Hedgehog通路在缺氧誘導(dǎo)腦膠質(zhì)瘤轉(zhuǎn)移中的作用及分子機(jī)制研究
傅之梅 胡兵偉 王錢東 馬婷婷


[摘要] 目的 探討Hedgehog通路在缺氧誘導(dǎo)腦膠質(zhì)瘤轉(zhuǎn)移中的作用及分子機(jī)制。 方法 選擇2018年1月~2019年8月浙江省立同德醫(yī)院腦膠質(zhì)瘤患者5例,取其腦膠質(zhì)瘤細(xì)胞,隨機(jī)分為對(duì)照組、抑制劑處理組、乏氧組和乏氧加抑制劑處理組。各組細(xì)胞均完成48 h培養(yǎng),采用Westernblot檢測(cè)各組細(xì)胞Smoothened(SMO)、神經(jīng)膠質(zhì)瘤相關(guān)癌基因同源蛋白1(GLI1)和上皮間質(zhì)轉(zhuǎn)化(EMT)E-cadherin基因表達(dá),采用BrdU-ELISA法檢測(cè)細(xì)胞增殖,采用Transwell法檢測(cè)細(xì)胞侵襲作用。 結(jié)果 Westernblot檢測(cè)結(jié)果顯示,乏氧加抑制劑處理組SMO、GLI1及EMT相關(guān)蛋白表達(dá)水平低于乏氧組、抑制劑處理組與對(duì)照組(P<0.05);乏氧組SMO、GLI1及EMT相關(guān)蛋白表達(dá)水平高于抑制劑處理組與對(duì)照組(P<0.05)。BrdU-ELISA法結(jié)果顯示,乏氧加抑制劑處理組不同時(shí)間點(diǎn)細(xì)胞增殖率低于乏氧組,高于抑制劑處理組與對(duì)照組(P<0.05)。Transwell法檢測(cè)結(jié)果顯示,乏氧加抑制劑處理組細(xì)胞侵襲率低于乏氧組,但明顯高于抑制劑處理組與對(duì)照組(P<0.05);乏氧組細(xì)胞侵襲率高于抑制劑處理組與對(duì)照組(P<0.05)。 結(jié)論 Hedgehog通路可能通過SMO、GLI1調(diào)控EMT,參與疾病的發(fā)生、發(fā)展,在缺氧誘導(dǎo)腦膠質(zhì)瘤轉(zhuǎn)移中發(fā)揮重要的作用,能為腦膠質(zhì)瘤轉(zhuǎn)移治療提供新的靶點(diǎn)。
[關(guān)鍵詞] Hedgehog通路;Smoothened;神經(jīng)膠質(zhì)瘤相關(guān)癌基因同源蛋白1;上皮間質(zhì)轉(zhuǎn)化;缺氧誘導(dǎo);腦膠質(zhì)瘤轉(zhuǎn)移
[中圖分類號(hào)] R739.41? ? ? ? ? [文獻(xiàn)標(biāo)識(shí)碼] A? ? ? ? ? [文章編號(hào)] 1673-9701(2020)32-0020-04
[Abstract] Objective To explore the role and molecular mechanism of Hedgehog pathway in hypoxia-induced glioma metastasis. Methods From January 2018 to August 2019, a total of 5 patients with glioma from Tongde Hospital of Zhejiang Province were selected as subjects. Glioma cells were collected and randomly divided into a control group, an inhibitor-treated group, a hypoxia group, and a hypoxia combined with inhibitor-treated group. The cells in each group were given 48 hours of culture. The expression of Smoothened(SMO), glioma-associated oncogene homologous protein 1(GLI1) and epithelial mesenchymal transformation(EMT) E-cadherin genes were detected by Westernblot, cell proliferation was detected by BrdU-ELISA method, and cell invasion was detected by Transwell method. Results Westernblot test results showed that the expression levels of SMO, GLI1 and EMT-related proteins in the hypoxia combined with inhibitor-treated group were lower than those in the hypoxia group, inhibitor-treated group and control group(P<0.05); the expression levels of SMO, GLI1 and EMT-related proteins in the hypoxia group were higher than those in the inhibitor-treated group and the control group(P<0.05); the results of BrdU-ELISA showed that the cell proliferation rate in the hypoxia combined with inhibitor-treated group at different time points was lower than that in the hypoxia group, but was higher than that in the inhibitor-treated group and control group(P<0.05); Transwell test results showed that the cell invasion rate in the hypoxia combined with inhibitor-treated group was lower than that in the hypoxia group, but was significantly higher than that in the inhibitor-treated group and control group(P<0.05); the cell invasion rate in the hypoxia group was higher than that in the inhibitor-treated group and control group(P<0.05). Conclusion Hedgehog pathway may participate in the occurrence and development of EMT through SMO and GLI1 regulations, which play important roles in hypoxia-induced glioma metastasis and can provide a new target for the treatment of glioma metastasis.
[Key words] Hedgehog pathway; Smoothened; Glioma-associated oncogene homologous protein 1; Epithelial mesenchymal transformation; Hypoxia-induced; Glioma metastasis
膠質(zhì)瘤是臨床常見的惡性腫瘤,在中樞神經(jīng)系統(tǒng)腫瘤中較為常見,占顱腦腫瘤的35.26%~60.96%,其發(fā)病率占腦腫瘤首位,死亡率居第二位[1]。由于膠質(zhì)瘤具有高浸潤(rùn)生長(zhǎng)的生物學(xué)特性,導(dǎo)致手術(shù)無法保證腫瘤的完全切除,術(shù)后復(fù)發(fā)率近90.0%,且隨著手術(shù)次數(shù)、復(fù)發(fā)次數(shù)的增加,惡性程度具有增加趨勢(shì)。缺氧是腫瘤微環(huán)境的基本特征之一,能促進(jìn)腫瘤侵襲和轉(zhuǎn)移作用[2-3]。臨床研究顯示,介導(dǎo)缺氧應(yīng)答的主要轉(zhuǎn)錄因子-缺氧誘導(dǎo)因子1α(HIF-α)在腦膠質(zhì)瘤等多種腫瘤中過表達(dá),且與腦膠質(zhì)瘤的轉(zhuǎn)移、預(yù)后有關(guān)[4-5]。上皮間質(zhì)轉(zhuǎn)化(EMT)是將具有極性的上皮細(xì)胞轉(zhuǎn)換成具有活性能力、能在細(xì)胞基質(zhì)間自由移動(dòng)的間質(zhì)細(xì)胞的過程[6]。Suh[7]的研究表明,Hedgehog通路中的鋅指轉(zhuǎn)錄因子GLI1及上游轉(zhuǎn)膜蛋白SMO亦是上述缺氧/JDAC3調(diào)節(jié)的靶基因,哺乳動(dòng)物Hedgehog通路由三種HH配體激發(fā),能與受體轉(zhuǎn)膜蛋白Patched1(PTCH1)結(jié)合,從而阻斷PTCH1對(duì)轉(zhuǎn)膜蛋白SMO的抑制功能,釋放SMO活性,實(shí)現(xiàn)下游靶基因的調(diào)控作用[8-9]。因此,本研究以腦膠質(zhì)瘤細(xì)胞為研究對(duì)象,探討Hedgehog通路在缺氧誘導(dǎo)腦膠質(zhì)瘤轉(zhuǎn)移中的作用及分子機(jī)制,現(xiàn)報(bào)道如下。
1 資料與方法
1.1 一般資料
選擇2018年1月~2019年8月浙江省立同德醫(yī)院腦膠質(zhì)瘤患者5例。取其腦膠質(zhì)瘤細(xì)胞,隨機(jī)均分為對(duì)照組、抑制劑處理組、乏氧組和乏氧加抑制劑處理組。本研究經(jīng)醫(yī)院醫(yī)學(xué)倫理委員會(huì)批準(zhǔn),所有患者知情同意。
1.2 方法
1.2.1 材料與設(shè)備? 胎牛血清RMPI-1640培養(yǎng)基(美國(guó)HyClone公司)、小牛血清(杭州四季青生物工程材料有限公司)、Transwell小室(美國(guó)Becton Dickinson)、人工基質(zhì)膠Mateigel(美國(guó)Becton Dickinson)、青/鏈霉素,RIPA裂解液、BCA法蛋白濃度測(cè)定試劑盒(碧云天公司)。
1.2.2 細(xì)胞處理[10-11]? 取腦膠質(zhì)瘤細(xì)胞,將細(xì)胞接種在濃度為10.0%的胎牛血清RMPI-1640培養(yǎng)基中,放置在37℃、濃度為5%的CO2細(xì)胞培養(yǎng)箱中培養(yǎng),待細(xì)胞融合80.0%以上時(shí),開始傳代培養(yǎng)。培養(yǎng)前去除原培養(yǎng)液,并利用PBS緩沖液連續(xù)進(jìn)行2次漂洗。向獲得的溶液中加入濃度為0.25%的胰蛋白酶消化液進(jìn)行消化,倒置顯微鏡下觀察細(xì)胞形態(tài),待細(xì)胞回縮、變圓后,將消化液吸出,并加入完全培養(yǎng)液終止消化。利用吸管反復(fù)、輕柔吹打培養(yǎng)瓶底,使細(xì)胞充分脫離瓶壁。取細(xì)胞懸液15 mL放置在離心管中,連續(xù)進(jìn)行8 min離心,離心速度1000 rpm,去除上清液;向獲得的細(xì)胞懸液中加入完全培養(yǎng)基重懸細(xì)胞,以1∶2進(jìn)行傳代培養(yǎng),每3天換液一次,待細(xì)胞融合90.0%時(shí)再次傳代,取第三代對(duì)數(shù)生長(zhǎng)的細(xì)胞,將其隨機(jī)分裝在不同的試管中進(jìn)行分組。對(duì)照組不采取任何措施處理及干預(yù),向細(xì)胞中加入培養(yǎng)基進(jìn)行常規(guī)培養(yǎng),連續(xù)完成48 h培養(yǎng);抑制劑處理組在常規(guī)培養(yǎng)24 h后加入SANT1、GANT61處理48 h;乏氧組在收細(xì)胞前24 h由常規(guī)培養(yǎng)改為低氧(1%O2)培養(yǎng),連續(xù)完成48 h培養(yǎng);乏氧加抑制劑處理組在低氧培養(yǎng)24 h后加抑制劑處理48 h。各組細(xì)胞處理后放置在培養(yǎng)箱中進(jìn)行培養(yǎng),培養(yǎng)箱為37℃、5%CO2、飽和濕度[12]。
1.2.3 檢測(cè)方法? (1)典型基因標(biāo)志表達(dá):采用Western blot檢測(cè)各組細(xì)胞Smoothened(SMO)、神經(jīng)膠質(zhì)瘤相關(guān)癌基因同源蛋白1(GLI1)和上皮間質(zhì)轉(zhuǎn)化(EMT)標(biāo)志蛋白E-cadherin表達(dá)[13]。具體步驟包括:①蛋白質(zhì)的提取;②蛋白質(zhì)SDS-PAGE凝膠電泳;③蛋白質(zhì)轉(zhuǎn)印;④封閉;⑤一抗、二抗孵育;⑥顯影。將最終處理后的PVDF膜,以β-actin作為內(nèi)參物,根據(jù)正確的位置、方法放置在發(fā)光板(蛋白面板)上,并加入少許發(fā)光液,選擇合適的曝光條件,完成顯影并保存。(2)細(xì)胞增殖:采用BrdU-ELISA法檢測(cè)細(xì)胞增殖。取各組干預(yù)后12、24、36及48 h的細(xì)胞,以20 000/孔培養(yǎng)在96孔細(xì)胞培養(yǎng)板中,以梯度濃度CWE完成72 h處理,根據(jù)BrdU試劑盒說明,完成細(xì)胞的固定、沖洗,加入相應(yīng)的抗體、洗滌后顯色,在微孔板分光光度計(jì)上以450 nm波長(zhǎng)度數(shù)進(jìn)行測(cè)定[14]。(3)細(xì)胞侵襲能力:采用Transwell法檢測(cè)細(xì)胞侵襲能力。取各組干預(yù)后的細(xì)胞,以1×105個(gè)/孔的密度接種在24孔板Transwell中,每孔中設(shè)置復(fù)孔5個(gè),并且在上室加入200 μL DMEM∶F12培養(yǎng)基、下室加入600 μL DMEM∶F12培養(yǎng)基,并在37℃細(xì)胞培養(yǎng)箱中連續(xù)完成48 h培養(yǎng),吸取下室中培養(yǎng)液,加入結(jié)晶紫染色液100 μL/孔,連續(xù)完成10 min染色,染色完畢后采用PBS進(jìn)行2次洗滌,400倍顯微鏡下連續(xù)統(tǒng)計(jì)5個(gè)視野的細(xì)胞并取平均值[15]。
1.3觀察指標(biāo)
①典型基因表達(dá):記錄乏氧加抑制劑處理組、乏氧組、抑制劑處理組、對(duì)照組各典型基因的表達(dá)水平。②增殖率:記錄各組干預(yù)后12、24、36及48 h的細(xì)胞增殖率。③細(xì)胞侵襲率:記錄各組細(xì)胞干預(yù)后12 h的細(xì)胞侵襲率。
1.4 統(tǒng)計(jì)學(xué)分析
數(shù)據(jù)應(yīng)用SPSS22.0統(tǒng)計(jì)學(xué)軟件進(jìn)行分析,計(jì)量資料用(x±s)表示,采用t檢驗(yàn);計(jì)數(shù)資料用[n(%)]表示,采用χ2檢驗(yàn),P<0.05為差異有統(tǒng)計(jì)學(xué)意義。
2 結(jié)果
2.1 各組典型基因標(biāo)志表達(dá)比較
Westernblot檢測(cè)結(jié)果顯示,乏氧加抑制劑處理組SMO、GLI1及EMT相關(guān)蛋白E-cadherin表達(dá)水平分別為(0.32±0.05)、(0.41±0.08)、(0.35±0.06),低于乏氧組的(0.78±0.12)、(0.80±0.14)、(0.79±0.13),低于抑制劑處理組的(0.57±0.11)、(0.62±0.14)、(0.59±0.12),低于對(duì)照組的(0.60±0.13)、(0.69±0.16)、(0.67±0.15),差異有統(tǒng)計(jì)學(xué)意義(P<0.05);乏氧組SMO、GLI1及EMT相關(guān)蛋白E-cadherin表達(dá)水平高于抑制劑處理組與對(duì)照組,差異有統(tǒng)計(jì)學(xué)意義(P<0.05)。見表1、圖1。
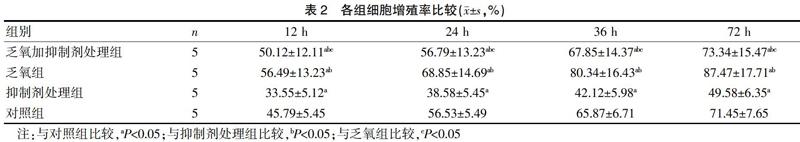
2.2 各組細(xì)胞增殖率比較
BrdU-ELISA法結(jié)果顯示,乏氧加抑制劑處理組不同時(shí)間點(diǎn)細(xì)胞增殖率低于乏氧組,高于抑制劑處理組與對(duì)照組,差異有統(tǒng)計(jì)學(xué)意義(P<0.05);乏氧組不同時(shí)間點(diǎn)細(xì)胞增殖率高于抑制劑處理組與對(duì)照組,差異有統(tǒng)計(jì)學(xué)意義(P<0.05)。見表2。
2.3各組細(xì)胞侵襲率比較
Transwell法檢測(cè)結(jié)果顯示,乏氧加抑制劑處理組細(xì)胞侵襲率為(58.56±5.67)%,低于乏氧組的(78.57±6.83)%,但明顯高于抑制劑處理組的(27.58±5.31)%與對(duì)照組的(37.59±7.21)%,差異有統(tǒng)計(jì)學(xué)意義(P<0.05);乏氧組細(xì)胞侵襲指數(shù)高于抑制劑處理組與對(duì)照組,差異有統(tǒng)計(jì)學(xué)意義(P<0.05)。見表3。
3 討論
腦膠質(zhì)瘤是由大腦和脊髓膠質(zhì)細(xì)胞癌變產(chǎn)生的、最常見的原發(fā)性顱腦惡性腫瘤,其發(fā)病率占顱內(nèi)腫瘤的35.2%~61.0%,主要由膠質(zhì)細(xì)胞演化而來,具有發(fā)病率高、復(fù)發(fā)率高及死亡率高等特點(diǎn),影響患者的健康和生活。缺氧是腦膠質(zhì)瘤轉(zhuǎn)移的基本特征,持續(xù)的缺氧能促進(jìn)腫瘤侵襲與轉(zhuǎn)移[16]。而介導(dǎo)缺氧應(yīng)答的主要轉(zhuǎn)錄因子在腦膠質(zhì)瘤中呈高表達(dá),其表達(dá)水平與腦膠質(zhì)瘤的轉(zhuǎn)移、預(yù)后存在相關(guān)性[17]。目前,臨床對(duì)于腦膠質(zhì)瘤的病因尚未明確,可能與腫瘤本身存在緊密聯(lián)系,其中主要包括病毒感染、化學(xué)、電磁輻射及環(huán)境等因素。臨床研究顯示,HIF-1α/HIF-1β轉(zhuǎn)錄復(fù)合體的穩(wěn)定與活化激活下游靶基因,調(diào)控腦膠質(zhì)瘤細(xì)胞的增殖、血管生成及上皮間質(zhì)轉(zhuǎn)化(EMT),能促進(jìn)腫瘤的轉(zhuǎn)移[18]。本研究中Westernblot檢測(cè)結(jié)果顯示,乏氧加抑制劑處理組SMO、GLI1及EMT相關(guān)蛋白E-cadherin表達(dá)水平低于乏氧組、抑制劑處理組與對(duì)照組(P<0.05),乏氧組SMO、GLI1及EMT相關(guān)蛋白E-cadherin表達(dá)水平高于抑制劑處理組與對(duì)照組(P<0.05),說明Hedgehog通路在腦膠質(zhì)瘤轉(zhuǎn)移中呈高表達(dá),且乏氧狀態(tài)下腫瘤轉(zhuǎn)移率最高,能加劇腫瘤的轉(zhuǎn)移。Hedgehog通路最早在研究果蠅基因突變時(shí)被發(fā)現(xiàn),在脊椎動(dòng)物中其信號(hào)通路成員較多,如膜受體Ptch、配體Hh、Smo及下游轉(zhuǎn)錄因子Gli。當(dāng)配體處于功能狀態(tài)時(shí),Shh、Ptch結(jié)合接觸,能抑制Smo受體,從而引起下游轉(zhuǎn)錄因子激活,能促進(jìn)細(xì)胞的增殖激活。而當(dāng)配體處于無功能狀態(tài)時(shí),Ptch對(duì)受體能發(fā)揮良好的抑制作用,引起Gli發(fā)生水解、失效。因此,激活Hh對(duì)配體的發(fā)育具有重要的作用。本研究中BrdU-ELISA法的結(jié)果顯示,乏氧加抑制劑處理組不同時(shí)間點(diǎn)細(xì)胞增殖率低于乏氧組,高于抑制劑處理組與對(duì)照組(P<0.05),說明乏氧能促進(jìn)腦膠質(zhì)瘤細(xì)胞的增殖,而給予細(xì)胞抑制劑,則能在一定程度上抑制細(xì)胞的增殖。楊寧等[19]的研究結(jié)果顯示,N-Shh能刺激腦膠質(zhì)瘤細(xì)胞的侵襲、轉(zhuǎn)移,而Hh信號(hào)通路特異性阻斷劑的干預(yù)可降低細(xì)胞的侵襲和轉(zhuǎn)移。本研究中Transwell法檢測(cè)結(jié)果顯示,乏氧加抑制劑處理組細(xì)胞侵襲率低于乏氧組,但明顯高于抑制劑處理組與對(duì)照組(P<0.05),乏氧組細(xì)胞侵襲率高于抑制劑處理組與對(duì)照組(P<0.05),說明乏氧加抑制劑處理能降低腦膠質(zhì)細(xì)胞的侵襲和轉(zhuǎn)移,而乏氧則能促進(jìn)腦膠質(zhì)細(xì)胞的侵襲。姚軍利等[20]的研究顯示,阻斷Hh信號(hào)通路能抑制腦膠質(zhì)瘤細(xì)胞的侵襲,可能與Glil抑制血管內(nèi)皮生長(zhǎng)因子的表達(dá)有關(guān),從而抑制腫瘤的侵襲和轉(zhuǎn)移。
綜上所述,Hedgehog通路可能通過SMO、GLI1調(diào)控EMT,參與疾病的發(fā)生、發(fā)展,在缺氧誘導(dǎo)腦膠質(zhì)瘤轉(zhuǎn)移中發(fā)揮重要的作用,能為腦膠質(zhì)瘤轉(zhuǎn)移治療提供新的靶點(diǎn)。
[參考文獻(xiàn)]
[1] 朱宏明.Notch信號(hào)通路介導(dǎo)EMT與腫瘤侵襲轉(zhuǎn)移的研究進(jìn)展[J].腫瘤學(xué)雜志,2018,24(8):808-812.
[2] Xue TQ,Jiang SZ,Li MX,et al.The unwanted cell migration in the brain:Glioma metastasis[J].Neurochemical Research,2017,42(4):1-17.
[3] 常亞男,陳紅,段潔,等.Hedgehog信號(hào)通路拮抗劑GANT61對(duì)子宮頸癌裸鼠移植瘤生長(zhǎng)的作用及其機(jī)制[J].中華婦產(chǎn)科雜志,2018,53(5):331-334.
[4] 李俊東,郝長(zhǎng)來.Hedgehoge信號(hào)通路在多發(fā)性骨髓瘤中的作用[J].國(guó)際輸血及血液學(xué)雜志,2017,40(2):174-177.
[5] Livia G,Noriyuki K,A.Sorana M,et al.A hematogenous route for medulloblastoma leptomeningeal metastases[J].Cell,2018,172(5):1050-1062.
[6] 曹曼卿,高君蓉,黃佳飛,等.缺氧誘導(dǎo)因子2α對(duì)含CUB結(jié)構(gòu)域蛋白1的調(diào)控及在肝癌轉(zhuǎn)移中的作用[J].中華腫瘤雜志,2017,39(1):18-23.
[7] Suh CH,Kim HS,Jung SC,et al.Diffusion-weighted imaging and diffusion tensor imaging for differentiating high-grade glioma from solitary brain metastasis:A systematic review and meta-analysis[J].American Journal of Neuroradiology,2018,39(7):1208.
[8] 朱江,任梅,許治國(guó).miR-375對(duì)缺氧誘導(dǎo)的人視網(wǎng)膜微血管內(nèi)皮細(xì)胞功能的抑制作用及其機(jī)制[J].中華實(shí)驗(yàn)眼科雜志,2017,35(8):695-702.
[9] Xiang Z Kebin Z,Chunhui L,et al.Nobiletin inhibits invasion via inhibiting AKT/GSK3β/β-catenin signaling pathway in slug-expressing glioma cells[J].Oncology Reports,2017,37(5):2847.
[10] 閆義濤,王曉麗,谷圓圓,等.藏紅花素通過HIF-1α/VEGF通路對(duì)缺氧誘導(dǎo)的視網(wǎng)膜色素上皮細(xì)胞血管新生的抑制作用[J].中國(guó)免疫學(xué)雜志,2019,(16):1957-1961.
[11] Hiromasa K,Naito K,Tsuyoshi S,et al.Oral squamous cell carcinoma-derived sonic hedgehog promotes angiogenesis[J].Anticancer Research,2017,37(12):6731-6737.
[12] 毛漢丁,程崗,張劍寧.缺氧誘導(dǎo)因子-1α與核轉(zhuǎn)錄因子-κB在創(chuàng)傷性腦損傷中的作用及其串聯(lián)機(jī)制綜述[J].解放軍醫(yī)學(xué)院學(xué)報(bào),2019,(4):391-394.
[13] Saud AA,Goutam M,Ram IM.Coadministration of polymeric conjugates of docetaxel and cyclopamine synergistically inhibits orthotopic pancreatic cancer growth and metastasis[J].Pharmaceutical Research,2018,35(1):17.
[14] 霍小東,張洪典,馬釗,等.缺氧誘導(dǎo)因子-1α在食管鱗癌上皮-間質(zhì)轉(zhuǎn)化中的作用及機(jī)制[J].中華消化外科雜志,2017,16(1):83-89.
[15] 蘇雅珍,張莉蕓,馬丹,等.Hedgehog信號(hào)通路在風(fēng)濕性疾病中的研究進(jìn)展[J].中華風(fēng)濕病學(xué)雜志,2018,22(9):636-638.
[16] Renhui Y,Jiugeng F,Shaochun Y,et al.miR-484/MAP2/c-Myc-positive regulatory loop in glioma promotes tumor-initiating properties through ERK1/2 signaling[J].Journal of Molecular Histology,2018,49(2):1-10.
[17] 馬義麗,王樂,李明霞.缺氧誘導(dǎo)因子1α及血管內(nèi)皮生長(zhǎng)因子在新生大鼠缺氧性肺動(dòng)脈高壓發(fā)病機(jī)制中的作用[J].中國(guó)新生兒科雜志,2017,32(1):64-68.
[18] Shan Y,Kong W,Zhu A,et al.Long noncoding RNA CCAL promotes gastric cancer cell proliferation and migration in a myc dependent way[J].Pharmazie,2018,73(1):42-48.
[19] 楊寧,路強(qiáng),袁夢(mèng)克,等.黃芩素對(duì)缺氧誘導(dǎo)的視網(wǎng)膜神經(jīng)膠質(zhì)細(xì)胞中脯氨酸羥化酶2表達(dá)的抑制作用[J].中華實(shí)驗(yàn)眼科雜志,2017,35(11):990-996.
[20] 姚軍利,劉蘇,呂游,等.脊髓神經(jīng)元sonichedgehog信號(hào)通路在小鼠嗎啡耐受中的作用[J].中華麻醉學(xué)雜志,2017,37(2):175-179.
(收稿日期:2019-12-09)

