mL). They also found an increase in vitamin D levels after therapy with cyclosporine eye drops 1% although this increase was lower than that of healthy controls.
Another study done by Bozkurtetal[6], mean 25 (OH) D3 level in the VKC group was 11.02±5.16 ng/mL, and in the controls was 15.99±7.36 ng/mL which was significantly lower. They found severe deficiency (<10 ng/mL) in 48.3% and levels <20 ng/mL in 93.1% of the VKC children. This could be attributed to the fact the patients with VKC have intense photophobia and most often avoid direct exposure to sunlight, wear sunglasses or hats to decrease the exposure. Also, people having increased pigmentation, such as those belonging to India, Middle East or Africa, are more predisposed to deficiency of vitamin D.
In our study, there was a significant reduction in the time spent outside in the VKC group (1.07±0.76h, with a median of 1h) as compared to the controls (2.08±0.72h, with a median of 2h). The time spent outside correlated with the vitamin D levels, with higher vitamin D levels in children who spent more time outside (r=0.478,P<0.001), but it did not show a significant correlation with the VKC score (correlation coefficient -0.047,P=0.8038). Bozkurtetal[6]also found a significant difference in the time spent outdoors between the VKC group (229.5±101.2min) and the controls (160±65.9min), and they found a significant correlation between 25 (OH) D3 levels and the time spent outside.
The most important source of vitamin D is the sun, UV B radiation from the sunlight helps in converting the naturally occurring 7-Dehydrocholesterol (present in the skin) to cholecalciferol (Vit D3). Dietary products like fish and meat are also minor sources of vitamin D3. Cholecalciferol is a biologically inactive form, and is converted to the biologically active 1, 25 dihydroxycholecalciferol by two reactions, firstly 25-hydroxylation in the liver, followed by 1 alpha hydroxylation in the kidneys. The synthesis of Vit D3 in the skin depends upon the amount of UV B radiation absorbed, which further depends upon the geographical latitude, season, type of clothing worn, use of sunscreen and the amount of skin pigmentation[33-35].
Several recent studies have found the presence of vitamin D target cells and vitamin D hydroxylase activity (i.e. the ability to convert 25 hydrocholecalciferol to 1, 25 dihydrocholecalciferol) in numerous ocular cells, which suggests that the ocular tissue may have a role in activation and regulation of Vitamin D metabolism[36-39]. In the eye, administration of vitamin D has been found to decrease retinal inflammation and neovascularization, decrease the macrophage number and dose dependent inhibition of capillary endothelial cell morphogenesis[40-41].
Vitamin D has also been shown to inhibits the expression of IgE on B cells and its deficiency is associated with eosinophilia, increased serum IgE levels and sensitization to allergen[42-45]. In a few studies on asthma patients, low vitamin D levels correlated with the elevated serum IgE levels, severity of asthma and the number of eosinophils, and supplementation with Vitamin D was shown to increase the steroid responsiveness in those who were steroid resistant[43-50].
In our study the mean VKC score was 10.33±2.62 (median 10, range: 7-15) and it showed significant correlation with severity of VKC (P=0.001). The VKC score and the serum vitamin D levels did not show a statistically significant correlation (correlation coefficient -0.342,P=0.0646).
In the studied population, children with VKC had a significantly lower serum vitamin D levels as compared to the healthy children, which correlated with time spent outside. Positive correlation was found between the increasing severity of disease and the severity of vitamin D deficiency. Majority of the patients had severe vitamin D deficiency which seems to develop secondary to decreased sunlight exposure.
The possibility of vitamin D deficiency should be kept in mind, and appropriately managed in VKC patients, as it may contribute to the pathophysiology of the disease. However, further studies are warranted to establish vitamin D deficiency as a risk factor in VKC and understand the course of remission of the disease after giving vitamin D supplementation.

