Coexistence of breakpoint cluster region-Abelson1 rearrangement and Janus kinase 2 V617F mutation in chronic myeloid leukemia: A case report
Xue-Bing Shi, Ji-Fa Jiang, Feng-Xiang Jin, Wei Cheng
Abstract
Key words: Chronic myeloid leukemia; JAK2 V617F; BCR-ABL1; Imatinib;Myeloproliferative neoplasm; Case report
INTRODUCTION
Chronic myeloid leukemia (CML) is a hematologic malignant neoplasm with clonal proliferation of hematopoietic cells. The specific molecular biologic feature of typical CML corresponds to a translocation between chromosome 9 and chromosome 22[t(9;22)(q34;q11)], named the Philadelphia (Ph) chromosome, which leads to breakpoint cluster region-Abelson1 (BCR-ABL1) rearrangement[1]. The Ph chromosome and/orBCR-ABL1rearrangement are necessary for the diagnosis of typical CML[1]. Janus kinase 2 (JAK2) V617F mutation is an important biomarker in the diagnosis of myeloproliferative neoplasms (MPNs). According to the literature, the mutation rate ofJAK2V617F is 90%-95% in polycythemia vera (PV) and about 60% in both essential thrombocythemia (ET) and primary myelofibrosis (PMF)[2]. However,BCR-ABL1-positive CML withJAK2V617F mutation is very uncommon. Herein, we present a case of CML with both theBCR-ABL1rearrangement andJAK2V617F mutation.
CASE PRESENTATON
Chief complaints
On May 29, 2018, a 45-year-old Chinese woman with a history of marked thrombocytosis for 20 d was admitted to the Department of Hematology and Oncology, Tongling People's Hospital (Anhui Province, China).
History of present illness
She had been treated with antibiotics for 3 wk for lobar pneumonia in another hospital before admission to our hospital. Peripheral blood count showed a platelet count of 586 × 109/L at the beginning of anti-infective therapy, which increased to 1109 × 109/L when her pneumonia resolved. She attended our department for hematological evaluation.
History of past illness
She had no past history of surgery, anemia or malignant neoplasms and was not taking any medication.
Personal and family history
She was married, and her spouse and daughter were both healthy. The family history was unremarkable.
Physical examination upon admission
Physical examination showed that the splenic inferior margin was 2 cm under the left arcus costarum.
Laboratory examinations
The concentration of lactate dehydrogenase was 364 U/L. Peripheral blood count showed a leukocyte count of 11.46 × 109/L, hemoglobin of 121 g/L, platelet count of 1582 × 109/L and neutrophil count of 7.63 × 109/L. Peripheral blood smear examination showed 2% blasts, 1% myelocytes, 70% mature neutrophils, 3%eosinophils, 7% basophils, 13% lymphocytes and 4% monocytes (Table 1). Bone marrow cytomorphologic examination revealed mild granulocytic hyperplasia of 49%, including 1.5% myelocytes, 5.5% metamyelocytes, 10.5% stab nuclear neutrophils, 22% segmented neutrophils, 1.5% eosinophils, 3% basophils and 5%blasts (Table 1). The leukocyte alkaline phosphatase score was 135 and leukocyte alkaline phosphatase positivity was 92%. Immunophenotyping analysis by flow cytometry revealed 5% blast cells. The reagents applied in flow cytometry mainly consisted of antibodies against CD10, CD19, CD5, CD7, CD13, CD33, HLA-DR, CD38,CD34, CD16, CD11b, CD117, CD36, CD64, CD56, CD14, CD20, CD8, CD3, CD2, CD4,cMPO, cCD22, cCD3, TCRab, TCRgd, CD45RA, CD45RO, CD15,CD11c, CD43 and CD45. Cytogenetic analysis using both the G-banding and R-banding technique demonstrated a karyotype of 46, XX, t(9:22)(q34;q11.2) in 20/20 metaphases examined. The rearrangement ofBCR-ABL1(P210) was detected by fluorescent polymerase chain reaction (commonly known as PCR), and theBCR-ABL1/ABL1ratio was 32.31%. Moreover, theJAK2V617F mutation was identified by PCR and Sanger DNA sequencing, and the mutation percentage, which was calculated as [copynumberJAK2V617F/ (copy-numberJAK2V617F+ copy-numberwild-typeJAK2)], was 10%. Bone marrow biopsy examination showed active proliferation of granulocytic cells and marked hyperplasia of megakaryocytes (Figure 1A). The proliferative megakaryocytes had small cell bodies and decreased karyolobism. Additional immunohistochemistry of bone marrow cells exhibited CD34 (2%+), CD117 (5%+), MPO partial +, CD235a minority +, CD61 + for megakaryocytes and a few scattered CD138 +. Gomori staining was positive (++ - +++) (Figure 1B).
Imaging examinations
Color Doppler ultrasound examination showed mild splenomegaly.
FINAL DIAGNOSIS
The patient was diagnosed with CML (chronic phase, Sokal 1.68, high risk) andJAK2V617F mutation.
TREATMENT
Due to severe thrombocytosis, the patient was treated with hydroxyurea (0.5-2.0 g/d),aspirin (0.1 g/d) and platelet separation. On the sixth day of hospitalization, she was administered imatinib (0.4 g/d) due to the detection of theBCR-ABL1rearrangement.Her platelet count rapidly decreased, and hydroxyurea and aspirin were discontinued successively.
OUTCOME AND FOLLOW-UP
On July 11, 2018, her peripheral blood counts were as follows: leukocytes 3.44 × 109/L,neutrophils 2.11 × 109/L, hemoglobin 117 g/L and platelets 130 × 109/L, and she was discharged from the hospital. After leaving hospital, she continued to take imatinib(0.4 g/d). During regular follow-up, her peripheral blood counts were in the normal reference range, and spleen size returned to normal within 2 mo. After 3 mo of imatinib therapy, bone marrow aspiration was reexamined. Mutation of theABL1kinase domain was negative. Chromosomal karyotype was 46, XX in all 20 metaphases by G-banding, while the karyotype of 46, XX, t(9;22)(q34;q11.2) was identified in 1/16 metaphases by R-banding. TheBCR-ABL1/ABL1ratio decreased to 0.216% andBCR-ABL1(IS) was 0.143%, but the percentage ofJAK2V617F mutation increased to 15%. The patient had an optimal response to imatinib therapy and is continuing to take imatinib.
DISCUSSION
MPNs are clonal disorders of hematopoietic stem cells, and they can be divided intoBCR-ABL1-negative MPN and Ph chromosome and/orBCR-ABL1positive CML according to the 2016 World Health Organization classification system for hematopoietic and lymphoid tissue tumors. The former mainly includesJAK2/CALR/MPLmutated MPNs (PV, ET and PMF), chronic neutrophilic leukemia,chronic eosinophilic leukemia and unclassified MPN[2]. As an important marker in thediagnosis ofJAK2/CALR/MPLmutated MPNs, theJAK2V617F mutation has often been reported in PV, ET and PMF, but rarely in typical CML.
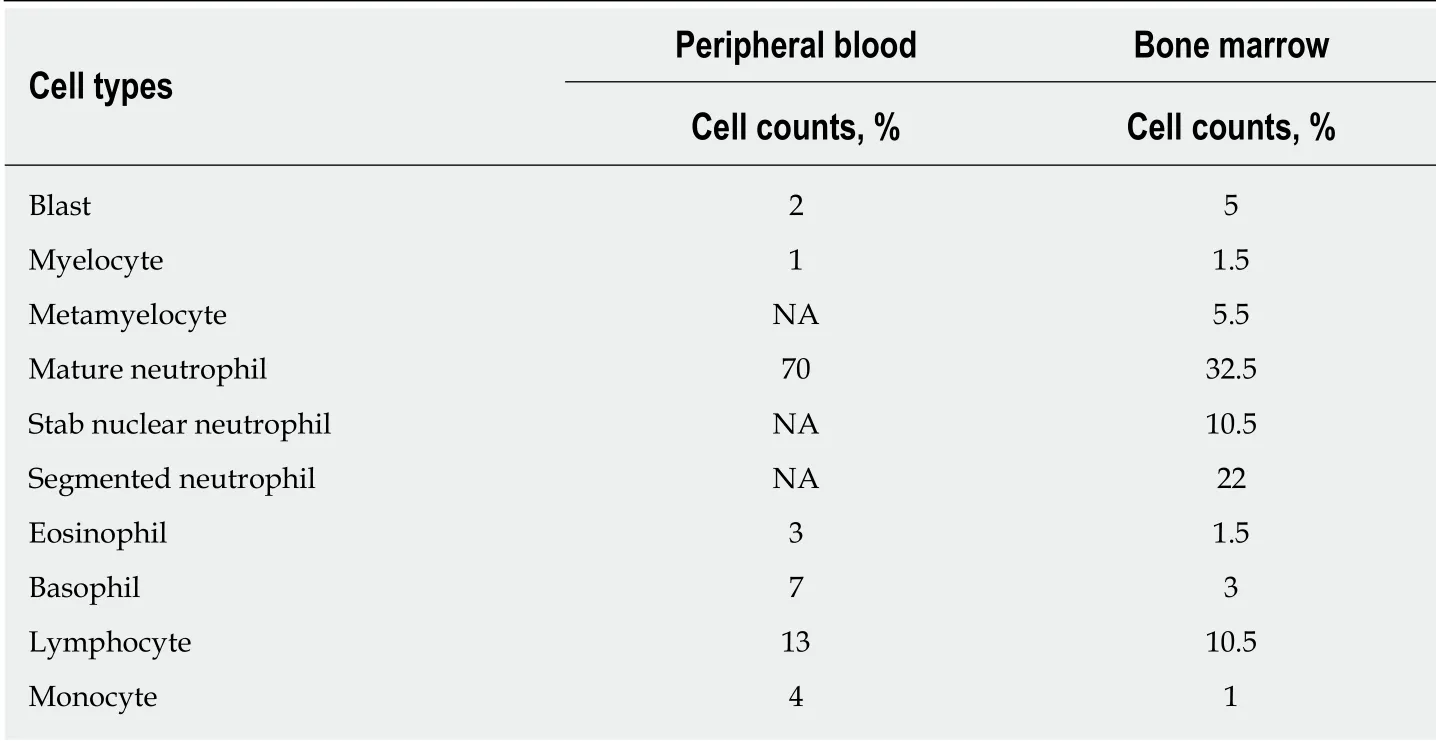
Table 1 Differential cell counts in peripheral blood and bone marrow
In recent years, a few studies have reported thatBCR-ABL1rearrangement/Ph chromosome andJAK2V617F mutation can coexist in CML patients[3-14]. However,some of these studies failed to examineJAK2status at the time of initial diagnosis of CML, but detectedJAK2V617F mutation with a decrease inBCR-ABL1translocation level during treatment with tyrosine kinase inhibitors (TKIs)[3-9], while others discovered concomitantBCR-ABL1rearrangement andJAK2V617F mutation when CML was diagnosed and before administration of TKIs[5,10-14]. The CML patients with aJAK2V617F mutation not only had typical CML characteristics but also had notable thrombocythemia[5-7,9-11], and thrombocytosis even persisted in some patients after obtaining a complete cytogenetic response, major molecular response or deep molecular response after TKI therapy[5,7,11]. Most studies indicated that following TKI treatment, the mutation rate ofJAK2V617F increased with a decrease inBCR-ABL1transcript level in this category of CML patients[7,8,12,13]. Only one study showed thatJAK2V617F mutation gradually decreased and then disappeared, accompanied by a reduction inBCR-ABL1rearrangement[10]. As reported in the literature,JAK2V617F mutation affected the curative effect in CML patients, andJAK2 V617F-positive CML patients often had a suboptimal response to TKIs[9,10,13]. Pahoreet al[14]demonstrated that 26.7% of 45 CML patients had aJAK2V617F mutation, and the risk of early disease progression in patients with aJAK2V617F mutation was significantly higher than that in patients without theJAK2V617F mutation.
There is no optimal treatment strategy forJAK2V617F-positive CML patients. As described in published reports, TKIs are preferentially administered in this subset of patients[3-12]. To our knowledge, it is unclear whether such cases can benefit from theJAK2inhibitor ruxolitinib.
In our patient, bone marrow examination revealed the coexistence ofBCR-ABL1rearrangement andJAK2V617F mutation before imatinib was administered, and the patient also presented with marked megakaryocytic hyperplasia and myelofibrosis.Following hospitalization, peripheral blood primarily showed a marked increase in platelet count. The patient achieved complete hematological response following 2 mo of imatinib treatment. After 3 mo of imatinib treatment, the proportion of Ph chromosome-positive cells was 6.25% in all metaphases andBCR-ABL1(IS) was 0.143%, which suggested that the optimum response had been obtained. However, theJAK2V617F mutation rate rose from 10% to 15%. The marked thrombocytosis observed at diagnosis and identification of theJAK2V617F mutation level increasing in pace with the decrease inBCR-ABL1transcript level during imatinib therapy were consistent with previously reported observations[5-9,11-13]. We hypothesize that the coexistence ofBCR-ABL1rearrangement andJAK2V617F mutation originates from two different clones that grow independently. Although our patient has favorable treatment efficacy at present, theJAK2V617F mutation level is still increasing and bone marrow fibrosis is still present. Thus, the long-term prognosis of this patient may be poor, and extended follow-up is required.
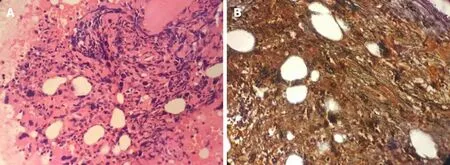
Figure 1 Bone marrow biopsy. A: Hematoxylin and eosin staining shows active proliferation of granulocytic cells and marked megakaryocytic hyperplasia (400 ×); B:Gomori staining is positive (++ to +++) (400 ×).
CONCLUSION
With the rapid development of molecular biology, a few CML patients with aJAK2V617F mutation have been reported recently, but such cases are relatively rare. The specific pathogenesis, optimal treatment and prognosis of this special type of CML are currently still ambiguous, and further large-sample studies are urgently needed.Moreover, further research to determine whether theJAK2mutation is associated withBCR-ABL1translocation in these patients is required. Attention should be paid to the detection of theJAK2mutation during the diagnosis and treatment of CML in order to timely identifyJAK2mutation-positive CML patients and guide the formulation of treatment strategies.REFERENCES
1 Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring.Am J Hematol2016; 91: 252-265 [PMID: 26799612 DOI: 10.1002/ajh.24275]
2 Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, Orazi A, Tefferi A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion.Blood Cancer J2018; 8: 15 [PMID: 29426921 DOI: 10.1038/s41408-018-0054-y]
3 Kr?mer A, Reiter A, Kruth J, Erben P, Hochhaus A, Müller M, Cross NC, Jones AV, Ho AD, Hensel M.JAK2-V617F mutation in a patient with Philadelphia-chromosome-positive chronic myeloid leukaemia.Lancet Oncol2007; 8: 658-660 [PMID: 17613428 DOI: 10.1016/S1470-2045(07)70206-1]
4 Inami M, Inokuchi K, Okabe M, Kosaka F, Mitamura Y, Yamaguchi H, Dan K. Polycythemia associated with the JAK2V617F mutation emerged during treatment of chronic myelogenous leukemia.Leukemia2007; 21: 1103-1104 [PMID: 17301812 DOI: 10.1038/sj.leu.2404591]
5 Darling HS, Kumar R, Kapoor R, Singh J, Verma T. BCR-ABL and JAK2V617F Mutation Co-existence,Rare or Just Unexplored.Indian J Hematol Blood Transfus2017; 33: 633-635 [PMID: 29075087 DOI:10.1007/s12288-017-0781-4]
6 Pagnano KB, Delamain MT, Magnus MM, Vassallo J, DE Souza CA, DE Almeida D, Lorand-Metze I.Concomitant essential thrombocythemia with JAK2 V617F mutation in a patient with chronic myeloid leukemia with major molecular response with imatinib and long-term follow-up.Oncol Lett2016; 12: 485-487 [PMID: 27347169 DOI: 10.3892/ol.2016.4631]
7 Pastore F, Schneider S, Christ O, Hiddemann W, Spiekermann K. Impressive thrombocytosis evolving in a patient with a BCR-ABL positive CML in major molecular response during dasatinib treatment unmasks an additional JAK2V617F.Exp Hematol Oncol2013; 2: 24 [PMID: 24007855 DOI:10.1186/2162-3619-2-24]
8 Hussein K, Bock O, Seegers A, Flasshove M, Henneke F, Buesche G, Kreipe HH. Myelofibrosis evolving during imatinib treatment of a chronic myeloproliferative disease with coexisting BCR-ABL translocation and JAK2V617F mutation.Blood2007; 109: 4106-4107 [PMID: 17449802 DOI:10.1182/blood-2006-12-061135]
9 Hassan A, Dogara LG, Babadoko AA, Awwalu S, Mamman AI. Coexistence of JAK2 and BCR-ABL mutation in patient with myeloproliferative neoplasm.Niger Med J2015; 56: 74-76 [PMID: 25657500 DOI: 10.4103/0300-1652.149177]
10 Campiotti L, Appio L, Solbiati F, Ageno W, Venco A. JAK2-V617F mutation and Philadelphia positive chronic myeloid leukemia.Leuk Res2009; 33: e212-e213 [PMID: 19589593 DOI:10.1016/j.leukres.2009.06.011]
11 Lewandowski K, Gniot M, Wojtaszewska M, Kandu?a Z, Becht R, Paczkowska E, M?dra? E, Wasilewska E, Iwo?a M. Coexistence of JAK2 or CALR mutation is a rare but clinically important event in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors.Int J Lab Hematol2018; 40: 366-371[PMID: 29508552 DOI: 10.1111/ijlh.12798]
12 Xu W, Chen B, Tong X. Chronic myeloid leukemia patient with co-occurrence of BCR-ABL junction and JAK2 V617F mutation.Int J Hematol2014; 99: 87-90 [PMID: 24293258 DOI:10.1007/s12185-013-1480-z]
13 De Roeck L, Michaux L, Debackere K, Lierman E, Vandenberghe P, Devos T. Coexisting driver mutations in MPN: clinical and molecular characteristics of a series of 11 patients.Hematology2018; 23:785-792 [PMID: 29993347 DOI: 10.1080/10245332.2018.1498182]
14 Pahore ZA, Shamsi TS, Taj M, Farzana T, Ansari SH, Nadeem M, Ahmad M, Naz A. JAK2V617F mutation in chronic myeloid leukemia predicts early disease progression.J Coll Physicians Surg Pak2011;21: 472-475 [PMID: 21798133]
 World Journal of Clinical Cases2019年9期
World Journal of Clinical Cases2019年9期
- World Journal of Clinical Cases的其它文章
- Crizotinib-induced acute fatal liver failure in an Asian ALK-positive lung adenocarcinoma patient with liver metastasis: A case report
- Rare variant of pancreaticobiliary maljunction associated with pancreas divisum in a child diagnosed and treated by endoscopic retrograde cholangiopancreatography: A case report
- Adult-onset mitochondrial encephalopathy in association with the MT-ND3 T10158C mutation exhibits unique characteristics: A case report
- Nerve coblation for treatment of trigeminal neuralgia: A case report
- Management of the late effects of disconnected pancreatic duct syndrome: A case report
- Sofosbuvir/Ribavirin therapy for patients experiencing failure of ombitasvir/paritaprevir/ritonavir + ribavirin therapy: Two cases report and review of literature
