Lesson Ninety- two Arrhythmia- induced cardiomyopathy
Tachycardia-induced Cardiomyopathy
Tachycardia-induced cardiomyopathy(T-CM)refers to the presence of a reversible LV dysfunction solely due to increase in ventricular rates, regardless of tachycardia origin. The risk of developing T-CM depends not only on the type, but also the duration and rate of tachycardia. A study reported T-CM in 2.7%patients referred for radiofrequency ablation(RFA);however,it also included patients referred for premature ventricular contractions ablation. T-CM has been reported in 10% of patients with atrial tachycardia(AT), and as high as 37% of patients with incessant AT. Moreover, permanent junctional reciprocating tachycardia appears to have the highest association with T-CM(20% to 50%)as it frequently presents as an incessant supraventricular tachycardia.
Pathophysiology and Mechanism
Animal models have been key to understand the pathophysiology and mechanism of T-CM. Similar to humans,animals exposed to persistent tachycardia using a continuous rapid atrial or ventricular pacing develop heart failure (HF)symptoms, left ventricular(LV)systolic dysfunction and dilatation, decrease in LV dP/dtmax and myocardial blood flow,and increase in LV wall stress and end-diastolic pressure and volume.Dilatation tends to be biventricular with mild thinning or no associated hypertrophy or change in heart mass. The progression of these physiological changes include a decrease in systemic blood pressure and increase in LV and pulmonary artery pressure, which plateaus at 1 week, while cardiac output, ejection fraction, and volumes continue to deteriorate the following 4 weeks with development of symptomatic HF within 2 to 3 weeks.
T-CM is characterized by structural and functional myocardial changes. Similar to human studies, T-CM models have also demonstrated electrical remodeling and abnormal Ca homeostasis thought to be responsible for impaired excitation-contraction coupling and diastolic dysfunction. Only total Ca cycling, Ca channel inhibition,and basal ATPase activity have demonstrated a statistical correlation with decrease in left ventricular ejection fraction(LVEF).
Clinical Presentation, Diagnosisi, and Imaging Features
Clinical studies have found a variable time from onset of arrhythmia symptoms to development of T-CM,ranging from 3 to 120 days with an overall LVEF of 32%. Regardless of tachyarrhythmia, HF symptoms will manifest earlier at higher tachycardia rates, such as patients with persistent atrial flutter or tachycardia with 2:1 AV conduction with rates >150 beats/min.A recent clinical study found a more severe LV dysfunction(LVEF 29.3±6.6%)in T-CM when compared with dilated and inflammatory CM (32.1±10.2% and 41.9±12.9%,respectively;P<0.001)
Major reported symptoms include palpitations(29%), HF class Ⅲto Ⅳ(47%), and syncope/presyncope(12%), while the remaining may have no symptoms. Sudden cardiac death is uncommon but has been reported in up to 8%to 12%despite treatment and resolution of cardiomyopathy.
Echocardiogram or cardiac magnetic resonance can assist in excluding other etiologies. T-CM is characterized by a dilated CM (increased LV end-diastolic dimension and area)with moderate to severe biventricular systolic dysfunction and normal LV septal and posterior wall thickness (lack of hypertrophy). Mitral insufficiency may be present due to LV and mitral annular dilatation with lack of leaflet coaptation.
Treatment
A major feature of T-CM is its reversibility once tachycardia is eliminated. Thus, the mainstay treatment consists of suppression of tachycardia based on the culprit arrhythmia1with antiarrhythmic drugs(AADs)and/or RFA.
Elimination of tachyarrhythmia not only resolves LV function within 4 to 12 weeks, but also improves heart failure symptoms by at least 1 New York Heart Association functional class in most patients.Unfortunately, the recovery of T-CM is not always complete. Histopathological abnormalities, diastolic dysfunction, and ventricular dilatation with a hypertrophic response may persist despite normalization of LVEF.
Prnmature Ventricular Contraction-Cardiomyopathy
Premature ventricular contractions-cardiomyopathy(PVC-CM)is defined as the development of LV dysfunction caused solely by frequent PVCs. A PVC burden≥10% is often considered high and significant enough to trigger PVC-CM.The prevalence of PVC-CM has been reported at 7% among patients with frequent PVC burden >10%. Clinical studies have reported a diagnosis of PVC-CM in 9%to 30%of patients referred for RFA of PVC.
Potential Mechanism(s)of PVC-CM
The primary cause of contractile dysfunction in PVC-CM appears to be disorders of the calcium-induced calcium release mechanism itself,with alterations of dyad(L-type Ca channel and Ryanodine receptor)function proposed as a potential mechanism.Similar to other cardiomyopathies, this PVC-CM model has revealed electrophysiological remodeling.Histopathological abnormalities are distinct without evidence of increased inflammation or apoptosis and minimal or no fibrosis. Mitochondrial studies have demonstrated no changes in oxidative phosphorylation.These findings are supported clinically by the lack of scar on cardiac magnetic resonance imaging of patients with PVC-CM.These findings further confirm a primary functional abnormality as a primary mechanism of this reversible CM. Whether all the cellular and molecular changes are in response to the CM rather than the cause of the CM remains unclear.
Predictors of PVC-CM
PVC burden has been shown to be a major predictor of PVC-CM. Two main studies have shown that PVC burden >16% and 24% best identifies patients with a diagnosis of PVC-CM. Although these and other studies suggest that a PVC burden of at least 10% is required to induce PVC-CM, other studies question this minimal PVC threshold,because they have shown improvement in LV function with PVC burden as low as 6% to 8% . The length of ambulatory ECG monitoring has important implications, because increasing the duration from a 24-h to a 7-day ambulatory Holter monitor can doubled the number of patients who reach the 10%threshold.
Some other PVC features have been found to be independent predictors for PVC-CM such as male sex,lack of symptoms or duration of palpitations >30 months, variability of PVC coupling interval, QRS duration of PVC >150 ms, and epicardial origin.PVC-CM index2, including PVC burden, PVC-QRS width, and epicardial origin, has been developed in an attempt to identify patients with high probability of PVC-CM.
Clinical Presentation, Diagnosis, and Imaging Features
The time course for the development of PVC-CM is unclear, but it is estimated to occur within months up to several years. Although animal studies with persistent high PVC burden(33% to 50%)develop CM within 4 weeks, human studies are not consistent in part due to the unclear onset and variability of PVCs.
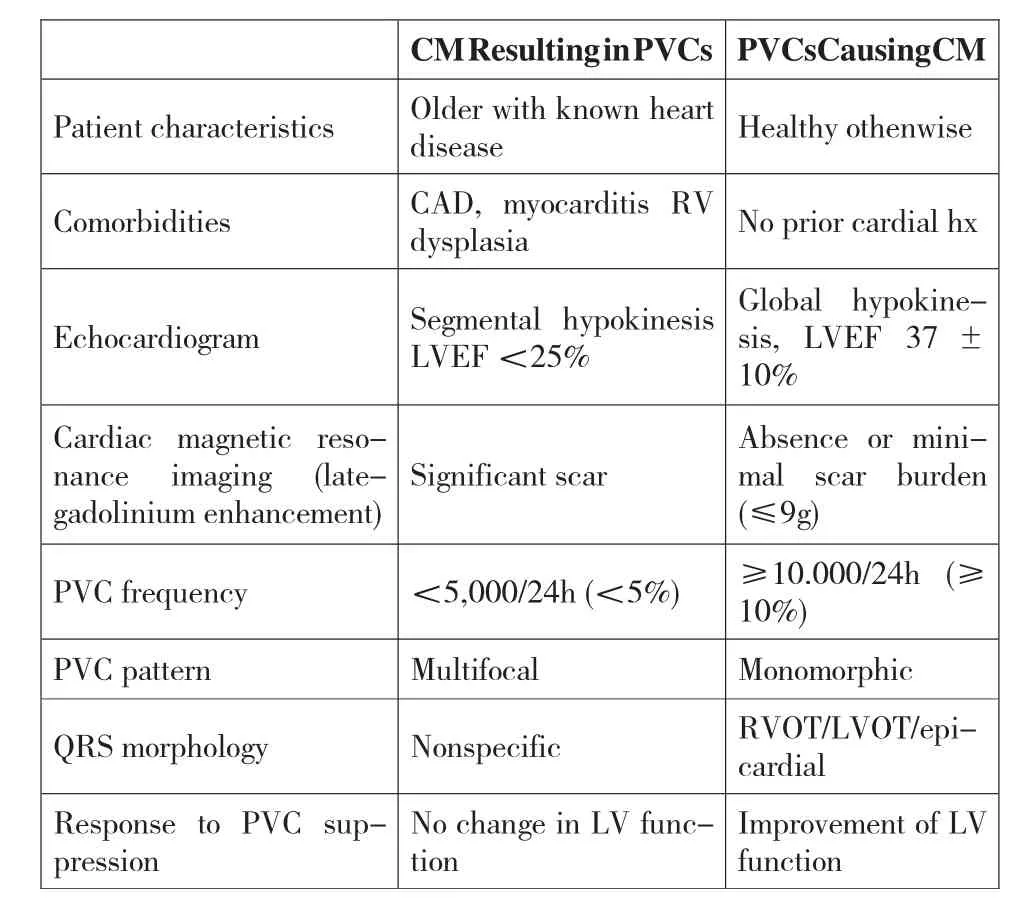
Table 1 Clinical and PVC Features to Identify PVC-CM
PVC-CM may have a wide range of presentations,from asymptomatic or vague symptomatology to heart failure and even syncope. It is unclear why some patients have symptoms related to PVCs while others do not, but a PVC coupling interval ratio <0.5 (PVC CI ratio: PVC coupling interval/Sinus coupling interval)has been proposed as an important marker of symptoms.
PVC-CM is a diagnosis of exclusion, to be suspected in patients with frequent PVCs >10% ,especially in nonischemic CM.A challenge is to identify when PVCs are the etiology of a CM or just "innocent bystanders" in patients with CM. Even if PVCs are the result of CM, these PVCs, if frequent, may contribute to and further worsen CM and HF symptoms; this is referred to as "superimposed" PVC-CM. In selected cases, echocardiographic and PVC features can help identify these patients (Table 1). PVC-CM is characterized by mild to moderate LV systolic dysfunction,LV dilatation,mild mitral regurgitation,and LA enlargement, which resolved within 2 to 12 weeks after elimination of PVCs. Cardiac imaging is key to identify LV dysfunction and prompt suspicion of PVC-CM in patients with high PVC burden(≥10%)(Table 1). Cardiac magnetic resonance with lategadolinium enhancement has the advantage of identifying scar and quantifying scar burden, which in turn potentially predicts the response to PVC suppression.
Treatment
Currently,a PVC suppression strategy with RFA or AADs is a widely accepted intervention to treat a CM that might be caused or exacerbated by frequent PVCs.However, the treatment of frequent PVCs(≥10%burden)without LV dysfunction (LVEF ≥50%),symptoms, or idiopathic ventricular fibrillation is less clear. PVC suppression is considered successful if burden is decreased by >80% of baseline PVCs, as it likely represents a true effect of treatment rather than spontaneous PVC variability.
PVC suppression in PVC-CM has been shown to improve LV function,LV dilatation,mitral regurgitation,and BNP levels. The mean improvement of LVEF after RFA in most studies is between 10% and 15% even in superimposed PVC-CM. A prospective study demonstrated a significant decrease in BNP levels while primary prophylaxis ICD implantation was avoided in 80% of all patients with PVC burden >13% due to significant improvement of LVEF after successful RFA.Another study found that 81%of patients with abnormal baseline estimated glomerular filtration rate had significant improvement in renal function after RFA of PVCs.
詞 匯
Pathophysiology n.病理生理學
leaflet n.&v.瓣葉,散頁印刷品,傳單,小冊子;散發傳單
coaptation n.對合,接合,適應
histopathological adj.組織病理學的
apoptosis n.細胞凋亡,程序性細胞死亡
mitochondrial adj.線粒體的
oxidative adj.氧化的,具有氧化特性的
phosphorylation n.磷酸化
implication n. 含意,暗指,牽連,牽涉
vague adj.模糊的,微小的,不明確的,不具體的
symptomatology n. 癥狀學,癥候學
innocent adj.無辜的,清白的,無罪的,無辜受害的;n.無辜者,單純的人
superimpose v.疊加,疊映,使重疊
注 釋
1.culprit arrhythmia 本文中指導致心肌病的心律失常,culprit在冠心病心肌梗死介入治療中常譯作“罪犯”,在此可譯作“致病”,即致病心律失常。
2.PVC-CM index 指PVC-CM 指數,由PVC 負荷(0-1)×PVC-QRS 寬度(毫秒)×常數C(結構性心臟疾病為1.28,心電圖提示心外膜起源為2)計算所得,≥39 分預測發生心肌病的風險高。
參考譯文
第92 課 心律失常所致心肌病心動過速所致心肌病
心動過速所致心肌病(T-CM)是指因心室率加快而引起的可逆性左心室功能不全,不考慮心動過速的起源。發生T-CM 的風險不但取決于心動過速的類型,也取決于心動過速的頻率和持續時間。有報道T-CM 占射頻消融患者的2.7%。不過,這包含行室性期前收縮消融的患者。據報道10%的房性心動過速患者發生T-CM,這在頑固性房性心動過速患者高達37%。此外,持續性結性折返型心動過速因其常為頑固性室上性心動過速,T-CM 的發生率最高(20%~50%)。
病理生理與機制
動物模型是了解T-CM 病理生理和機制的關鍵。與人類相似,連續心房或心室起搏引起的持續性心動過速可誘發動物心力衰竭癥狀、左心室收縮功能不全和擴張,降低左心室dP/dtmax 和心肌血流,增加左心室室壁張力、舒張末壓力和容量。心臟擴張涉及雙心室,伴輕微室壁變薄或不伴肥厚或心臟質量變化。這些生理學變化的進展包括動脈血壓降低、左心室和肺動脈壓增加,一周時這些變化達到穩定,而心搏出量、左心室射血分數(LVEF)和容量在隨后的4 周繼續惡化,在2~3 周內即出現心力衰竭癥狀。
T-CM 特征表現為心肌結構和功能變化。與人類相似,T-CM 模型也證實電重構和鈣穩態異常,認為這導致了興奮-收縮偶聯障礙和舒張功能異常。已證實只有總鈣循環、鈣通道抑制和基礎ATP 酶活性與LVEF 降低存在統計學意義的關聯。
臨床表現、診斷和圖像特征
臨床研究發現從心律失常癥狀到發生T-CM 的時間變化不一,從3d 到120d,總體上LVEF 為32%。除了快速型心律失常,較快速率的心動過速如持續性心房撲動或2:1 順傳室率>150 次/min 的心動過速較早出現心力衰竭癥狀。近期臨床研究發現,與擴張型心肌病或炎癥性心肌病[分別為(32.1±10.2)%和(41.9±12.9)%]相比,左心室功能不全更為嚴重[LVEF(29.3±6.6)%]。
主要癥狀包括心悸(29%),心功能Ⅲ到Ⅳ級(47%),暈厥或先兆暈厥(12%),其余為無癥狀。猝死不常見,但有報道盡管心肌病得到治療并緩解,仍然達到8%~12%。
心臟超聲或心臟磁共振檢查有助于排除其他病因。T-CM 特征表現為擴張性心肌病(左心室舒張末徑和面積增大)、中到重度雙心室收縮功能障礙、左心室間隔和后壁厚度正常(無肥厚)。由于左心室和二尖瓣環增大瓣尖閉合不佳而出現二尖瓣關閉不全。
治療
T-CM 的主要特征隨心動過速消除而逆轉。因此,治療重點是基于致病心律失常用抗心律失常藥物或射頻消融手術抑制心動過速。
消除快速心律失常不但可以在4~12 周內恢復左心室功能,而且多數患者至少可改善心力衰竭癥狀達NYHA 1級。遺憾的是T-CM 不總能完全恢復。盡管LVEF 正常了,組織病理異常、舒張功能不全及伴隨肥大反應的心室擴張會持續存在。
室性期前收縮所致心肌病
室性期前收縮所致心肌病(PVC-CM)是指頻發PVC 引起的左心室功能不全。通常認為PVC 負荷≥10%時足以引起PVC-CM。有報道PVC 負荷>10%的患者PVC-CM 的發生率為7%。臨床研究發現行PVC 射頻消融的患者中9%到30%診斷為PVC-CM。
PVC-CM 的潛在機制
PVC-CM 收縮功能不全的主要原因是鈣誘導的鈣釋放機制的自身異常,潛在的機制是dyad(L 型鈣通道和雷諾定受體)功能改變。類似于其他心肌病,PVC-CM 模型揭示電生理重構。組織病理學異常確無炎癥或凋亡增加的依據,輕微或無纖維化。 線粒體研究證實無氧化磷酸化變化。臨床PVC-CM 患者磁共振心臟顯像缺乏疤痕支持這些發現。這些發現進一步證實這種可逆性心肌病的主要發病機制為原發心功能異常。是否所有細胞和分子變化是對心肌病的反應抑或是心肌病的原因尚不清楚。
PVC-CM 的預測指標
PVC 負荷已成為預測PVC-CM 的主要指標。兩大研究顯示PVC 負荷>16%和>24%能很好的鑒別出PVC-CM 的患者。盡管這些及其他研究提示要誘發PVC-CM,PVC 負荷至少達10%,其他研究對這最小的PVC 負荷提出質疑,因為他們證實PVC 負荷低至6%到8%的患者處理后左心室功能得到改善。動態心電圖監測時長具有重要價值,因為將監測時間從24h 延長至7d,達到10%閾值(即負荷值達10%)的患者數量倍增。
發現能獨立預測PVC-CM 的一些其他PVC 特征有男性、缺乏癥狀或心悸超過30 個月、PVC 聯律間期多變、PVC QRS 間期>150 ms 及心外膜起源。 PVC-CM 指數,包括PVC 負荷、PVC-QRS 寬度和心外膜起源,已形成以期鑒別PVC-CM 高發可能性的患者。
臨床表現、診斷和影響特征
尚不清楚發生PVC-CM 需多長時間。估計發生在數月至數年間。雖然動物研究持續高負荷(33%至50%)PVC 4 周內誘發心肌病,人類研究并不一致,部分原因為不知PVC 何時開始以及它的可變性。
PVC-CM 表現形式寬泛,從無癥狀到或輕微癥狀到心力衰竭甚至暈厥。尚不清楚為什么有些患者出現PVC 相關癥狀而另外的不出現,不過,已認為PVC 聯律間期比<0.5(PVC 聯律間期比:PVC 聯律間期/竇律聯律間期)是癥狀的重要標志。
PVC-CM 是一排他性診斷,對于PVC>10%的患者要考慮,特別是非缺血性心肌病。面臨的挑戰是對于心肌病患者,PVC 是心肌病的病因還是“無辜的旁觀者”。即使PVC 是心肌病所致,這些PVC 如果頻發,可促進或進一步惡化心肌病和心力衰竭癥狀,這稱作“疊加型”PVC-CM。在選擇性病例,心超和PVC 特征有助于鑒別這些患者(表1)。PVC-CM 特征為輕中度左心室收縮功能異常、左心室擴大、輕度二尖瓣反流、和左心房擴大,這些會在消除PVC 后2 至12 周內得到緩解。心臟影像在鑒別高負荷PVC(≥10%)患者左心室功能不全及考慮PVC-CM 中起到關鍵作用(表1)。心臟磁共振延遲釓強化有益于識別疤痕并定量分析疤痕負荷,這反過來可預測對PVC 抑制的反應。
治療
當前,采用射頻消融術或抗心律失常藥物抑制PVC 的方案已成為治療PVC 誘發或加重心肌病的廣為接受的干預方法。不過,對于不伴左心室功能不全、癥狀、或特發性心室顫動的頻發PVC(負荷≥10%)治療尚不清晰。認為當PVC 較基礎狀態下降80%以上時治療是成功的,這反映治療的正真作用而非PVC 自發變異。
PVC-CM 抑制PVC 能改善左心室功能、左心室擴張、二尖瓣反流和BNP 濃度。多數研究表明射頻消融后LVEF 的改善平均達10%~15%,即使疊加的PVC-CM 也如此。一項前瞻性研究證實所有PVC 負荷>13%的患者在成功射頻消融后BNP 水平顯著下降,由于LVEF 明顯改善而使80%患者免于預防性植入ICD。另一研究發現81%有基礎估測腎小球濾過率異常的患者,在PVC 射頻消融后腎功能得以明顯改善。
表1 鑒別PVC-CM 的臨床和PVC 特征

