在紫外和可見光下具有高催化活性的珊瑚狀金紅石型二氧化鈦的制備
朱 捷 葛奉娟 陳 艷 徐 艷 張學楊 鄒偉欣 董 林*,
(1徐州工程學院化學化工學院,徐州 221111)
(2徐州工程學院環境工程學院,徐州 221111)
(3介觀化學教育部重點實驗室,南京大學化學化工學院,南京 210093)
0 Introduction
With the development of modern industry,the depletion of fossil fuels and the environmental pollutions have become serious problems.The development of renewable and clean energies become one of the most emergent research subjects[1-2].Conversion of solar energy into hydrogen (H2)is considered asa perspective approach to solve these problems.Water splitting is an eco-friendly process that can be operated at ambient temperature and pressure[3-6].Since1972,Fujishima and Honda has reported photoassisted electrochemical water splitting on single-crystal titania and platinum electrodes[7],up to now,a large number of photocatalytic materials,with the enhanced light harvesting,photogenerated charge separation and transport,are designed for the photocatalytic hydrogen production,including binary metal oxides[8-14],complex metal oxides[15-18],metal sulfides[19-21]and metal-free materials[22-26].
TiO2has been widely used as a good photocatalyst in H2production from water splitting,due to its advantages of being cheap,stable,nontoxic and environmentally friendly.Generally,TiO2has four polymorphs,including anatase,rutile,brookite and TiO2(B).These four polymorphs are used in different fields on the basis of their different physical and chemical properties,but the most frequently studied TiO2based photocatalysts are anatase and rutile.Anatase,as it has higher surface area,more oxygen vacancies and lower conduction band (CB)potential,usually has better photocatalytic activity.However,anatase has poor visible light adsorption,which limits its application.It is reported that rutile has narrower band gap and faster charge carrier mobile,which make it a good candidate for photocatalytic reactions.There are still some shortcomings for rutile TiO2,such as lower surface area,less oxygen vacancies and faster e--h+recombination[27].Therefore,there are very few literatures that using pure rutile TiO2as photocatalysts,especially in hydrogen production field.Even for those anatase-rutile combined catalysts,the catalysts with best activity usually compose only a small part of rutile[28-32].Particularly,the commercial catalyst P25(Degussa)is composed of about 71%anatase and 29%rutile.On the other hand,the morphology of TiO2is an important factor for its photocatalytic activities.Some researchers prepared coral-like TiO2with anatase phase or anatase-rutile mixed phases which have higher discoloration rate of organic dye solution[33-34].However,the hydrogen generation by water splitting was seldom reported.Herein,we prepared coral-like rutile TiO2in diethylene glycol solution by solvothermal method.The sample has high surface area and outstanding photocatalytic activities on H2production from water splitting.
1 Experimental
1.1 Chem icals
Titanium ビ butoxide (TBOT,>99.0%),diethylene glycol(DG,>99.0%),chloroplatinic acid hexahydrate(H2PtCl6·6H2O,AR),triethanolamine (TEOA,98%)and commercial rutile TiO2(Rut-c for short,99.8%)were purchased from Aladdin.Hydrochloric acid(HCl,36%~38% (w/w))was purchased from Shanghai Lingfeng.P25 was purchased from Degussa Corporation.
1.2 Preparation of coral-like rutile TiO2
The coral-like rutile TiO2was prepared by solvothermal method in reference[35]with some modification.In a typical synthesis,2 mL TBOT was dissolved in 30 mL DG.After ultrasonication for 30 min,10 mL HCl(5 mol·L-1)was added dropwise.The mixed solution was ultrasonicated for further 30 min before transferred into a 50 mL Teflon-lined stainless-steel autoclave.The autoclave was heated to 150℃and maintained for 24 h.Then the autoclave was cooled down naturally to ambient temperature.The sample was centrifuged,washed several times by deionized water (DI)and ethanol and dried at 80℃overnight.The sample was denoted as Rut-dg.As comparison,the DG solution was replaced by ethanol or ethylene glycol,and the resulted samples were denoted as Rut-et and Rut-eg.
1.3 Characterization
Scanning electron microscopy (SEM)experiment was performed on a Philips XL30 electron microscope operated at beam energy of 10.0 kV.Transmission electron microscopy (TEM)images were taken on a JEM-2100 instrument at an acceleration voltage of 200 kV.The crystal structures were identified by X-ray diffraction (XRD)with Philips X′Pert Pro diffractometer by Ni-filtered Cu Kα radiation (λ=0.154 18 nm).The X-ray tube was operated at 40 kV and 40 mA.The scanning range was 10°~80°.Brunauer-Emmet-Teller (BET)surface area was measured by nitrogen adsorption-desorption at 77 K on Micrometrics ASAP-2020 adsorption apparatus.The FT-IR spectra were collected from 400 to 4 000 cm-1at the spectral resolution of 4 cm-1on Nicolet 5700 FT-IR spectrometer.UV-Vis diffuse reflectance spectroscopy(UVVis DRS)were recorded in the range of 200~800 nm by a Shimadzu UV-2401 spectrophotometer with the reference of BaSO4.
1.4 Hydrogen production test
The photocatalytic hydrogen evolution reaction was carried out in a top-irradiation vessel connected to a gas-closed glass system under an irradiation of 300 W Xe lamp with or without the 420 nm cut-off filter,to test the activity under visible or UV light.50 mg catalyst was dispersed in 100 mL aqueous solution(90 mL DI water and 10 mL TEOA.H2PtCl6aqueous solution was added which was in-situ reduced to Pt during the reaction (3%(w/w)Pt).The temperature of solution was kept around 6℃.The reaction system was sealed and evacuated for 30 min before irradiation.The amount of generated hydrogen was determined by a gas chromatograph.
2 Results and discussion
2.1 M orphology of Rut-dg
The morphology of Rut-dg was investigated by SEM.Fig.1(a,b)showed the spherical particles with uniform diameters at about 800~1 000 nm with corallike surface.The intersecting surface of the broken spheres (Fig.1c)exhibited the radical pore passages in it,and the branches could be estimated to have a length of~200 nm and a diameter of~10 nm.
The phase structure of the Rut-dg was characterized by XRD (Fig.1d).The sample showed typical diffraction peaks at about 27.40°,35.84°,41.22°and 54.28°.Compared with PDF No.78-2485,the peaks correspond with the (110),(101),(111)and (211)planes of rutile TiO2,respectively.Meanwhile,no peaks of anatase TiO2was observed,indicating that pure rutile structure was prepared by this method.According to Scherrer equation D=Kγ/(B cosθ),the mean particle size calculated though (110)plane diffraction peak was about 7.35 nm,where K is the Scherrer constant 0.89,B is the full width at half maximum (FWHM)at 2θ=27.4°, λ is the wavelength of X ray (0.154 18 nm).Combined with the SEM result,this size should be the diameter of the coral branches.
The detailed structure of the coral branches was examined by TEM in Fig.1(e,f).It can be clearly seen that the diameters of the branches were about 7~10 nm,which is consistent with the SEM and XRD results.
As comparison,the XRD and SEM of Rut-et and Rut-eg were shown in Fig.S1.The samples were still rutile phase,while “coral branch” structures disappeared.The possible reason may be the higher viscosity of DG compared with ethanol and EG.The ion diffusion and nucleation process are hindered in DG solution,which constrains the isotropic growth of the crystal,leading to the branch structures.
The N2adsorption/desorption isotherm of Rut-dg exhibited a typeⅣisotherm with a H2hysteresis loop,demonstrating the mesoporous structures (Fig.S2).The average pore size calculated by BJH method was about 10 nm.The BET surface area of Rut-dg was 228 m2·g-1,which was more than 7 times of Rut-c (32 m2·g-1),indicating the branch structures enhanced the surface area dramatically.
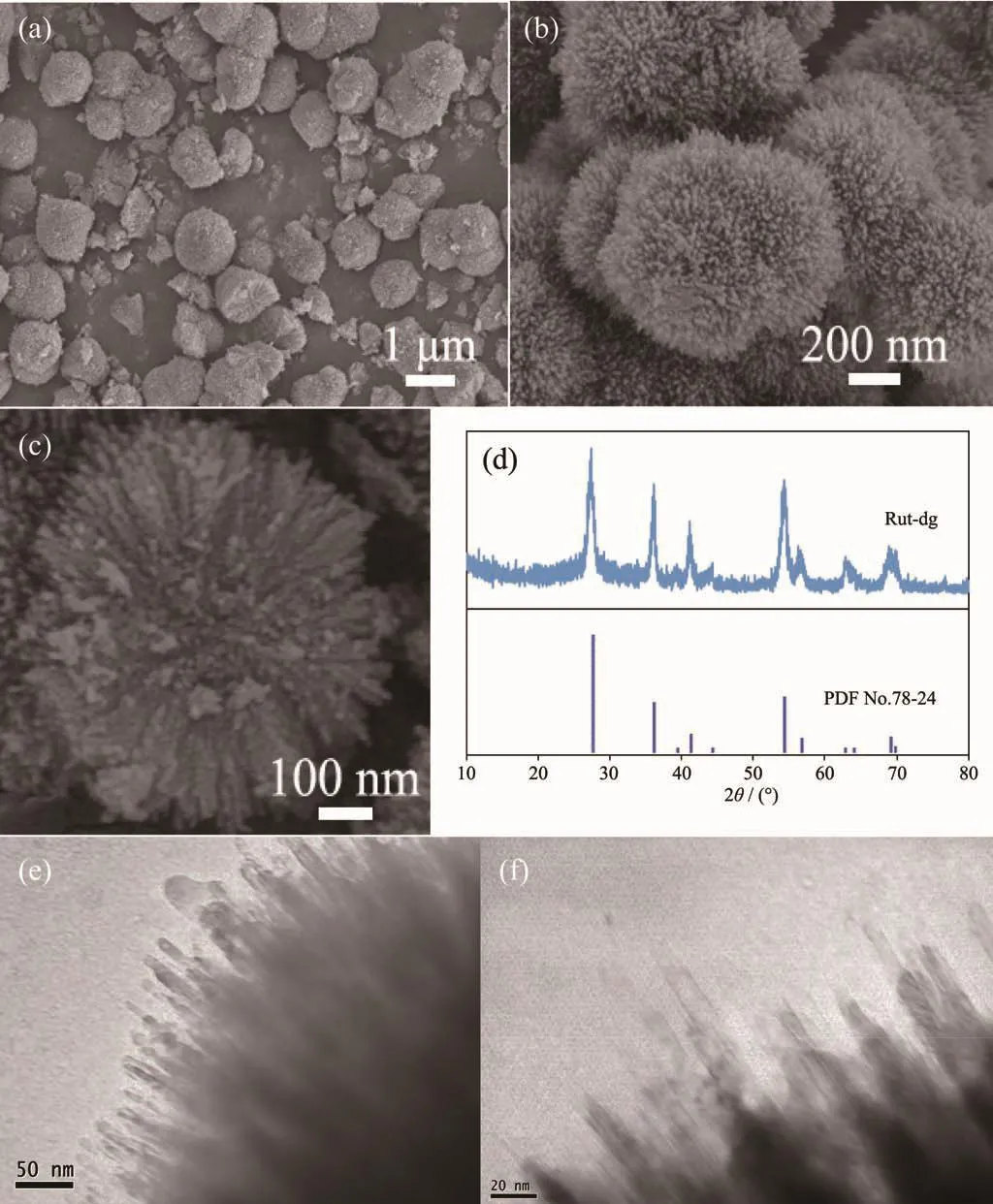
Fig.1 (a~c)SEM images (d),XRD pattern and (e~f)TEM images of Rut-dg samples
2.2 Photocatalytic hydrogen generation experiments
Fig.2 shows the hydrogen production of Rut-dg,Rut-c and P25 samples under UV (Fig.2a)or visible light irradiation (Fig.2b),with an aqueous solution containing 10%(V/V)TEOA as a scavenger and 3%(w/w)Pt as a cocatalyst.It can be seen that under UV irradiation,Rut-dg had much higher hydrogen production (about 25 000 μmol·g-1·h-1after 2 h irradiation),which was about 50%higher than commercial P25 and 13 times of Rut-c.Under visible light,only Rutdg had an obvious photocatalytic activity about 270 μmol·g-1·h-1,while no obvious activities for Rut-c and P25 samples could be detected.
2.3 UV-Vis diffuse reflectance spectroscopy
The optical absorption properties of different samples were investigated by UV-Vis DRS (Fig.3).It can be seen that P25 and Rut-c had no obvious adsorption under visible light (>420 nm).For Rut-dg,the tangent line intersected the wavelength axis at about 421 nm,the values for Rut-c and P25 were 415 and 409 nm,respectively.The maximum absorption wavelength of Rut-dg had only slight redshift.However,it should be noted that the adsorption intensity of Rut-dg decreased slowly with the increase of wavelength and still maintained at about 20%~40%of the highest value under visible light range (420~500 nm).Thus,Rut-dg has considerable activity under visible irradiation.
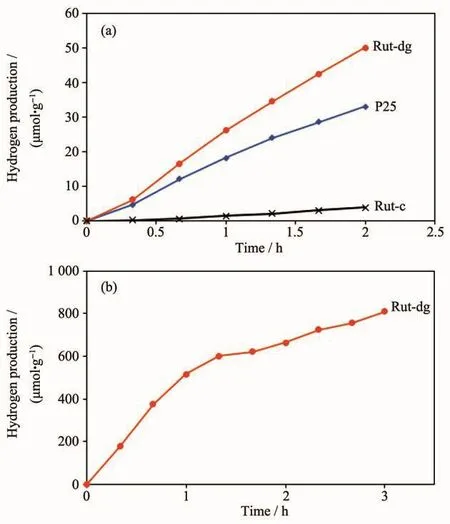
Fig.2 H2 production of(a)Rut-dg,P25 and Rut-c samples under UV irradiation and (b)Rut-dg sample under visible light
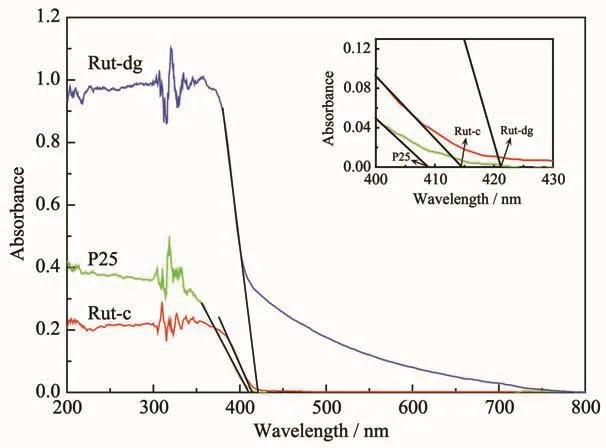
Fig.3 UV-Vis DRSof Rut-dg,P25 and Rut-c
2.4 FT-IR spectra analysis
Organic compounds,such as alcohols (e.g.,methanol,ethanol,isopropanol,etc.)and organic acids(formic acid,acetic acid,etc.),could be used as electron donors in photocatalytic process and promote the hydrogen evolution substantially.FT-IR spectra of Rut-dg and Rut-c were recorded to determine whether existed organics on the surface of the catalysts.As shown in Fig.4,thepeaksat~1 632 cm-1were commonly attributed to the stretching vibrations of C=C bonds or bending vibrations of H-O-H bonds.As no characteristic peaks of C-C,C-H or C-O bonds could be observed in the infrared spectrum,the peaks at 1 632 cm-1should be attributed to absorbed water on the surface of samples.The peaks at 3 379 cm-1should be O-H bending vibration of absorbed water.The FT-IR result showed that no detectable organics existed on the surface of the Rut-dg,which means that the better photocatalytic activity compared with Rut-c should be attributed to the special morphology of Rut-dg sample.It should be noted that the peaks intensity of Rut-dg was much higher than Rut-c.The possible reason should be Rut-dg has coral-like morphology and hydrophilic surface with a large number of hydroxyls on the surface,which leads to capillary condensation,thus more water molecules are absorbed on the surface of Rut-dg.
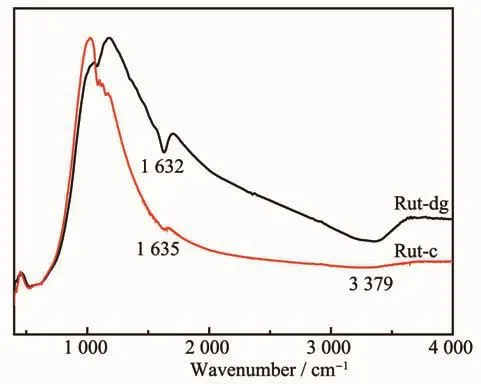
Fig.4 FT-IR of Rut-dg and Rut-c
2.5 Reaction mechanism analysis
The Rut-dg was calcined at 300℃for 1 h before photocatalytic test under UV irradiation.Obvious sintering of the branch structures could be observed in Fig.5a,meanwhile,the BET surface area decreased sharply to109 m2·g-1.From the N2adsorption-desorption isotherm,it could be clearly seen that the isotherm exhibited a typeⅡisotherm,indicating the collapse of the mesoporous structures (Fig.S2).Accordingly the hydrogen production decreased by 15%~25% (Fig.5b),further proving that the coral-like structure is the key factor for high activity.
The coral-like structure can increase the hydrogen production owing to the following potential reasons (Fig.6):
(1)As discussed in SEM and BET sections,Rutdg has coral-like structure with high surface area.Compared with Rut-c,the high surface area equates to more surface-active sites in unit volume.The surfaceactive sites are required for the adsorption of H2O,which is a key step in photoreactivity between TiO2and water[36].Meanwhile,the coral structures also facilitate the adsorption of water,as discussed above.
(2) Rut-dg coral-like surface with branches diameter was less than 10 nm.According to the literature,when the grain size of titania decreases below 15 nm,the photocatalytic performance will increase abruptly because of quantum size effect and faster charge transfer[37].
(3)The higher surface area of Rut-dg makes more water adsorption and photogenerated carriers excited than Rut-c under same irradiation.Furthermore,the coral structures reduce the transfer distance of photon-generated carriers dramatically and so facilitate the oriented movement and efficient separation of electrons and holes,increase the probability of their reaching the surface without e--h+recombination and enhance the activity remarkably[38].
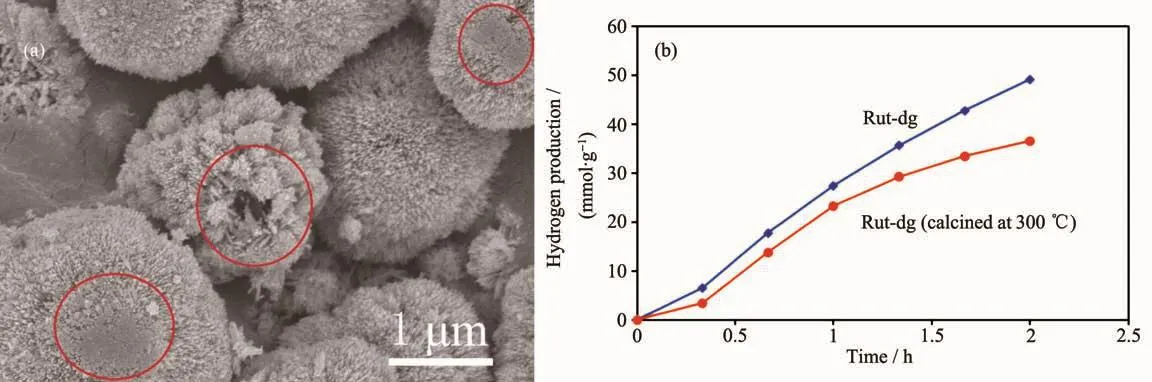
Fig.5 (a)SEM image of Rut-dg calcined at 300 ℃ and (b)hydrogen production under UV irradiation of Rut-dg samples before and after calcination
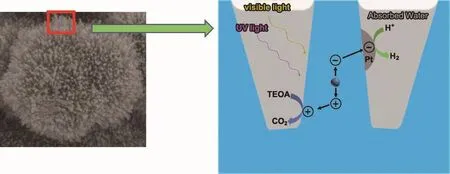
Fig.6 Schematic of the possible hydrogen evolution mechanism over Rut-dg catalyst
3 Conclusions
In summary,the coral-like rutile TiO2was synthesized by solvothermal method in DG solution.The coral branch structure increased the surface area of the sample substantially and facilitated the e--h+separation,which lead to its superior performance on photocatalytic water splitting under both UV and visible light.
Acknow ledgements:The financial support of the Key Research Project of Social Development of Xuzhou (Grant No.KC17154)is gratefully acknowledged.
Supporting information is availableat http://www.wjhxxb.cn

