BaO·4B2O3·5H2O納米材料的制備及其對聚丙烯阻燃性能的熱分解動力學方法評價
苗靜,郭睿鳳,劉志宏
陜西師范大學,化學化工學院,陜西省大分子重點實驗室,西安 710062
1 Introduction
Fiber, resin and woodetc. have widely used in industry and lives. But there has been a need to ensure that these products are safe from the danger of fire1. Flame retardant is one of the most important additives for combustible polymer to improve its fire resistance2-5. Borate is considered as a type of highly efficient inorganic halogen-free flame retardant because of its smoke suppression, low toxicity and good thermal stability. For example,2ZnO·3B2O3·3H2O, 4ZnO·B2O3·H2O and 2CaO·3B2O3·5H2O are the widely used fire retardant materials6. However, the sizes for prepared borates were usually in the range of micrometer,which made them disperse in a polymer matrix difficultly,restraining their uses as fire retardant materials. So, the application of borate nanomaterials as flame retardant has been thought as an effective method.
Until now, most investigations about preparation of borate nanomaterials as flame retardant have concentrated on the zinc borates7-13. In recent years, the preparation of alkaline earth borate nanomaterials as flame retardant, such as calcium borates for 2CaO.3B2O3.H2O, 2CaO.B2O3.H2O, and CaO.3B2O3.4H2O14-16,and magnesium borates for 2MgO.B2O3.1.5H2O17, were also reported. However, the preparation of barium borate nanomaterials as flame retardant was not reported, though there was a report for preparation of nanometricβ-barium metaborate18.
In this work, the preparation of a new barium borate BaO·4B2O3·5H2O nanosheet and nanoribbon will be reported.The influences of sizes for loading BaO·4B2O3·5H2O nanomaterials on the flame retardant properties of polypropylene have been investigated by unique thermal decomposition kinetics method.
2 Experimental section
2.1 Chemicals
All chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd., China. All chemicals were of analytic reagent grade and were used directly without further treatment, and the detailed purities are given as follows: H3BO3(≥ 0.995),Ba(CH3COO)2(≥ 0.990), Ba(NO3)2(≥ 0.995),(NH4)2B10O6·8H2O (≥ 0.990), Na2B4O7.10H2O (≥ 0.995), and BaCl2.2H2O (≥ 0.995). Polypropylene (≥ 0.990) was purchased from Aladdin, and its molecular weight was 3700.
2.2 Preparation of BaO·4B2O3·5H2 O nanomaterials with different sizes
BaO·4B2O3·5H2O nanosheet (S1): In a typical synthesis route,Ba(NO3)2(2.615 g) and (NH4)2B10O6·8H2O (5.447 g) with the molar ratio Ba : B = 1 : 10 were mixed with 60 mL of deionized water under vigorous magnetic stirring for 1 h. Subsequently, the teflon-lined stainless-steel autoclave (V= 100 mL) was maintained at 160 °C for 24 h.
BaO·4B2O3·5H2O nanoribbon (S2): A mixture solution of 2.55 g Ba(CH3COO)2, 3.70 g H3BO3and 10 mL deionized water was stirred until homogeneous, which was transferred to a 50 mL stainless steel autoclave and reacted at 180 °C for 12 h.
BaO·4B2O3·5H2O bulk (S3): A mixture solution of 2.44 g BaCl2.2H2O, 0.61 g H3BO3,3.81 g Na2B4O7.10H2O and 60 mL deionized water was stirred until homogeneous, which was placed in a 100 mL stainless steel autoclave and reacted at 120°C for 24 h.
After cooled to room temperature, the above three BaO·4B2O3·5H2O samples were obtained after the white precipitates being centrifuged, washed with distilled water and ethanol two times, respectively, and dried in an oven at 80 °C for 12 h.
2.3 Instruments and methods of characterization
The crystalline structures were analyzed by X-ray diffraction spectrometer (XRD, D8-ADVANCE, Bruker, Germany; with Cu Kα radiation atλ= 0.15418 nm; the scanning range was fixed at 10°-70° at a scanning rate of 0.01 (°)·s?1) and Fourier transform infrared spectroscopy (FTIR, TENSOR 27, Bruker, Germany;KBr pellets). Thermogravimetric analysis (TGA) and differential scanning calorimety (DSC) were performed using a simultaneous thermal analyzer (TGA/DSC 3+, METTLER,Switzerland) under nitrogen atmosphere (50 mL·min?1) at a heating rate of 10 °C·min?1. Morphologies and sizes of samples were analyzed using scanning electron microscope (SEM, SU-8020, Hitachi, Japan) with an acceleration voltage of 5 kV.
2.4 Flame retardant performance evaluation
TGA, DSC, non-isothermal decomposition kinetics methods and the limited oxygen index (LOI) method (determined by an oxygen index type combustion tester, JF-3, Shine Ray, Nanjing,China) were used to study the flame retardant properties. 100 g polypropylene (PP) was selected as the blank sample. 90 g PP and 10 g prepared barium borate samples were alternately mixed in a small amount into the double-drum mixing machine at 165 °C. In order to mix evenly, the distance of the drum was adjusted. The finished mixture sample was cooled and removed after mixing for 30 min, then a size of 100 mm × 10 mm × 4 mm gauge was cut with a guillotine according to GB/T2406.2-2009 standard.
2.5 Mechanical property test
Mechanical test for tensile strength was carried out by using universal material testing machine (RGT-10, Mingkesi, China).Rectangular PP strips with dimension of 100 mm (l) × 10 mm(w) were tested at stretch speed of 5 mm?min?1with a grip separation of 4 mm. This test was carried out with 7-10 times and then reported the mean values.
3 Results and discussion
3.1 Characterization
The XRD patterns of samples S1, S2 and S3 are given in Fig.1, which are identical. The main diffraction data for characteristicdvalues are 0.7122, 0.6276, 0.5330, 0.4703,0.3611, 0.3071, 0.2498, 0.2103 and 0.2097 nm, which correspond to the those of the JCPDS standard card (File No. 97-002-1417) for BaB8O11(OH)419.
The FT-IR spectra of the prepared samples are given in Fig.2, which exhibit the following absorption peaks, and those for sample S3 are assigned20. The broad bands at 3537 cm-1and 3435 cm-1support the presence of hydroxyl groups (O―H) in the structure. The band at 1647 cm-1(bending of H―O―H)shows it contains the crystal water. The bands at 1373 cm?1and 1321 cm?1are assigned to asymmetric stretching vibration of B(3)―O in group BO3 plane triangle. The bands at 1209 cm?1and 1151 cm?1are the in-plane bending vibration of B―O―H.The bands at 1022 cm?1and (873, 817) cm?1are assigned to asymmetric and symmetric stretching of B(4)―O in group BO4tetrahedron. The bands at 733 cm?1and 681 cm?1are out-ofplane bending vibration of B(3)―O.
The simultaneous TG-DSC curves of prepared sample (Fig. 3)indicate that there exist two mass loss stages. The first mass loss is 10.61% from 50 to 250 °C, which corresponds to the loss of 3 crystal water, and can be compared with the calculated value of 10.35%. The second mass loss is 6.51% from 250 to 700 °C,which corresponds to the loss of 2 structural water, and can be compared with the calculated value of 6.89%. In the DSC curve,the endothermic peak at 186.2 °C is related to the dehydration.The endothermic peak at 424.8 °C is related to the loss of 2 structural water.
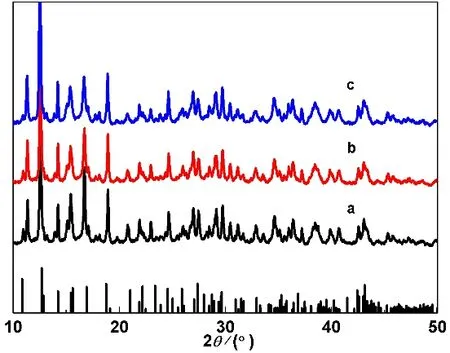
Fig. 1 XRD patterns of prepared samples: (a) S1, (b) S2, (c) S3.
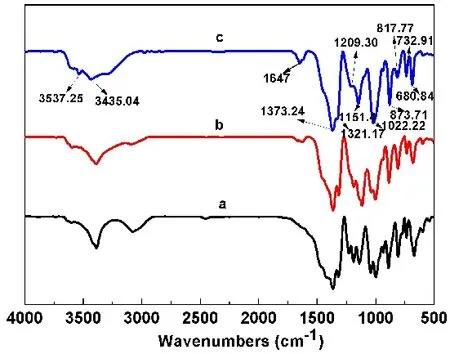
Fig. 2 FT-IR spectra of prepared samples: (a) S1, (b) S2, (c) S3.
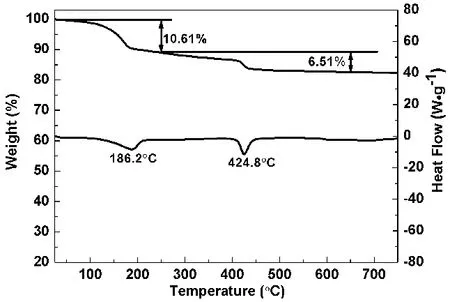
Fig. 3 TG-DSC curves of prepared sample S1 under N2 atmosphere at a heating rate of 10 °C·min-1.
Above characterizations show that the present hydrothermal prepared sample is a new compound, and its structure should be BaB8O11(OH)4·3H2O according to the reported structure of BaB8O11(OH)4 obtained by low temperature flux method19.
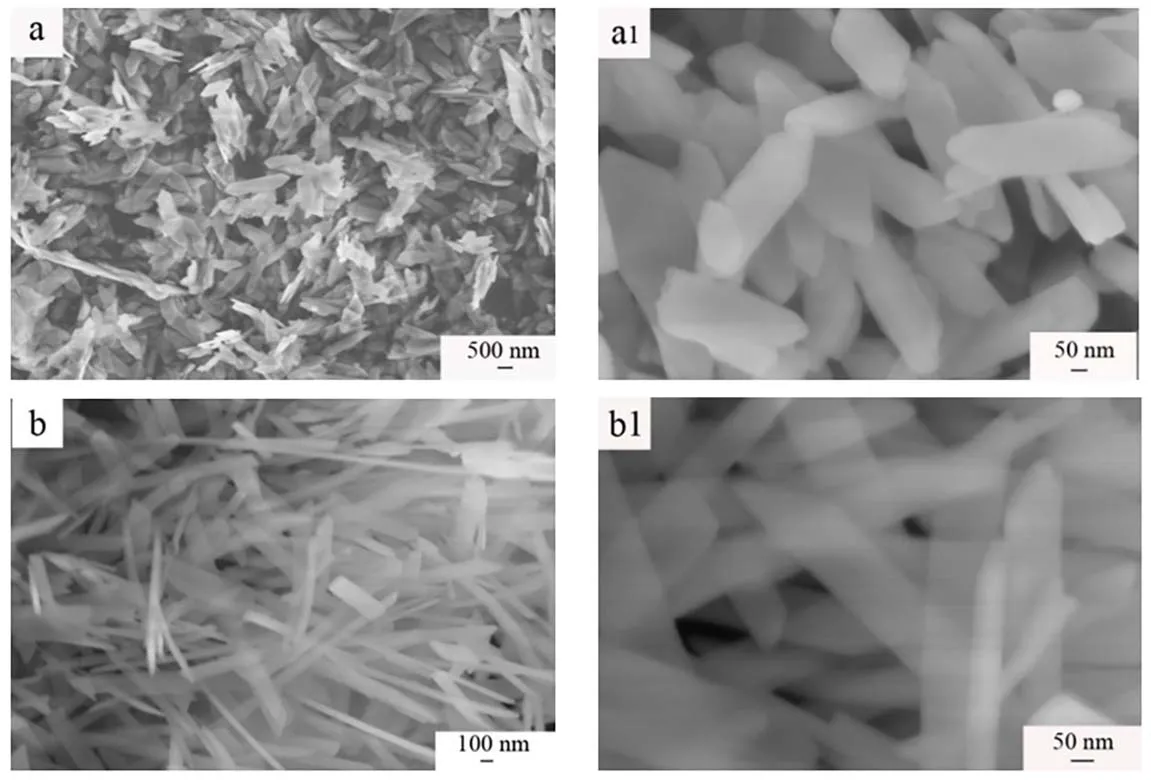
Fig. 4 SEM images of prepared samples: (a, a1) S1, (b, b1) S2.
3.2 Sizes of prepared samples
The typical SEM images (Fig. 4) clearly show that the sample S1 presents a uniform nanosheet with the thickness of 20 nm.Sample S2 presents homogeneous nanoribbon with the thickness of 20 nm and the broad of 100-150 nm. It can be seen that the sample S1 is shorter than sample S2.
3.3 Flame retardant evaluation
3.3.1 TG method
As shown in TG curves (Fig.5), PP and PP/BaO·4B2O3·5H2O(doped with 10% different morphologies) composite materials fast disintegrate from 400 to 500 °C. It can be seen that the pure PP nearly completely disintegrate. The mass losses at 500 °C are gradually decreased, that is 99.46% for PP, 95.87% for PP with 10% prepared sample S3, 91.82% for PP with 10% prepared sample S2, and 91.34% for PP with 10% prepared sample S1. It indicates the improvement of the flame retardant properties from S3 to S1 samples.
3.3.2 DSC method
From the DSC curves (Fig. 6), it can be seen that each curve has two endothermic peaks. The first endothermic peak should be the melting of the PP, and the second peak should be the disintegrating of the PP. The enthalpy changes (ΔrH) for the second peak are 382.4 J?g?1fora, 412.62 J?g?1forb, 431.17 J?g?1forcand 475.93 J?g?1ford, respectively, which indicate the improvement of the flame retardant property from microstructure to nanoribbon and then to nanosheet.
3.3.3 Limited oxygen index (LOI) method
The LOI values for PP and PP/BaO·4B2O3·5H2O composites(doped with 10% prepared samples S3, S2 and S1) are 19.8, 21.9,22.2 and 22.6, respectively. The increasing LOI means the improvement of flame retardant properties. This result consists with that of above thermal analysis test.
3.3.4 Non-isothermal decomposition kinetic method
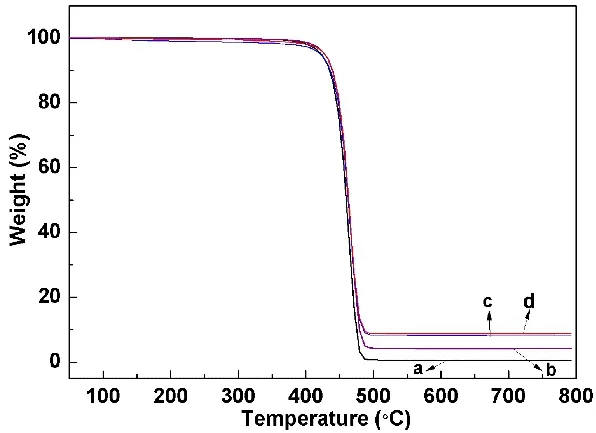
Fig. 5 TG curves of mixture samples under N2 atmosphere at a heating rate of 10 °C?min-1: (a) PP, (b-d) PP with 10% prepared samples S3, S2 and S1, respectively.
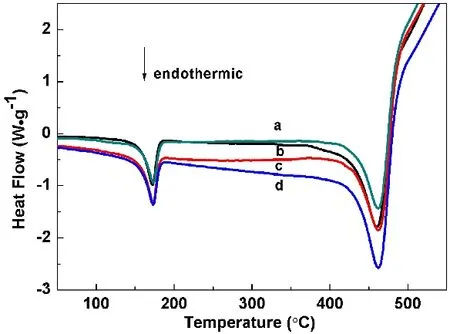
Fig. 6 DSC curves of mixture samples under N2 atmosphere at a heating rate of 10 °C?min-1: (a) PP, (b-d) PP with 10% prepared samples S3, S2 and S1, respectively.
Considering the near TG mass losses and the near LOI values for PP with 10% prepared BaO·4B2O3·5H2O nanosheet (S1) and nanoribbon (S2), their flame retardant properties need to be further evaluated by non-isothermal decomposition kinetic method, as shown in Fig. 7. In order to obtain kinetic parameters in the process of thermal decomposition of PP/BaO·4B2O3·5H2O composite, the following Kissinger’s model was used referring to literatures21,22, in whichTpis the peak temperature (K) in the DTG curve,βis the linear heating rate (K·min?1),Ris the gas constant (J·mol?1·K?1),Eais the apparent activation energy(J·mol?1), andAis the pre-exponential factor (s?1).
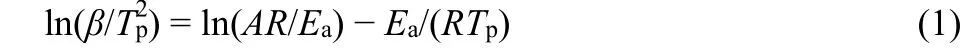
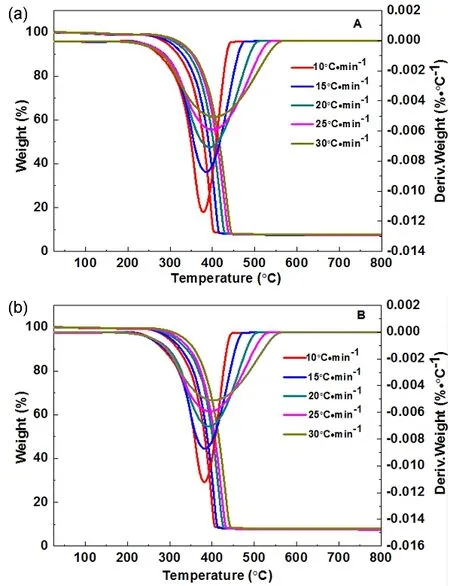
Fig. 7 TG and DTG curves of the PP/BaO·4B2O3·5H2O composites under air atmosphere at different heating rates: (A)nanosheet (S1), (B) nanoribbon (S2).
By plotting ln(β/Tp2) against 1/Tp, the values ofEa andAwere obtained from the slope ?Ea/Rand intercept ln(AR/Ea) of Eq. (1),which are given in Table 1.
It can be seen that the PP with 10% prepared BaO·4B2O3·5H2O nanosheet (S1) is of the biggerEaof 149.43 kJ·mol?1, which means the better flame retardant property.
To sum up, the flame retardant performances of prepared BaO·4B2O3·5H2O samples to PP increase gradually from bulk to nanoribbon and then to nanosheet, with the reducing of TG mass loss, the increasing of heat absorption in DSC under N2atmosphere, the increasing of apparent activation energyEa, as well as the increasing of LOI values, as shown in Table 2. This trend may be ascribed to their sizes being decreased accordingly,because the smaller size of sample can benefit its good dispersion in polymer and increase its contact area with polymer.
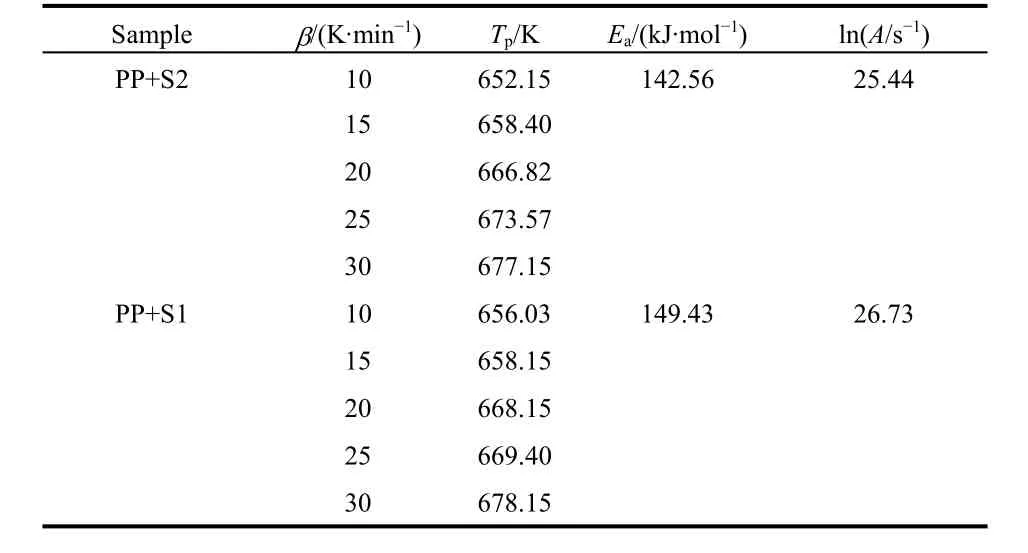
Table 1 Thermal decomposition kinetic parameters of PP with 10%prepared BaO·4B2O3·5H2O samples S1 and S2.

Table 2 All the test data of the flame retardant of different materials.
3.4 Mechanism of flame retardancy of BaO·4B2O3·5H2O nanosheet
Like other borate nanomaterials16, the better flame retardancy of BaO·4B2O3·5H2O nanosheet comes from the dehydration at higher temperature, the formed glassy borate as a layer, the formed water vapor diluting the surface oxygen concentration,the better dispersion in the PP, and the quick thermal decomposing rate.
Because the intumescent char layer could slow the heat and mass transfer between gas and condensed phases, and could also protect the underlying materials from further burning23,24, the formation of intumescent char layer should be the most important factor to achieve the much better flame retardancy of composite25. So, we have analyzed the after-flame chars of PP/BaO·4B2O3·5H2O composite by SEM images (Fig. 8).
It can be seen from Fig. 8 that the char layer is more compact and continuous for PP/BaO·4B2O3·5H2O nanosheet composite,compact but existing a few pores for PP/BaO·4B2O3·5H2O nanoribbon composite, and discontinuous for PP/BaO·4B2O3·5H2O microstructure composite. So the PP/BaO·4B2O3·5H2O nanosheet composite is of the best flame retardant behavior.
3.5 Assessment of mechanical properties

Fig. 8 SEM images of after-flame chars: (a) PP/S3, (b) PP/S2, (c) PP/S1.
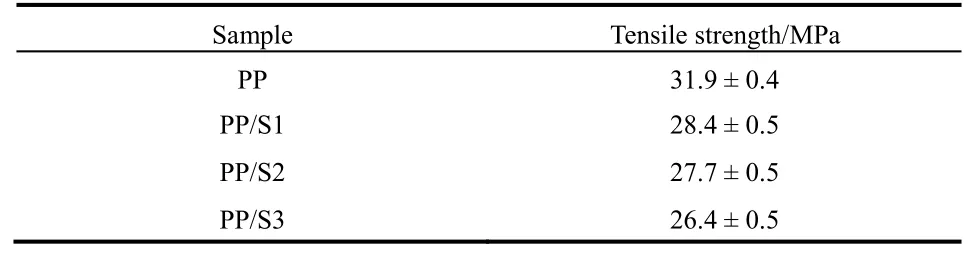
Table 3 Tensile strength of PP and PP with 10% prepared BaO·4B2O3·5H2O.
In order to inspect the influence of loading prepared inorganic flame retardant on the mechanical property of PP, we have assessed the tensile strength of materials. It can be seen from Table 3 that the tensile strength of PP was declined after loading the 10% prepared BaO·4B2O3·5H2O samples, which was resulted from the reducing of the compatibility after addition of the inorganic component to the organic system. It is also found that the tensile strength improve gradually from BaO·4B2O3·5H2O microstructure to nanoribbon and then to nanosheet. Moreover, the tensile strength of PP did not cause a great change because of the use of BaO·4B2O3·5H2O nanosheet though it decreased. So its application prospect as the flame retardant has not been affected greatly.4 Conclusions
In this work, the barium borate BaO·4B2O3·5H2O nanosheet and nanoribbon were prepared by hydrothermal method. The influence of loading BaO·4B2O3·5H2O nanomaterials on the flame retardancy of PP was investigated by using unique nonisothermal decomposition kinetic method. The results show that the loading prepared BaO·4B2O3·5H2O nanosheet sample has the best flame retardant performance, and also does not cause a great change in tensile strength of PP. So, BaO·4B2O3·5H2O nanosheet sample could be developed as a promising flameretardant material.

