Effects of silver nanoparticles on resistance characteristicsof the wetland plant Typha orientalis in a hydroponic system
Ma Yixuan Huang Juan Cao Chong Cai Wenshu Xiao Jun Yan Chunni
(School of Civil Engineering, Southeast University, Nanjing 210096, China)
Abstract:To probe the influence and the adverse-resistance characteristics of wetland plants in presence of silver nanoparticles (AgNPs), the changes in the physiological and biochemical characteristics (including the superoxide dismutase (SOD) activity, catalase (CAT) activity, peroxidase (POD) activity, soluble protein content, and chlorophyll content) of Typha orientalis exposed to different concentrations of AgNPs solutions (0, 0.1, 1, 20 and 40 mg/L) were explored. Meantime, the accumulation of silver content in these plants was revealed. The results show that under low concentrations of AgNPs, the SOD and POD activities in the leaves of Typha orientalis are strengthened to different degrees. However, high concentrations of AgNPs inhibit the activities of SOD and POD. Under the stress of different concentrations of AgNPs, the CAT activities are inhibited initially and later recovered to some extent. Under the stress of low concentrations of AgNPs, the soluble protein content in the leaves of Typha orientalis increases significantly, but decreases more significantly with increasing concentrations of AgNPs. Low concentrations of AgNPs promote chlorophyll synthesis in the leaves of Typha orientalis, but the chlorophyll content subsequently falls to pre-stress levels. In contrast, high concentrations of AgNPs cause a certain inhibition to generate chlorophyll. Meanwhile, the results show that the silver concentrations of plant tissues increase with the exposure of concentrations of AgNPs and they have a positive relationship with the exposure of concentrations of AgNPs.
Key words:silver nanoparticles (AgNPs); wetland plants; Typha orientalis; physiological and biochemical characteristics; antioxidase
Silver nanoparticles (AgNPs) have become one of the most widely used and quickly developed nanomaterials due to their high surfactivity, surface energy and catalytic performance. Globally, the total mass of the consumer products containing silver nanoparticles is approximately 320 t every year[1], which can be used in a wide range of applications, from consumer products (clothing, textiles and hygiene products, etc.) to disinfecting medical devices, home appliances, and water treatment[2]. With the increasing use of nanosilver products, the possibility of AgNPs released into the environment is also increasing. Previous research has indicated that AgNPs may enter the environment through sewage discharge and surface runoff[3-4]. As a conventional wastewater treatment ecological system, the constructed wetlands (CWs) are also facing AgNPs pollution[5]. In addition, the wetland plants are one of the most essential components in CWs, which have several properties related to the treatment process[6]. Many studies showed that wetland plants can promote the removal efficiency of pollutants such as chemical oxygen demand (COD), total nitrogen (TN) and total phosphorus (TP)[7]. AgNPs are one of the most toxic metallic nano particles to aquatic organisms[8-9]. Bao et al.[10]found that AgNPs can enter plant tissues and tend to accumulate predominantly in the apoplast of root tissues whereby a minor portion was transported to shoot tissues. Hence, it is necessary to evaluate the risk of toxic effects of AgNPs on the wetland plants.
The effects of AgNPs have been widely studied in many aspects, such as microorganisms, mammalian cells and plants[11-13]. In the terms of plants, there was some research about the toxic effects on crops, vegetables and forage grass, etc.[12-16]and the exposure of AgNPs can cause the gene damage, lower germination of seeds, the decrease of root elongation and reduction of biomass[11-12,17]. El-Temsah and Joner[18]treated ryegrass seed with 10 mg/L AgNPs and observed a 20% decrease in their germination. Meantime, other studies found that AgNPs affected the physiological and biochemical indices of plants such as superoxide dismutase (SOD) activity, catalase (CAT) activity, peroxidase (POD) activity, soluble protein content and chlorophyll content. When the plants suffered the stress, such as from heavy metals, the plant’s cell accumulated reactive oxygen species (ROS) such as hydroxyl radicals (OH·) and hydrogen peroxide (H2O2). It can lead to the increase in lipid peroxidation and protein, resulting in biological macromolecule (i.e. nucleic acid and enzyme) damage, which seriously interfere with the normal metabolism of plants[19]. AgNPs-contaminated plants initiate enzymatic and non-enzymatic protection systems to clear and remove the ROS, reducing or eliminating the damage within limits. Antioxidant enzymes (including SOD, POD and CAT) are the main removal systems of ROS in plants, which can reflect the adverse-resistance characteristics of plants[20]. In addition, soluble protein content is the most intuitive indicator of resistance genes and chlorophyll content can directly affect the intensity of photosynthesis and the rate of material synthesis[21]. Nair and Chung[15]studied the physiological and biochemical characteristics of mung beans treated with AgNPs, indicating that total chlorophyll showed a significant decrease at 50 mg/L AgNPs, and proline content showed a significant increase at 20 to 50 mg/L AgNPs. When studying the physiological and biochemical characteristics of Chinese cabbage, Baskar et al.[22]found that AgNPs showed an inhibiting effect on the cabbage at 100 mg/L of AgNPs, and significantly reduced the root length, stem growth and fresh weight when treated with 500 mg/L of AgNPs. At the same time, reactive oxygen species (ROS), the propanediol (MDA) content and proline content increased, and chlorophyll decreased at concentrations of 500 mg/L AgNPs.
Few studies have been reported on the effect of AgNPs on wetland plants, which are inevitably affected by AgNPs. Yin et al.[23]found that 40 mg/L gum arabic coated AgNPs significantly reduced the germination of three kinds of wetland plants which areScirpuscyperinus,JuncuseffusesandPhytolaccaamericana. Furthermore, even the research on the physiological and biochemical indices of wetland plants affected by AgNPs is rarely seen. On average, most concentrations of AgNPs tested in these studies were above 1 mg/L, which were regarded as high concentrations[24]. Therefore, it is necessary to monitor the changes in the physiological and biochemical characteristics of the wetland plants to probe the adverse-resistance characteristics of wetland plants in the presence of different concentrations of AgNPs which were divided into low concentrations (0, 0.1,1 mg/L) and high concentrations (20, 40 mg/L). In the meantime, it is of great significance to reveal the accumulation of silver content in plants under different concentrations of nanosilver stress.
Typhaorientalisis the macro-emergent aquatic plant which is the most common wetland plant and is frequently used in constructed wetland and ecological floating beds[25]. Meanwhile, it has a good removal capacity for COD, total nitrogen and total phosphorus in water and it is a potential feedstock for sustainable bioenergy production[26]. Hence,Typhaorientaliswas chosen to be a test plant in this study. Furthermore, all the test plants were cultivated in a hydroponic system. Compared with the complex environment of the soil culture system, the hydroponic system has a single and controllable relative influencing factor, which can more directly reflect the impact of nanosilver on the stress resistance of wetland plants.
The objectives of the present study are to investigate the changes in the physiological and biochemical characteristics of the wetland plants to probe their adverse-resistance characteristics in the presence of different concentrations of AgNPs. Furthermore, the silver concentrations of different plant tissues ofTyphaorientalisare revealed as well.
1 Materials and Methods
1.1 Nanomaterials
The monomer solution of silver nanoparticles (purchased from Shanghai Huzheng Nano Technology Co., LTD, Shanghai, China) used in this study has the following characteristics: The purity is greater than 99.99%, the average particle size is 15 nm and the solid content (Ag) is 2×10-3. The monomer AgNPs solution is a type of water-based silver-colloidal dispersion obtained by coating Ag particles with polyvinylpyrrolidone (PVP) using a physical chemistry method. The monomer solution of silver nanoparticles was observed by a transmission electron microscope (TEM). As shown in Fig.1, the particles are finely dispersed and uniform, with the diameters from 10 to 40 nm. The statistical information of the particle size is (24.31±9.72) nm.
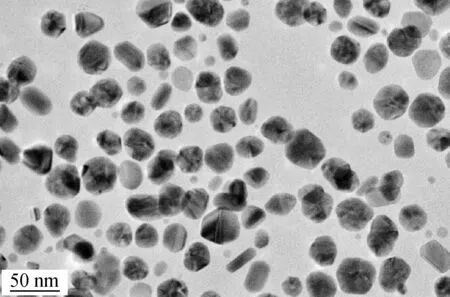
Fig.1 The TEM photograph of the nanometer silver solution
1.2 Test plants
As a typical wetland plant,Typhaorientaliswas selected to serve as the test plant for AgNPs stress. The test plantsTyphaorientaliswere purchased from a wetland in Shuyang, Jiangsu Province, China. All test plants were cultivated in an identical environment with identical concentrations of nutrients, which were diluted from Hoagland medium (calcium nitrate 945 mg/L, potassium nitrate 607 mg/L, ammonium phosphate 115 mg/L, magnesium sulfate 493 mg/L, iron salt solution 2.5 mL, and trace elements solution 5 mL). Only healthy and uniform plants were selected for the test.
1.3 Experimental design and methods
Different concentrations of AgNPs solutions (The initial concentrations were 0.1, 1, 20 and 40 mg/L) and pure water (control) were prepared. Uniformly growing plants were selected, washed by distilled water, and cultivated in AgNPs solutions of the different concentrations described above. Three uniform plants were cultivated in each concentration and their roots submerged in the solutions. Since the beginning of the experiment, the functional leaves (mature and healthy leaves) of three uniform plants at each concentration were sampled with a leaf sampler (sampling diameter: 5 mm) in the morning between 8:30 and 9:30 every 7 d or 14 d and the vertical distance from each sampling point to the top of each plant stem was about 20 mm.
To measure SOD activity, POD activity, and CAT activity, 0.2 g of leaf blade was taken from each sample which was sampled from the functional leaf of each plant, added to the precooling enzyme extract, and fully ground under the ice bath. Then, the supernatant of the mixture was taken after refrigerated centrifugation (12 000 r/min, 20 min). To measure the soluble protein content, 0.1 g of leaf blade from each plant was weighed and then ground into homogenate with 5 mL distilled water. After that, the supernatant of the mixture was taken after refrigerated centrifugation (3 000 r/min, 10 min). To measure chlorophyll content, 0.1 g of fresh broken sample were collected to extract chlorophyll from 80% acetone. After 28 d, all indices were examined every 14 d until the 57th day. The temperatures during the experiments were 24 to 32 ℃ during the day and 15 to 28 ℃ during the night.
In addition,the SOD activities were measured using the NBT photochemical reduction method[27]. The POD activities were measured using a guaiacol method[28], while the CAT activities were measured using a spectrophotometric method[29]. The soluble proteins were measured through Coomassie brilliant blue staining[30], and the chlorophyll content was measured using spectrophotometric methods[31].
At the end of the test, to determine the silver concentrations of different plant parts, the pretreatment of root tissues and shoot tissues were implemented, respectively, according to the previous description[32]. Then, the silver concentrations were measured according to the US Environmental Protection Agency (EPA) 200.8 by the ICP-AES (ICAP6300, Thermo Fisher Scientific, MA, USA).
1.4 Data analysis
To explore the changes in the physiological and biochemical characteristics (including the superoxide dismutase (SOD) activity, catalase (CAT) activity, peroxidase (POD) activity, soluble protein content, and chlorophyll content), change rates were adopted in this study. The change rate is calculated as the difference between the value of physiological and biochemical characteristics on the following days (7th, 14th, 21st, 28th, 42nd, 56th) and the value at the beginning of the experiment (1st), then divided by the value on the first day. The translocation factor evaluates the ability of plants to transport the AgNPs from underground to the ground and is calculated according to the formula which is given by Nouri et al[33].
whereFTrepresents the translocation factor;MLis the metal concentration in the shoot tissues; andMRis the metal concentration in the root tissues.
Origin software was used to chart the experimental data. SPSS 19.0 software was used for the statistical analyses. All values were expressed as mean of three parallel samples and standard deviation (n=3).
2 Experimental Results
2.1 Changes of the SOD activity at different concentrations of AgNPs
As shown in Fig.2, the SOD activities ofTyphaorientalisshowed different change trends at different concentrations of AgNPs. WhenTyphaorientaliswere cultivated in pure water, SOD activity had a modest decrease. Under 0.1 mg/L AgNPs stress, the SOD activity showed a significant increasing trend during 56 d, increasing by 34.8% compared with that before exposure of AgNPs. It indicated that adding low concentrations of AgNPs (0.1 mg/L) induced SOD activity. While exposure at 1 mg/L AgNPs, SOD activity initially increased and then gradually decreased to its initial values on the 56th day. The fluctuation caused by 1 mg/L AgNPs might be because the antioxidant enzyme systems of wetland plants provided defense against the oxidative stress of AgNPs, and finally their free radical scavenging system recovered. However, under stress caused by high concentrations of AgNPs (20 and 40 mg/L), the SOD activity showed significant and persistent decreases, decreased by 38.4% and 55.5%, respectively, during 56 d. Therefore, high concentrations of AgNPs can cause significant oxidative stress toTyphaorientalisand decrease the activity of SOD markedly.
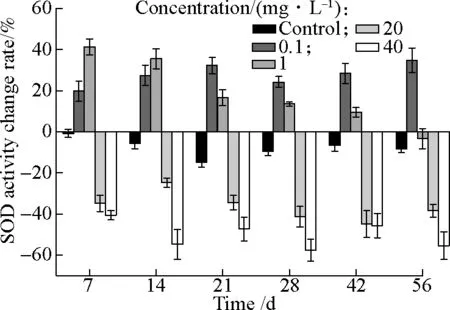
Fig.2 Change rates of SOD activity at different concentrations of AgNPs
2.2 Changes of the CAT activity at different concentrations of AgNPs
The CAT activity ofTyphaorientalisof control group (0 mg/L AgNPs) showed a decline trend and eventually reduced by 10.9% on the 56th day (see Fig.3). However, under the stress of different concentrations of AgNPs, the CAT activity ofTyphaorientalisshowed similar fluctuation trends. The activity of CAT showed a slight decline in the initial stage, followed by a gradual increase trend after 14 d. Meantime, the CAT activity of added AgNPs groups was significantly higher than that of the control group on the 28th day and the 42nd day. The enzyme CAT had higher activity in the later stage, which has the ability to catalyze H2O2to water and oxygen[34], thus it can protect cells from oxidative damage due to the stress of AgNPs[35].

Fig.3 Change rates of CAT activity at different concentrations of AgNPs
2.3 Changes of the POD activity at different concentrations of AgNPs
As shown in Fig.4, under stress by different concentrations of AgNPs, the POD activity showed a similar trend to the SOD activity. When cultivated in the hydroponic system, the POD activity stayed relatively steady, with change rates fluctuating between±20%. Under the stress of 0.1 and 1 mg/L AgNPs, the POD activity firstly increased by approximately 140% on the 21st day and the 28th day, and then gradually decreased to their original levels, which increased by 6.6% and 21.6% compared with the initial values (without AgNPs), respectively. As the important oxidoreductase in the plant, POD can catalyze the redox reaction of H2O2with various organic and inorganic hydrogen donors[36], and then scavenge excess free radicals. The increase of the POD activity in the middle stage indicated that the plant suffered oxidative stress from AgNPs. After that, the plant produced more POD to defend against stress. The POD activity decreased in the later stage, becauseTyphaorientalisprotected itself against oxidative stress caused by AgNPs through the synergy of antioxidases such as SOD, CAT and POD. Then, the free radical scavenging system of the plant can return to normal levels. However, at 20 mg/L AgNPs, the POD activity had a slight increase in the early stage and subsequently showed a decreasing trend, with a 36.7% decline during 56 d. After adding 40 mg/L AgNPs, the POD activity had a significant decrease compared with the control one, which is reduced by 55.7% on the 56th day.
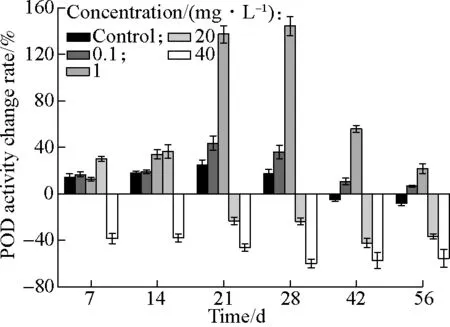
Fig.4 Change rates of the POD activity at different concentrations of AgNPs
2.4 Changes of the soluble protein content at different concentrations of AgNPs
When cultivated in the hydroponic system during the experimental period, the soluble protein content maintained a stable state, with a fluctuation in the range of ±10% (see Fig.5). At the AgNPs stress of 0.1 mg/L, the soluble protein content increased significantly, reaching to a maximum on the 21st day, with a 67.0% increase. However, when concentrations of AgNPs reached 1, 20, and 40 mg/L, the soluble protein content showed a decreasing trend with time, reducing by 47.6%, 62.7% and 61.9%, respectively, on the 56th day. When exposed to stress, the soluble protein content in the plant increases and can scavenge active oxygen[37], which can decrease the cellular water potential and enhance its water-retaining capacity to increase the stress tolerance of the plant. Therefore, it is concluded that the stress caused by low concentrations of AgNPs can induce the plantTyphaorientalisto synthesize new proteins to provideTypha

Fig.5 Change rates of the soluble protein content at different concentrations of AgNPs
orientaliswith a certain degree of tolerance. However, the soluble protein content obviously decreased when the plants were exposed to high concentrations of AgNPs.
2.5 Changes of the chlorophyll content at different concentrations of AgNPs
As shown in Fig.6, under the stress caused by low concentrations of AgNPs (0.1 and 1 mg/L), the chlorophyll content obviously increased by 83.58 % and 146.38% (max values) compared with that before exposure of AgNPs in the initial stages, respectively. At the end of the study, the chlorophyll content had no significant difference with control group. When exposed to high concentrations of AgNPs (20 and 40 mg/L), the total chlorophyll content showed an obvious fluctuation and a general decreasing tendency. The results indicated that the low concentrations of AgNPs had no inhibition effects on the synthesis of chlorophyll and could promote its production. However, high concentrations of AgNPs inhibited the synthesis of chlorophyll.

Fig.6 Change rates of chlorophyll content at different concentrations of AgNPs
2.6 Silver concentrations of the plant tissues
After being cultured with different concentrations of AgNPs for 56 d, the silver concentrations of root tissues and shoot tissues were measured, as shown in Fig.7. At the end of the experiment, some of the dosing AgNPs entered into the plant tissues, which was similar to the results of Bao et al[10]. Fig.7 shows that the silver contents of root tissues are 0.37, 0.59, 2.35 and 5.24 μg/g, respectively. However, the silver contents of shoot tissues are 0.12, 0.27, 0.87 and 0.98 μg/g, respectively. The results suggested that the silver concentrations of plant tissues increased with exposure to the concentrations of AgNPs, which had a positive relationship. The plant tissues exposed to silver concentrations at 0.1 and 1 mg/L AgNPs had no difference with each other. However, compared with the culture systems at 0.1 and 1 mg/L AgNPs, silver accumulation in plant tissues exposed to high concentrations of AgNPs (20 and 40 mg/L) had obvious differences and AgNPs absorbed by root and shoot tissues after exposure of 20 and 40 mg/L AgNPs were higher than those by other systems. As shown in Fig.7, more silver accumulated in the root tissues compared with shoot tissues, and only a minor portion of AgNPs was transported to shoot tissues. Meantime, the translocation factors were 0.34, 0.44, 0.37, 0.19, respectively.
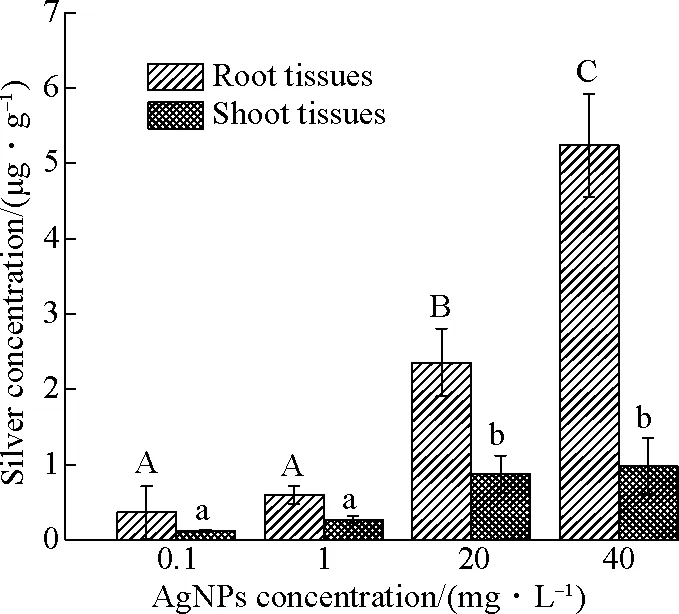
Fig.7 Silver concentration of plant tissues at different concentrations of AgNPs during 56 d exposure (Different letters show significant differences (p < 0.05))
3 Discussions
This study showed the impact of different concentrations of AgNPs on the physiological and biochemical characteristics of test plants in the aquatic systems.
In this study,the low concentrations of AgNPs had a role in promoting the increasing SOD and POD activities. However, the high concentrations of AgNPs can significantly inhibit the activity of SOD and POD ofTyphaorientalis. The study by Thwala et al.[38]on the stress of AgNPs on the antioxidant system of duckweed (Spirodelapunctuta) also showed higher inhibition on the SOD activity with increasing concentrations of AgNPs and the generation of ROS and H2O2increased after being exposed to AgNPs for 14 d. The variations of CAT activity were quite different from the enzymes SOD and POD. From the 1st to the 21st, the CAT activity was evidently suppressed at all exposure concentrations. However, after 28 d exposure to AgNPs, the CAT activity was significantly higher than the control, and the increasing degree was related to the concentrations of AgNPs. Baskar et al.[22]reported different concentrations of AgNPs on the physiological responses of Brassica rapa ssp. pekinensis, and the addition of high concentrations of AgNPs promoted the gene expressions of CAT and peroxidase and ROS production. These results were similar to those of the plants exposed to heavy metals[39].
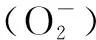
However,the variation of CAT activity was quite different from those of the enzymes SOD and POD. In the preliminary stage, CAT was inhibited slightly. It was probable that enzyme CAT did not play a major role in removing the ROS in antioxidant enzymes ofTyphaorientalis. SOD and POD and non-enzymatic protection systems played a major role in removing the ROS. Then, in the later stage, CAT activity was significantly higher than in the control one, because with more Ag accumulation inside the plants, the elevation of the CAT activity was induced to scavenge excess H2O2due to the stress of AgNPs. Nair et al.[44]found that upon exposure to AgNPs, the expression of CAT gene was upregulated into the plants ofChironomusriparius.
In this study, only 0.1 mg/L of AgNPs promoted the increasing trend of the soluble protein content. Under exposure to high concentrations of AgNPs, the soluble protein content was significantly lower than the control’s within the experimental period. The results suggested that the high AgNPs levels inhibited the ability of synthetic protein in the test plants. The reason might be that the AgNPs or Ag+released by AgNPs enter the plant tissues[10]and induce the production of ROS, destroying the cell and then reducing the protein capacity. The significant reduction in the chlorophyll content, as shown in this study, might be a result of excess lipid peroxidation of chloroplast membranes due to oxidative stress[45]. The previous study by Jiang et al.[46]also showed an obvious reduction in the chlorophyll content ofSpirodelapolyrhiza. Oukarroum et al.[47]found that under exposure of 10 mg/L AgNPs, the chlorophyll content significantly decreased, which was similar to the current results. As reported by Nair and Chung[15]and Qian et al.[48], the chlorophyll content decreased significantly when the plant suffered the stress of AgNPs, showing chlorotic leaves[49].
In this study, the results of ICP-AES revealed that the accumulation of silver content inTyphaorientaliswas increased with the increasing concentrations of AgNPs. The results of the present study coincided with the previous studies which revealed the silver content of Arabidopsis plants[10]andB.juncea[50]. The silver concentration in the plant treated with 40 mg/L AgNPs was eight times as high as that treated with 1 mg/L AgNPs, which might be responsible for strongly affecting the plant antioxidant enzymes (such as SOD, CAT, etc.) by promoting the production of ROS.
4 Conclusions
1)Typhaorientalisplants suffered significant inhibition under high concentrations of AgNPs, which was associated with the variation of physiological and biochemical characteristics such as decreased activity of antioxidant enzymes and the low level of soluble protein and chlorophyll biosynthesis.
2) Under low concentrations of AgNPs,Typhaorientalisshowed a certain degree of resistance to AgNPs stress with normal physiological and biochemical characteristics.
3) The silver concentrations of plant tissues increased with the exposure to concentrations of AgNPs, which had a positive relationship.
 Journal of Southeast University(English Edition)
2019年3期
Journal of Southeast University(English Edition)
2019年3期
- Journal of Southeast University(English Edition)的其它文章
- Experimental investigation of oil particles filtration on carbon nanotubes composite filter
- Projection pursuit model of vehicle emission on air pollution at intersections based on the improved bat algorithm
- Effect of waste concrete with different strengthon mechanical properties of recycled fine aggregate mortar
- Tensile behaviors of ecological high ductility cementitious composites exposed to interactive freeze-thaw-carbonation and single carbonation
- Travel time prediction model of freewaybased on gradient boosting decision tree
- Image denoising method with tree-structured group sparse modeling of wavelet coefficients
