由聯苯三羧酸和含氮配體構筑的鈷(Ⅱ)、鉛(Ⅱ)和鋅(Ⅱ)配合物的合成、晶體結構及熒光性質
黎 彧 鄒訓重 邱文達 莊文柳 趙 娜 成曉玲
(1廣東輕工職業技術學院, 佛山市特種功能性建筑材料及其綠色制備技術工程中心,廣州 510300)(2廣東工業大學輕工化工學院,廣州 510006)
0 Introduction
In recent years,the design and hydrothermal syntheses of metal-organic coordination polymers have attracted great interest in the field of coordination chemistry and organic chemistryowingtotheir intriguing architectures and topologies,as well as potential applications in catalysis,magnetism,luminescence and gas absorption[1-5].There are many factors,such as the coordination geometry of the metal centers,type and connectivity of organic ligands,stoichiometry,reaction conditions,template effect,presence of auxiliary ligands,and pH values influencing the structures of target coordination polymers during selfassembly[6-11].Among these factors,organic ligands play a very important role in constructing coordination polymers.
As we known,the multi-carboxylate biphenyl ligands have been certified to be of great significance as constructors due to their abundant coordination modes, which could satisfy different geometric requirements of metal centers[12-17].In order to extend our research in this field,we have selected biphenyl-2,4,4′-tricarboxylic acid(H3btc)asafunctional building block on account of the following considerations:(a)H3btc possesses three carboxyl groups that may be completely or partially deprotonated,depending on the pH value;(b)it is a flexible ligand allowing the rotation of two phenyl rings around the C-C single bond;(c)it can act as hydrogen-bond acceptor as well as donor,depending upon the degree of deprotonation.
Taking into consideration the above discussion,we herein report the syntheses,crystal structures,and luminescent properties of three Co(Ⅱ),Pb(Ⅱ) and Zinc(Ⅱ)complexes constructed from biphenyl tricarboxylic acid ligands.
1 Experimental
1.1 Reagents and physical measurements
All chemicals and solvents were of AR grade and used without further purification.Carbon,hydrogen and nitrogen were determined using an Elementar Vario EL elemental analyzer.IR spectra were recorded using KBr pellets and a Bruker EQUINOX 55 spectrometer.Thermogravimetric analysis(TGA)data were collected on a LINSEIS STA PT1600 thermal analyzer with a heating rate of 10℃·min-1.Excitation and emission spectra were recorded for the solid samples on an Edinburgh FLS920 fluorescence spectrometer at room temperature.
1.2 Synthesis of[Co(Hbtc)(phen)2(H 2O)]·3H 2O(1)
A mixture of CoCl2·6H2O (0.071 g,0.30 mmol),H3btc(0.086 g,0.30 mmol),phen(0.060 g,0.3 mmol),NaOH(0.024g,0.60mmol),and H2O(10 mL)wasstirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10 ℃·h-1.Yellow blockshaped crystals of 1 were isolated manually,and washed with distilled water.Yield:35% (based on H3btc).Anal.Calcd.for C39H32CoN4O10(%):C 60.39,H 4.16,N 7.22;Found(%):C 60.24,H 4.17,N 7.16.IR(KBr,cm-1):3 318w,2 921m,1 691w,1 546s,1 516m,1 417s,1 364m,1 335w,1 295w,1 218w,1 137w,1 102 w,1 050w,898w,869w,846m,770m,723s,676w,636w,548w.
1.3 Synthesis of[Pb(μ3-Hbtc)(2,2′-bipy)]n(2)
A mixture of PbCl2(0.083 g,0.30 mmol),H3btc(0.086 g,0.3 mmol),2,2′-bipy (0.047 g,0.3 mmol),NaOH (0.024 g,0.60 mmol),and H2O (10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10℃·h-1.Colorless block-shaped crystals of 2 were isolated manually,and washed with distilled water.Yield:60% (based on H3btc).Anal.Calcd.for C25H16PbN2O6(%):C 46.37,H 2.49,N 4.33;Found(%):C 46.51,H 3.51,N 4.30.IR(KBr,cm-1):3 656w,2 979w,1 679w,1 586s,1 540s,1 475w,1 417w,1 353s,1 312w,1 283w,1 178w,1 125 w,1 056w,1 003w,852w,822w,770m,693w,642w.
1.4 Synthesisof{[Zn3(μ2-btc)2(μ2-H 2O)(2,2′-bipy)3(H 2O)5]·8H 2O}n(3)
A mixture of ZnCl2(0.041 g,0.30 mmol),H3btc(0.057 g,0.2 mmol),2,2′-bipy (0.047 g,0.3 mmol),NaOH (0.024 g,0.60 mmol),and H2O (10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10℃·h-1.Colorless block-shaped crystals of 3 were isolated manually,and washed with distilled water.Yield:65%(based on H3btc).Anal.Calcd.for C60H64Zn3N6O26(%):C 48.65,H 4.35,N 5.67;Found(%):C 48.74,H 4.31,N 5.63.IR(KBr,cm-1):3 685w,2 927w,1 597m,1 574s,1 528m,1 475w,1 446m,1 376s,1 312w,1 248w,1 172w,1 097 w,1 061w,1 020w,932w,875w,822w,770m,735w,653w.The complexes are insoluble in water and common organic solvents,such as methanol,ethanol,acetone and DMF.
1.4 Structure determinations
Three single crystals with dimensions of 0.28 mm×0.21 mm×0.20 mm (1),0.27 mm×0.20 mm×0.18 mm(2),and 0.27 mm×0.24 mm×0.23 mm(3)were collected at 293(2)K on a Bruker SMART APEXⅡCCD diffractometer with Mo Kα radiation(λ=0.071 073 nm).The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-2014 program[18].All non-hydrogen atoms were refined anisotropically.All the hydrogen atoms were positioned geometrically and refined using a riding model.A summary of the crystallography data and structure refinements for 1~3 is given in Table 1.The selected bond lengths and angles for complexes 1~3 are listed in Table 2.Hydrogen bond parameters of complexes 1~3 are given in Table 3.
CCDC:1835223,1;1835224,2;1835225,3.
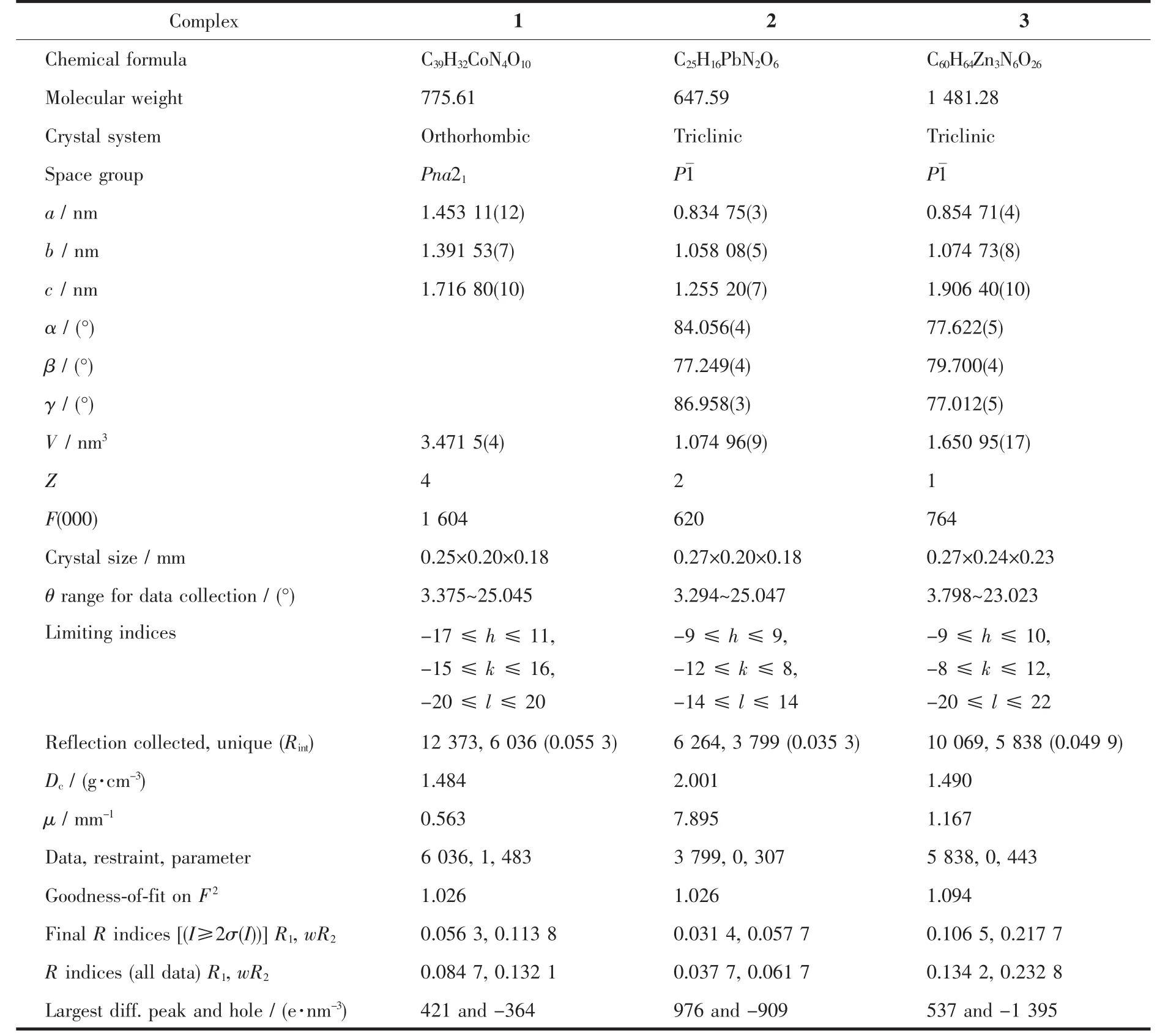
Table 1 Crystal data for complexes 1~3
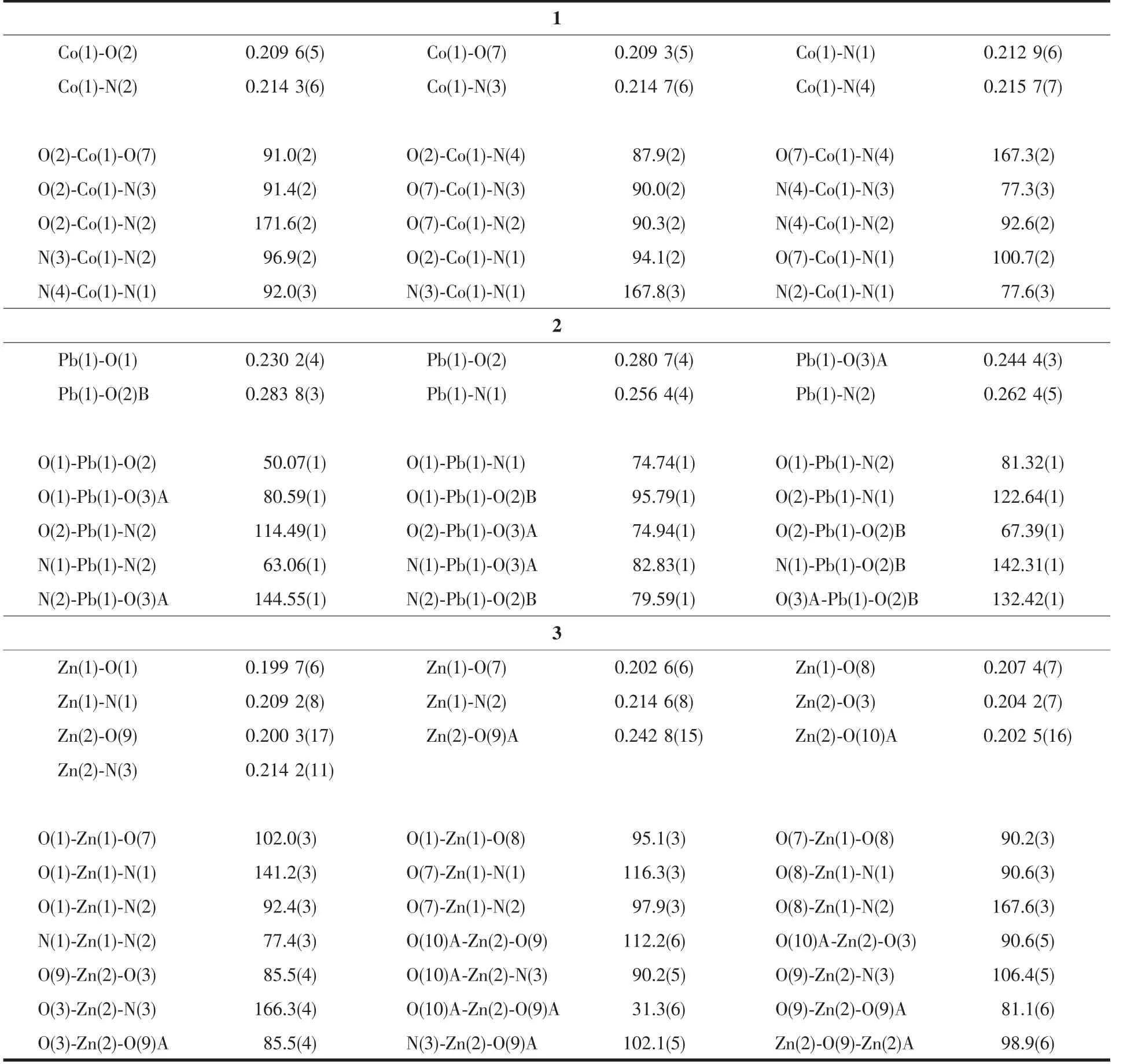
Table 2 Selected bond distances(nm)and bond angles(°)for complexes 1~3
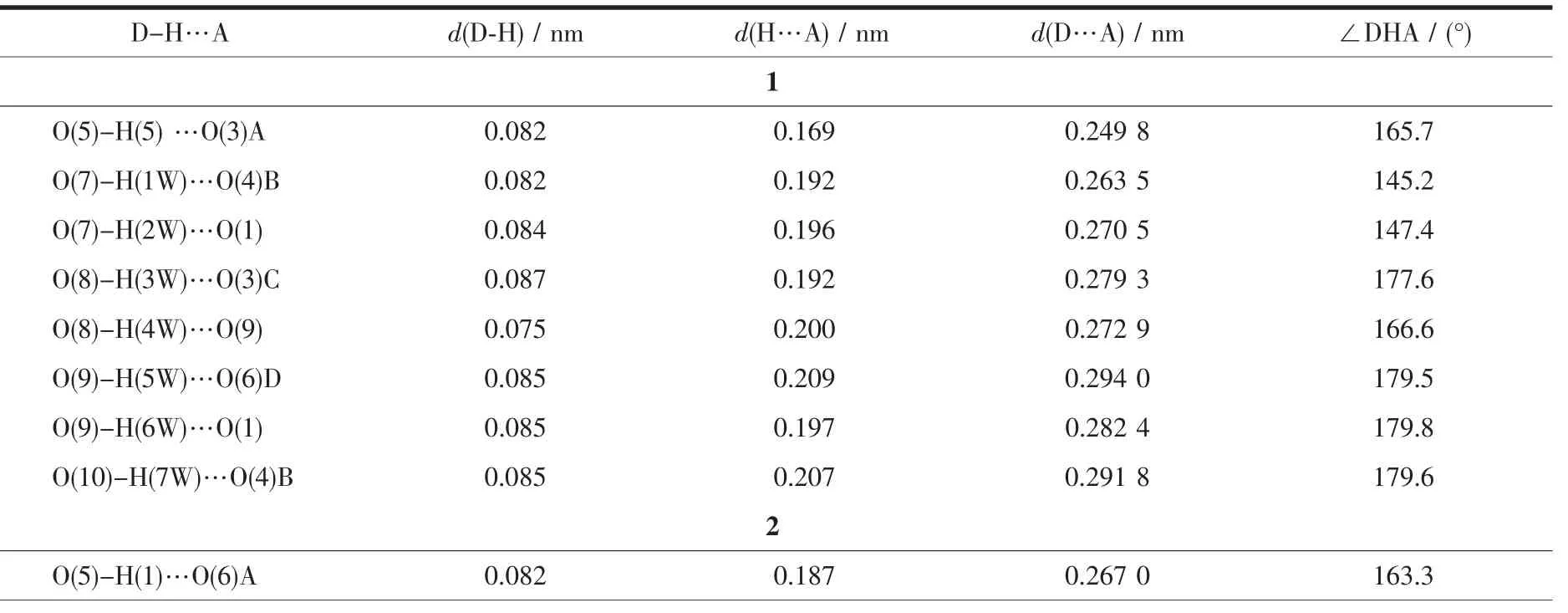
Table 3 Hydrogen bond parameters for complexes 1~3
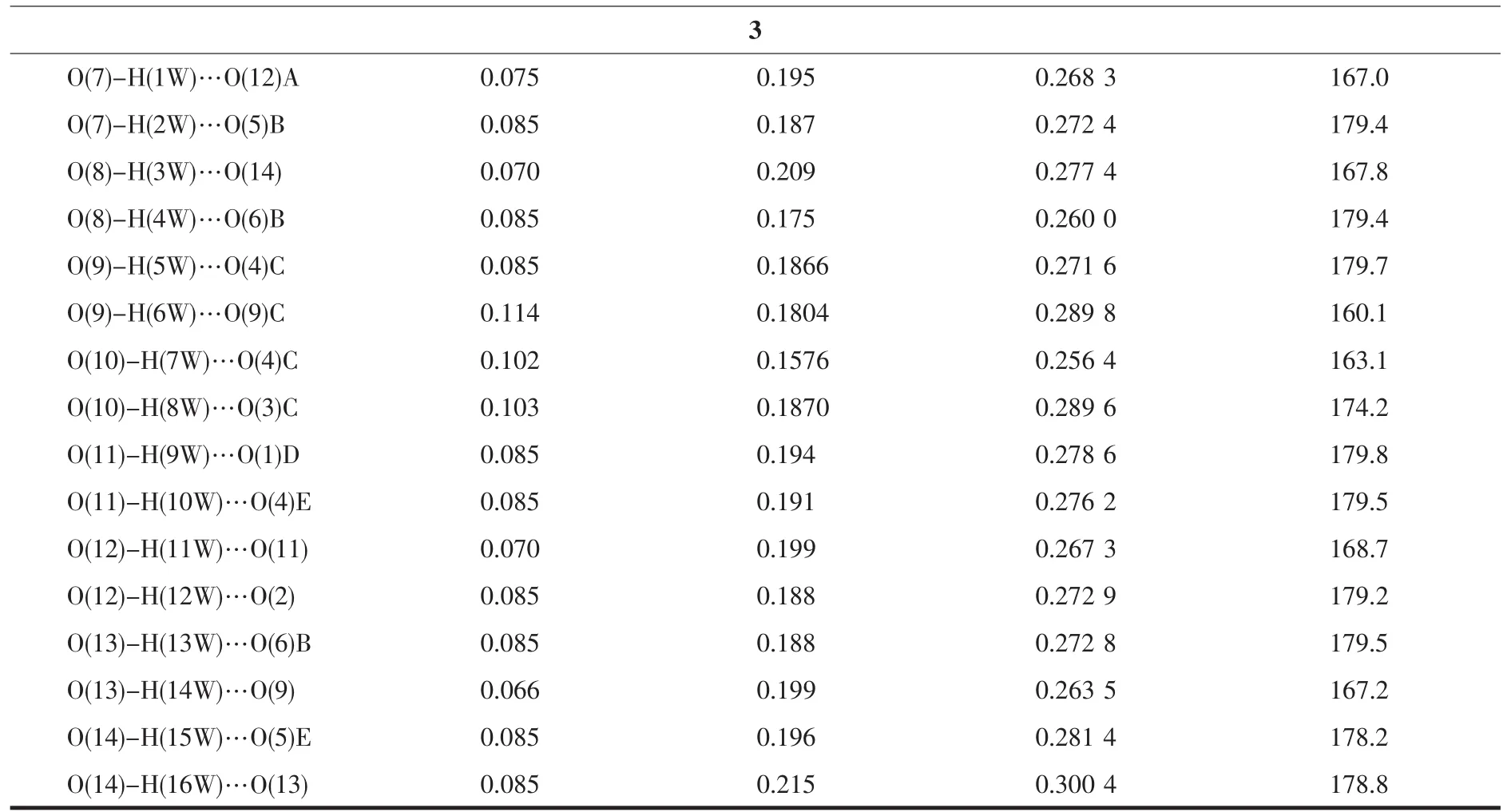
Symmetry codes:A:x,y-1,z;B:-x+1/2,y-1/2,z+1/2;C:x+1/2,-y+3/2,z;D:x+1/2,-y+1/2,z for 1;A:-x-1,-y+1,-z+2 for 2;A:x-1,y,z;B:x-1,y+1,z;C:-x,-y+2,-z+1;D:x+1,y,z;E:-x+1,-y+1,-z+1;F:x,y+1,z for 3.
2 Results and discussion
2.1 Description of the structure
2.1.1 [Co(Hbtc)(phen)2(H2O)]·3H2O(1)
Single-crystal X-ray diffraction analysis reveals that complex 1 crystallizes in the orthorhombic space group Pna21.This complex has a discrete monomeric structure with the asymmetric unit containing of one crystallographically unique Co(Ⅱ)ion,one Hbtc2-block,two phen moieties,one H2O ligand,and three lattice water molecules.As depicted in Fig.1,the sixcoordinated Co(Ⅱ)ion is surrounded by one carboxylate O atom from one Hbtc2-block,one O atom from H2O ligand,and four N atoms from two phen moieties,thus forming a distorted octahedral{CoO2N4}environment.The lengths of Co-O bonds range from 0.209 3(5)to 0.209 6(5)nm,whereas the Co-N distances vary from 0.212 9(6)to 0.215 7(7)nm;these bonding parameters are comparable to those found in other reported Co(Ⅱ)complexes[11-12,14].In 1,the Hbtc2-ligand adopts terminal coordination mode(modeⅠ,Scheme 1),in which the deprotonated carboxylate groups exhibit the monodentate or uncoordinated modes.The dihedral angle between two phenyl rings in the Hbtc2-is 56.32°.The discrete units of 1 are connected by means of O-H…O hydrogen bond to give rise to a 3D supramolecular framework(Fig.2 and Table 3).
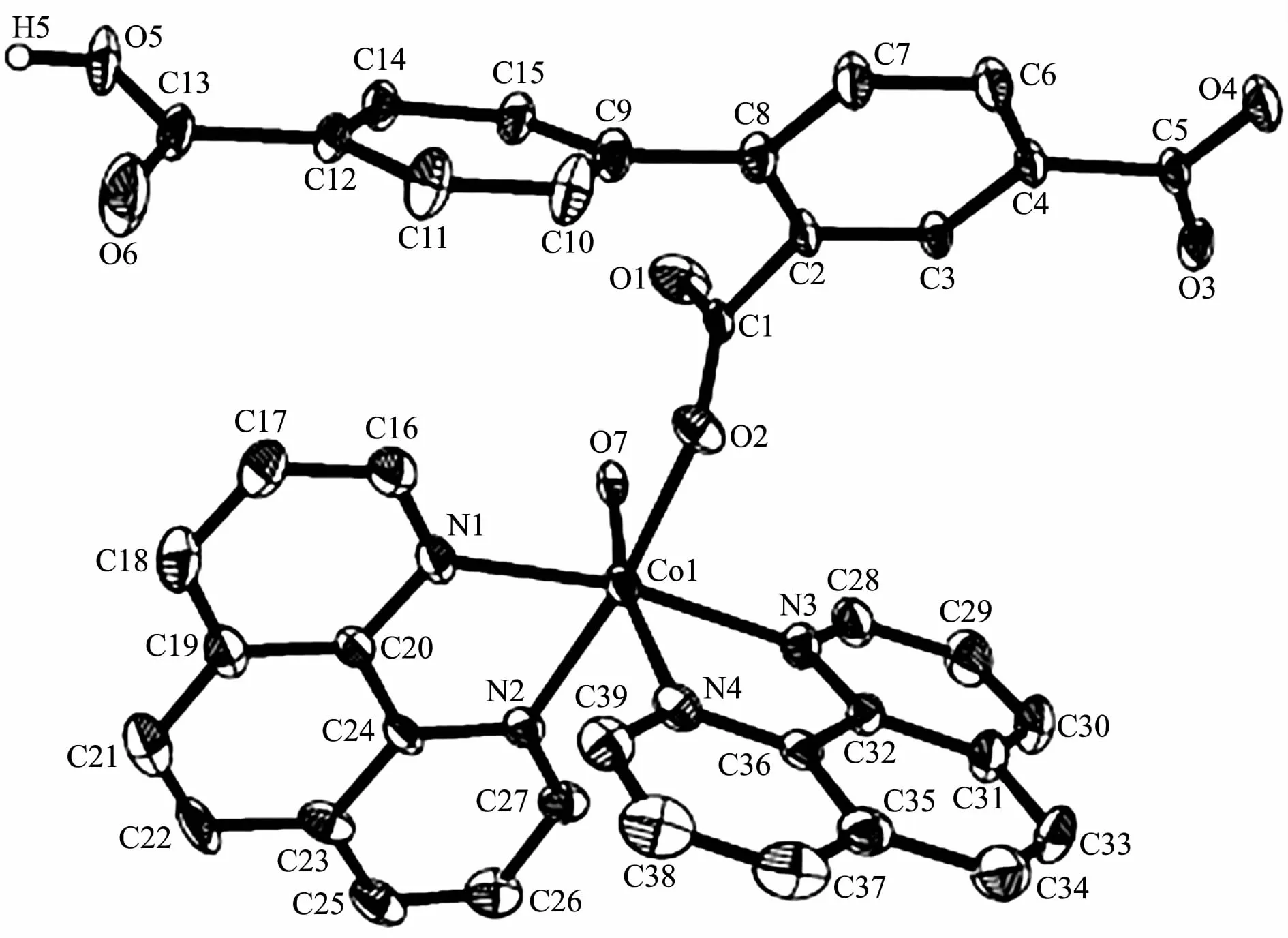
Fig.1 Drawing of the asymmetric unit of complex 1 with 30%probability thermal ellipsoids
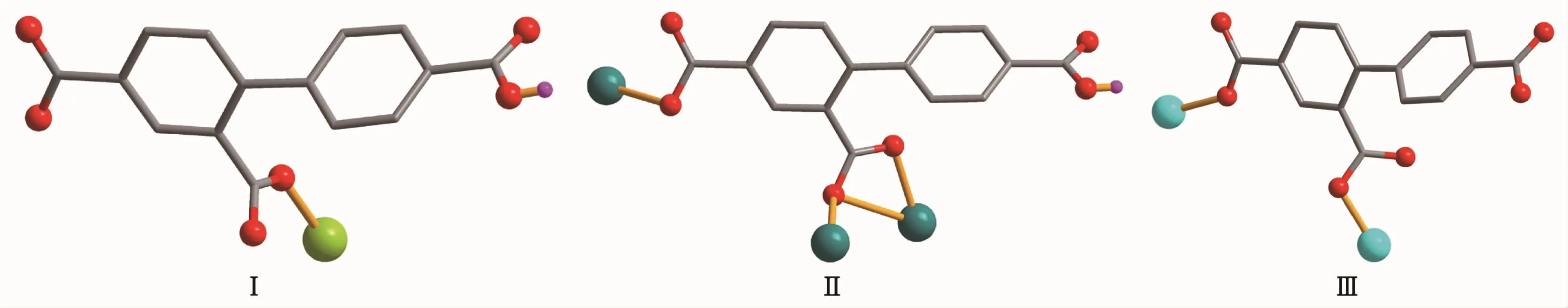
Scheme 1 Coordination modes of Hbtc2-/btc3-ligands in complexes 1~3
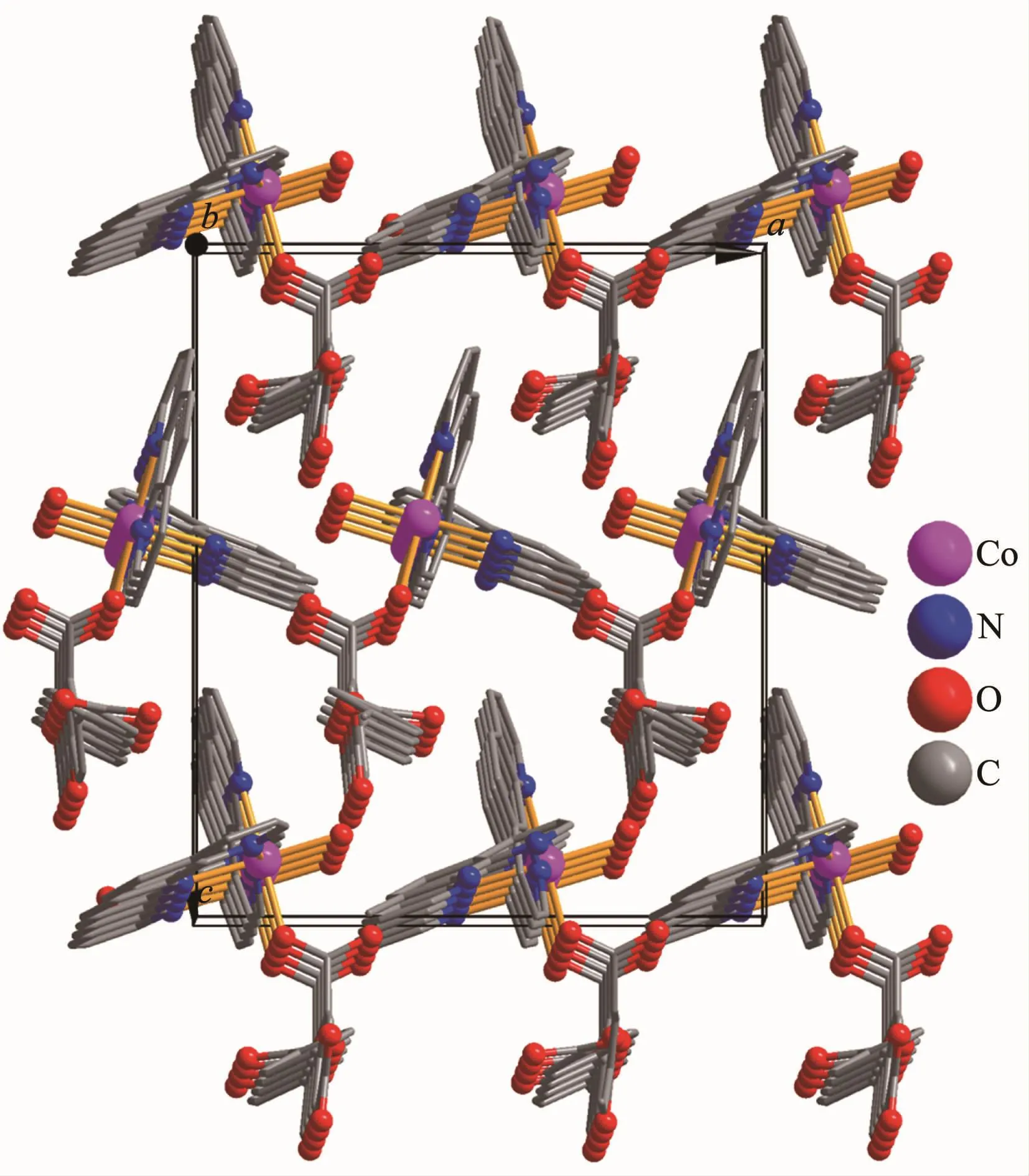
Fig.2 Perspective of 3D supramolecular framework parallel to the ac plane in 1
Complex 2 shows a 1D chain structure.The asymmetric unit of 2 consists of one crystallographically distinct Pb(Ⅱ) ion,one μ3-Hbtc2-block,and one 2,2′-bipy ligand.As shown in Fig.3,six-coordinate Pb1 center displays a distorted octahedral {PbO4N2}environment,filled by four carboxylate O atoms from three individualμ3-Hbtc2-blocks and two N atoms from one 2,2′-bipy ligand.The Pb-O distances range from 0.230 2(4)to 0.283 8(3)nm,whereas the Pb-N distances vary from 0.256 4(4)to 0.262 4(5)nm;these bonding parameters agree with those observed in other Pb(Ⅱ) complexes[12,14].In 2,the Hbtc2-block acts as a μ3-spacer and its deprotonated COO-groups take a monodentate or tridentate modes (modeⅡ,Scheme 1).In Hbtc2-,the dihedral angle between two benzene ringsis57.62°.The carboxylate groupsof Hbtc2-blocks link neighboring Pb(Ⅱ)ions to give a Pb2unit(Fig.4)with the Pb…Pb separation of 0.469 7(4)nm.Neighboring Pb2subunits are additionally interlinked by the Hbtc2-spacers to give a 1D chain(Fig.4).
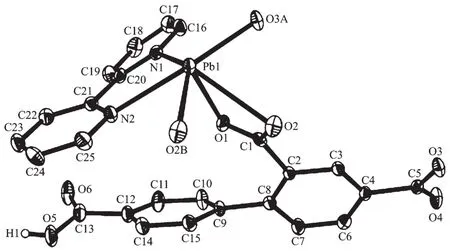
Fig.3 Drawing of the asymmetric unit of complex 2 with 30%probability thermal ellipsoids
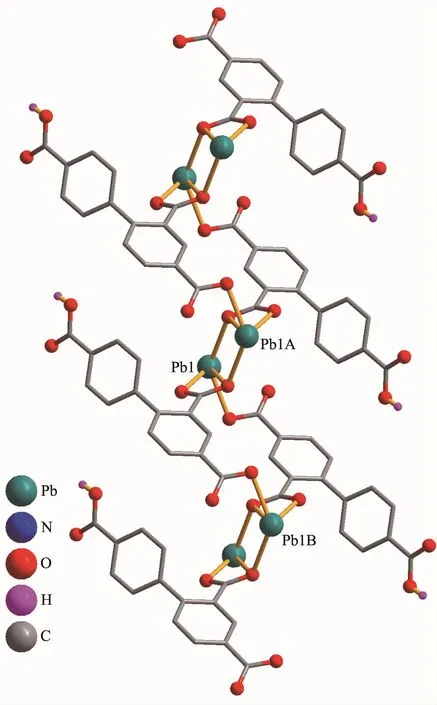
Fig.4 One-dimensional chain in complex 2
Complex 3 also exhibits a 1D chain structure.The asymmetric unit of 3 bears two crystallographically unique Zn(Ⅱ) ions (Zn1 with full occupancy;Zn2 with half occupancy),oneμ2-btc3-block,a half of oneμ2-H2O,two and a half of terminal coordinated water molecules,one and a half of 2,2′-bipy ligands,and four lattice water molecules.As shown in Fig.5,both Zn1 and Zn2 centers are fivecoordinated and feature distorted trigonal bipyramidal{ZnN2O3}or{ZnNO4}environments,respectively.Zn1 atom is surrounded by one carboxylate O atom from oneμ2-btc3-block,two O atoms from two H2O ligands,and two N atoms from one 2,2′-bipy moiety.The Zn2 atom is bound by one carboxylate O atom from one btc3-moiety,three O atoms from three water ligands,and one N atom from one 2,2′-bipy ligand.The Zn-O(0.199 7(6)~0.242 8(15)nm)and Zn-N(0.209 2(8)~0.214 6(8)nm)bond distances are in agreement with those reported for related zinc(Ⅱ) complexes[11,14,19].In 3,the btc3-block behaves as aμ2-spacer and its COO-groups adopt a monodentate or uncoordinated mode(modeⅢ,Scheme1).In btc3-,adihedral angle(between two aromatic rings)is 42.84°.Two O atoms from two water ligands bridge adjacent Zn2 atoms to form a dinuclear zinc unit(Fig.6)having a Zn…Zn distance of 0.338 1(7)nm.The Zn2units are linked by the 2,2′-bipy ligands to form a 1D chain(Fig.6).
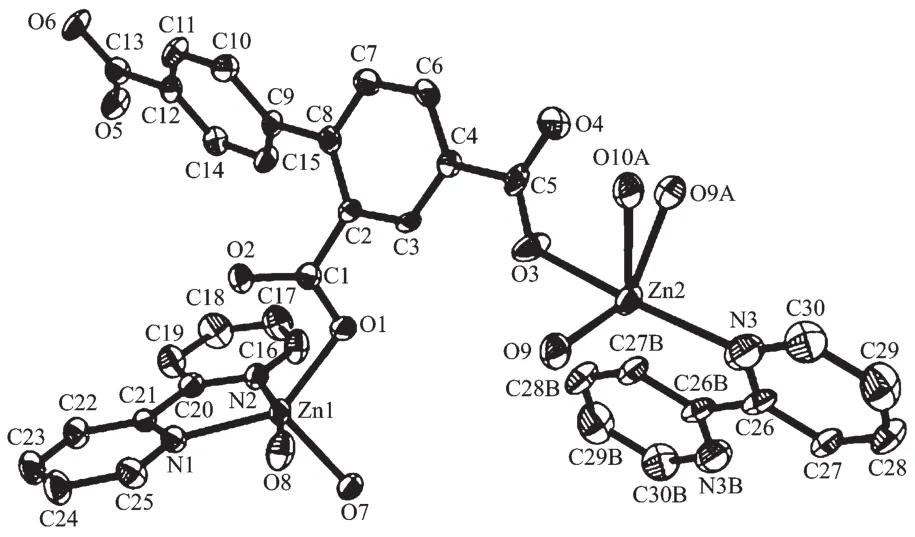
Fig.5 Drawing of the asymmetric unit of complex 3 with 30%probability thermal ellipsoids
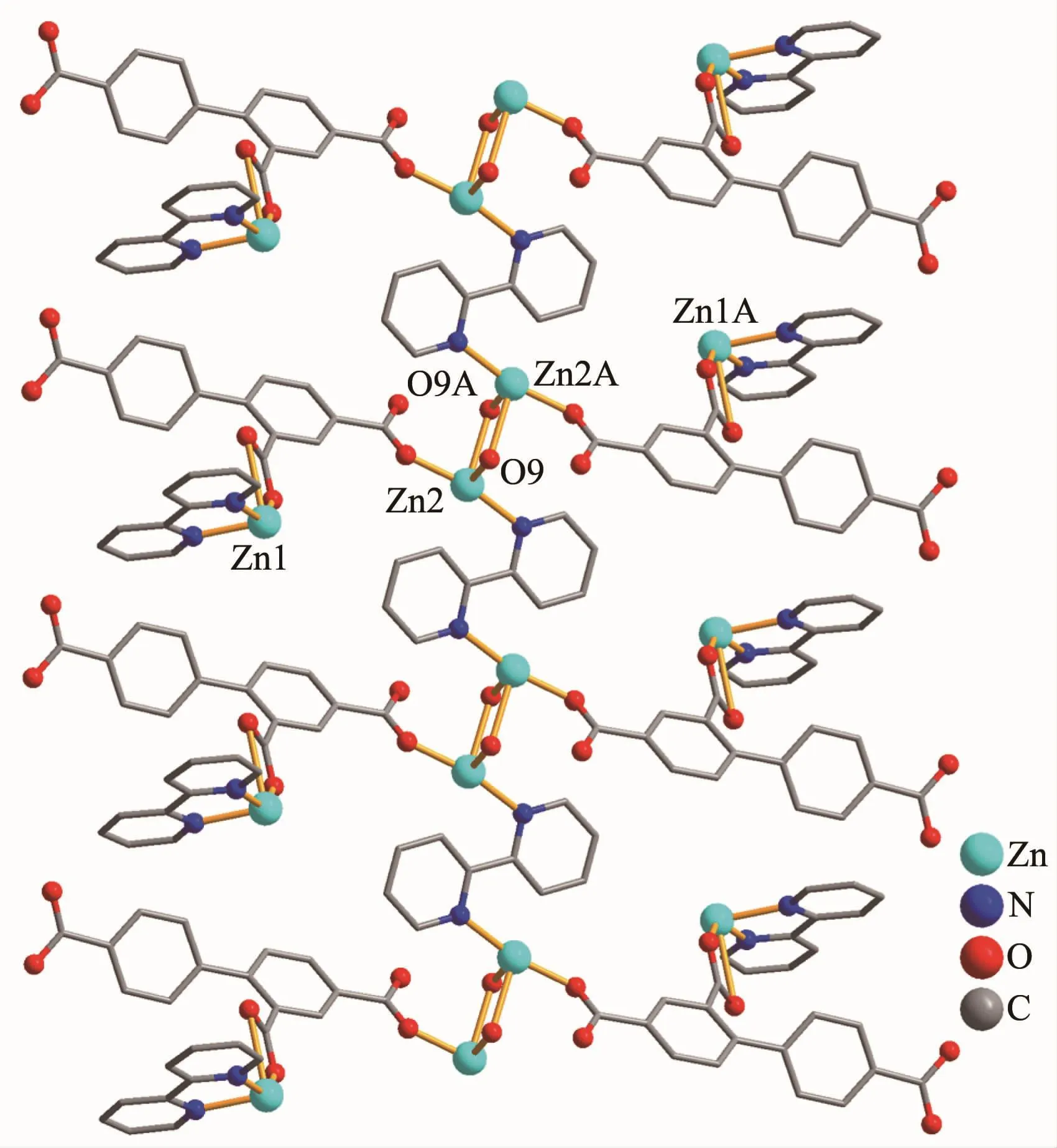
Fig.6 One-dimensional chain in complex 3
2.2 TGA analysis
To determine the thermal stability of complexes 1~3,their thermal behaviors were investigated under nitrogen atmosphere by thermogravimetric analysis(TGA).As shown in Fig.7,complex 1 lost its three lattice water molecules and one H2O ligand in the range of 59~291 ℃ (Obsd.8.9%;Calcd.9.3%),followed by the decomposition at 371℃.For complex 2,the TGA curve reveals that the sample is stable up to 339℃,followed by a decomposition on further heating.The TGA curve of 3 shows that eight lattice and six coordinated water molecules are released between 19~192 ℃ (Obsd.16.8%;Calcd.17.0%),and the dehydrated solid begins to decompose at 232℃.
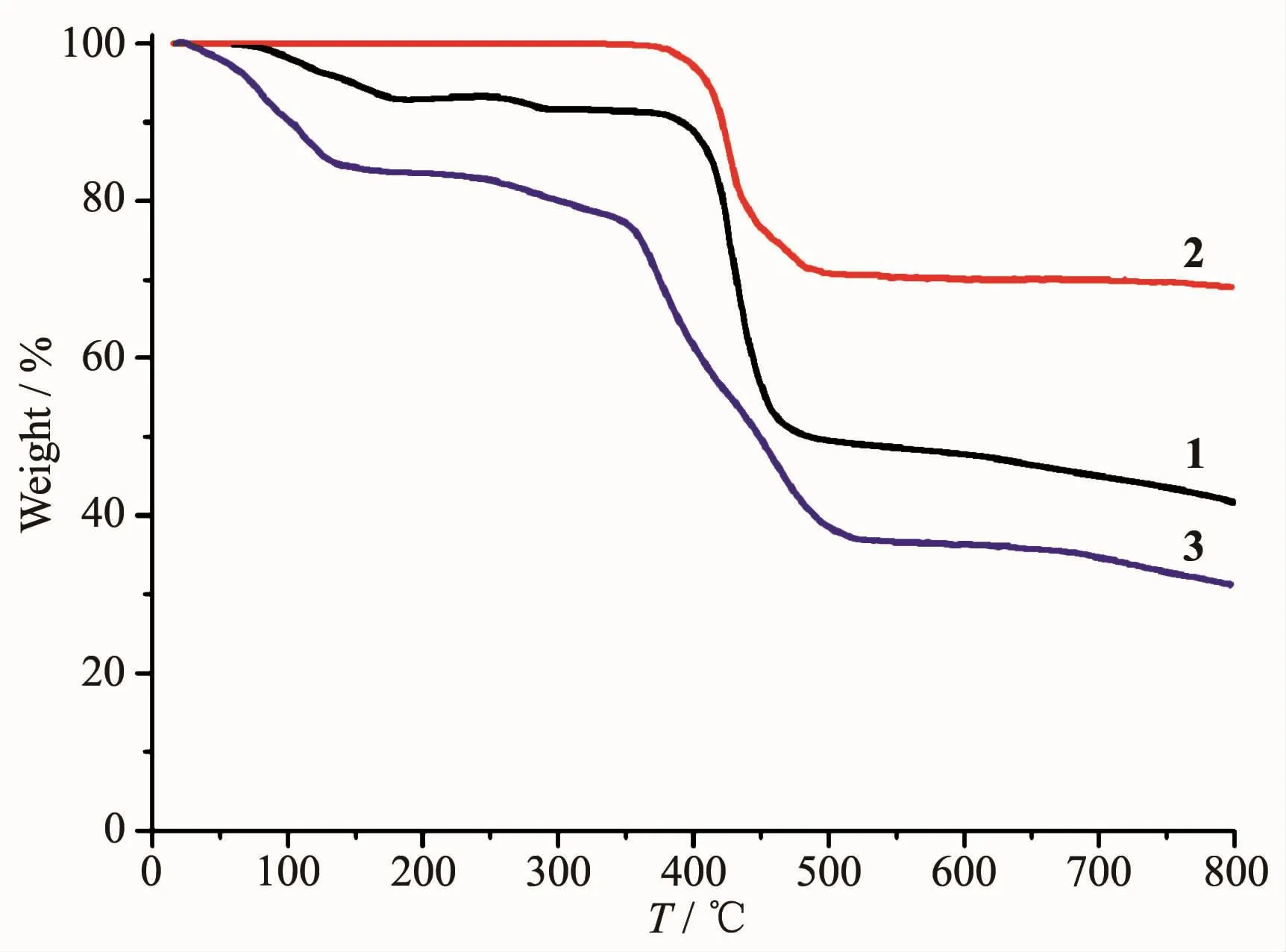
Fig.7 TGA curves of complexes 1~3
2.3 Luminescence properties
The emission spectra of H3btc and complexes 2 and 3 were measured in the solid state at room temperature,as depicted in Fig.8.The free H3btc ligand displays a weak photoluminescence with an emission maximum at 372 nm.For complexes 2 and 3,the more intense emission bands were observed with maximum at 369 nm for 2 and 415 nm(λex=300 nm in all cases)for 3.All bands can be assigned to an intraligand (π*→n or π*→π)emission[10,12,14].The enhancement of luminescence of complexes 2 and 3 can be attributed to the binding of ligands to the metal centers,which effectively increases the rigidity of the ligand and reduces the loss of energy by radiationless decay[10-11,13].
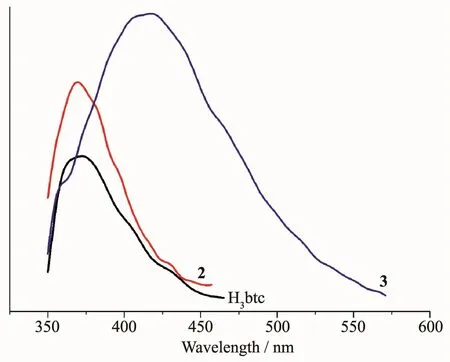
Fig.8 Solid state emission spectra of H3btc and complexes 2 and 3
3 Conclusions
In summary,three new complexes,namely[Co(Hbtc)(phen)2(H2O)]·3H2O(1), [Pb(μ3-Hbtc)(2,2′-bipy)]n(2),and{[Zn3(μ2-btc)2(μ2-H2O)(2,2′-bipy)3(H2O)5]·8H2O}n(3),have been synthesized under hydrothermal conditions.The complexes feature the 0D mononuclear and 1D chain structures,respectively.Luminescent properties of complexes 2 and 3 have also been studied.For complexes 2 and 3,the more intense emission bands were observed.

