射頻消融術后陣發性房顫患者心率減速力對復發的影響及臨床意義
張蕓蕓 周建國
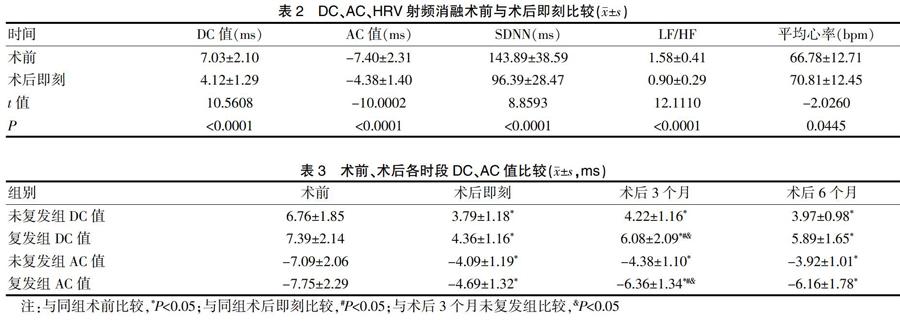
[摘要] 目的 探討射頻消融術后陣發性房顫患者心率減速力(DC)對復發的影響及臨床意義。 方法 選取2013年1月~2016年11月在心內科行左房環肺靜脈消融術(CPVI)治療陣發性房顫(PAF)的患者80例,分別在術前、術后3 d、3個月和6個月定期隨訪,行24 h動態心電圖檢查,根據術后3個月的隨訪結果將患者分為未復發組(56例)及復發組(24例)。記錄各時期復發組與未復發組患者的心率減速力、心率加速力(AC)和心率變異(HRV),并比較分析。 結果 (1)術后即刻患者與術前比較HRV、DC值、AC值絕對值均明顯降低(P<0.01);(2)與術前相比,無論復發組還是未復發組術后各時段DC值均下降(P<0.05),而在復發組,與術后即刻相比,術后3個月DC值升高(P<0.05)。未復發組,術后各時段DC值保持在較低水平,差異無統計學意義;(3)DC>4.5 ms患者的復發率53.5%(15/28)高于DC≤4.5 ms的17.3%(9/52),差異有統計學意義(P<0.01)。 結論 CPVI術后即刻未復發組與復發組DC值均降低,提示迷走神經功能降低;術后3~6個月復發組迷走神經功能恢復較快,未復發組迷走神經保持在較低水平;術后3~6個月DC值>4.5 ms患者復發率增高。
[關鍵詞] 陣發性心房顫動;射頻消融術;心率減速力;心臟自主神經
[中圖分類號] R541.75? ? ? ? ? [文獻標識碼] B? ? ? ? ? [文章編號] 1673-9701(2019)35-0011-04
Effect of heart rate deceleration on recurrence in patients with paroxysmal atrial fibrillation after radiofrequency ablation and its clinical significance
ZHANG Yunyun1? ?ZHOU Jianguo2
1.Department of Electrocardiogram, Lianyungang Hospital Affiliated to Nanjing University of Chinese Medicine, Lianyungang? ?222004, China; 2.Department of Radiology, Lianyungang Hospital Affiliated to Nanjing University of Chinese Medicine, Lianyungang? ?222004, China
[Abstract] Objective To investigate the effect of heart rate deceleration (DC) on recurrence in patients with paroxysmal atrial fibrillation after radiofrequency ablation and its clinical significance. Methods Eighty patients with paroxysmal atrial fibrillation (PAF) who underwent left atrial circumferential pulmonary vein ablation (CPVI) in the Department of Cardiology from January 2013 to November 2016 were enrolled. Regular follow-ups were conducted before surgery and 3 days, 3 months and 6 months after surgery. During the follow-ups, 24 h dynamic electrocardiography was performed. Patients were divided into the non-recurrent group (56 patients) and the recurrent group (24 patients) according to the follow-up results of 3 months after surgery. Heart rate deceleration, heart rate acceleration (AC) and heart rate variability (HRV) were recorded and compared between the recurrent and non-recurrent groups in each period. Results (1) The absolute values of HRV, DC and AC were significantly lower in patients immediately after surgery than those in patients before surgery (P<0.01). (2) The DC values after surgery were decreased in both the recurrent group and the non-recurrent group, compared with those before surgery (P<0.05). In the recurrent group, the DC values 3 months after surgery were higher than those immediately after surgery (P<0.05). In the non-recurrent group, the DC values remained at a lower level at each period after surgery, with no statistically significant differences. (3) The recurrence rate of patients with DC>4.5 ms was higher than that of patients with DC≤4.5 ms, which was 53.5% (15/28) and 17.3% (9/52), respectively. The difference was statistically significant (P<0.01). Conclusion Immediately after CPVI, the DC values of both the non-recurrent group and the recurrent group decreased, suggesting a decrease of vagus nerve function. The vagus nerve function recovers rapidly in the recurrent group 3~6 months after surgery. The vagus nerve function remained at a lower level in the non-recurrent group. The recurrence rate of patients with DC>4.5 ms increases 3~6 months after surgery.
[Key words] Paroxysmal atrial fibrillation; Radiofrequency ablation; Heart rate deceleration; Cardiac autonomic nerve
心房顫動(Atrial fibrillation,AF)是心律失常的常見病之一[1-2],目前臨床治療方法主要包括控制心室率、竇性心律以及導管射頻消融。左房環肺靜脈射頻消融術(Circumferential pulmonary vein isolation,CPVI)是目前AF消融的主流術式之一[3-4],CPVI術消融部位為肺靜脈-左房(PV-LA)相接處,重合于心房神經集中的地方,這種手術方式可能對心臟自主神經系統產生影響[5]。心臟的自主神經有迷走神經與交感神經,心率減速力(DC)及心率加速力(AC)能夠分別定量迷走與交感神經的張力。既往研究采用反映心臟自主神經的心率變異(HRV)等指標,其方法無法定量分析[6]。而用DC值研究陣發性房顫(Paroxysmal atrial fibrillation,PAF)患者射頻消融術后自主神經變化的文獻較少,本研究通過比較行CPVI術治療PAF患者術前及術后各時期復發與未復發患者的DC值變化,探討射頻消融術后陣發性房顫患者心率減速力對復發的影響及臨床意義。
1 資料與方法
1.1 一般資料
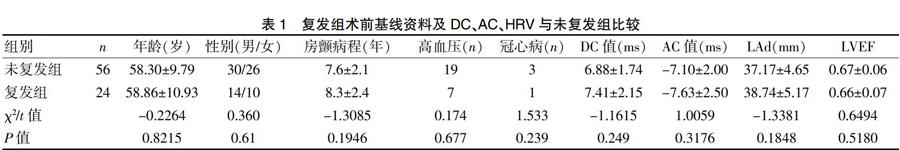
選取2013年1月~2016年11月于心內科行射頻消融術治療陣發性房顫的患者,納入標準:(1)年齡18~80歲;(2)符合2014年歐洲心臟病學會陣發性房顫診斷標準[7];(3)非結構性陣發性房顫且經兩種以上抗心律失常藥物治療后效果欠佳。排除標準:(1)安裝心臟起搏器者;(2)6個月內有心肌梗死或開胸手術史者;(3)甲狀腺機能障礙者。入組患者80例,其中男44例、女36例,平均(58.07±11.28)歲。根據隨訪患者消融術后3個月的結果將患者分為未復發組(56例)及復發組(24例)。兩組患者的年齡、性別、房顫病程、合并基礎疾病(高血壓、冠心病)、DC值、AC值、心超指標比較,差異均無統計學意義(P>0.05),具有可比性。見表1。本研究均經患者及家屬知情同意,院倫理委員會批準通過。
1.2 術前準備
所有患者的抗心律失常藥物均在術前停用,行24 h動態心電圖及超聲心動圖檢查。肺靜脈和左心房的結構經心臟CT掃描確定,除外左心耳及左房血栓,在術前48 h內行食道超聲心動圖檢查。
1.3 術后處理及隨訪
患者術后給予達比加群抗凝,可達龍維持竇性心律,質子泵抑制劑奧美拉唑20 mg qd抑酸,如有不適反應立即記錄常規心電圖。手術3 d后做動態心電圖檢查。于術后3個月及6個月評估手術療效及有無并發癥,定期復查心臟超聲、24 h動態心電圖及常規心電圖。
1.4 DC及HRV指標
在術前、術后3天、3個月和6個月全部患者均行動態心電圖檢查,通過動態心電分析系統把記錄回放,去除干擾、偽差,計算DC值、AC值和HRV值[8]。HRV時域指標為SDNN、rMSSD,頻域指標為低頻成分和高頻成分的比值(LF/HF)。
1.5 術后房顫復發定義
房顫射頻消融術后3個月為手術損傷期,故本研究將術后3個月患者出現心動過速的癥狀,或24 h動態心電圖中出現房顫、房撲或房速超過30 s作為術后復發。
1.6 統計學方法
采用SPSS22.0統計學軟件包進行數據統計分析,計量資料經檢驗符合正態發布,以均數±標準差(x±s)表示,采用兩獨立樣本t檢驗;ANOVA方差分析用于多組間比較;采用乘積極限法(Kaplan-Meier法)分析DC值對術后復發率的影響。P<0.05為差異有統計學意義。
2 結果
2.1 術后即刻DC、AC及HRV與射頻消融術前比較
80例患者中有24例于術后3個月出現復發,比率為30%。患者術后即刻與術前比較DC值、AC絕對值及HRV均顯著降低(P<0.01),平均心率升高(P<0.01)。見表2。
2.2 復發組術前、術后各時段DC值和AC值與未復發組比較
與術后3個月未復發組相比,復發組DC值和AC絕對值升高(P<0.05);與術前相比,無論復發組還是未復發組術后各時段DC值和AC絕對值均降低(P<0.05)。而在復發組,與術后即刻相比,術后3個月DC值和AC絕對值升高(P<0.05)。在未復發組,術后各時段DC值和AC絕對值保持在較低水平,差異無統計學意義(P>0.05)。見表3。
2.3 不同DC值對術后復發率的影響
DC≤4.5 ms提示迷走神經張力降低,將術后3~6個月患者分為DC≤4.5 ms者和DC>4.5 ms者。根據Kaplan-Meier復發分析結果顯示:DC>4.5 ms患者的復發率為53.5%(15/28),明顯高于DC≤4.5 ms的17.3%(9/52),差異有統計學意義(P<0.05)。
3 討論
AF的發生與心臟自主神經有關[9],患者一旦發生AF,心房的組織重構可促使其自身的維持和復發。AF臨床治療方法較多,導管消融治療的主要機制是破壞房顫的啟動基質及自主神經對心臟的調控,通過電隔離肺靜脈使心房達到去迷走效應。因此,迷走神經的損傷有利于提高肺靜脈誘導房顫消融的療效[10]。
目前,通過DC值評價迷走神經的功能備受關注,既往研究表明DC值可不受外界因素影響,實現對自主神經作用的直接檢測[11]。Patterson等[12]研究認為交感與副交感神經遞質可共同導致肺靜脈的快速放電,消融術中尚無法單獨去除迷走神經支配。雖然DC與AC都能定量測定迷走神經及交感神經,但DC測定結果與臨床循證醫學的結果更為符合[13],并且迷走神經張力的改變能促使房顫復發,即使刺激正常心臟的迷走神經也能使心房有效不應期縮短[14],故術后迷走神經張力的變化推薦應用DC值進行分析。Bauer等[15]通過臨床研究隨訪心肌梗死患者,提議以2.5 ms和4.5 ms作為分界線,分為低危值、中危值及高危值。DC≤4.5 ms提示迷走神經張力降低,本研究將術后3~6個月患者分為DC≤4.5 ms者和DC>4.5 ms者。與術前相比,術后即刻無論復發組還是未復發組患者DC值、AC絕對值均降低,提示術后迷走-交感神經的調節功能均下降。發現術后3個月及6個月的患者未復發者與復發者DC值均呈上升趨勢;在復發組,與術后即刻相比,術后3個月DC值升高(P<0.05);而在未復發組,術后各時段DC值保持在較低水平,差異無統計學意義(P>0.05),表明由肺靜脈誘導的房顫消融術后的療效可能與迷走神經持續損傷有關;與術后3~6個月DC≤4.5 ms患者相比,DC>4.5 ms患者的復發率較高。既往研究表明術后迷走神經損傷不是永久性的,存在自我修復功能[16],本研究結果與其類似。
綜上所述,CPVI術后即刻未復發組與復發組DC值均降低,提示迷走神經功能降低;術后3~6個月復發組迷走神經功能恢復較快,未復發組迷走神經保持在較低水平;術后3~6個月DC值>4.5 ms患者復發率增高。本研究樣本量不足,可能使統計學結果產生偏倚,有待加大樣本量進一步深入研究。
[參考文獻]
[1] Rademacher W,Seeck A,Surber R,et al. Multidimensional ECG-based analysis of cardiac autonomic regulation predicts early AF recurrence after electrical cardioversion[J]. J Electrocardiol,2016,45(2):116-122.
[2] Zhao R,Li D,Zuo P,et al. Influences of age,gender,and circadian rhythm on deceleration capacity in subjects without evident heart diseases[J]. Ann Noninvasive Electrocardiol,2018,20(2):158-166.
[3] Wang YP,Kuo TB,Lai CT,et al. Effects of breathing frequency on the heart rate deceleration capacity and heart rate acceleration capacity[J]. Eur J Appl Physiol,2017, 115(11):2415-2420.
[4] Gao L,Chen YD,Shi YJ,et al. Value of DC and DRs in prediction of cardiovascular events in acute myocardial infarctionpatients[J]. Zhonghua Yi Xue Za Zhi,2016,96(19):1519-1522.
[5] Kisohara M,Stein PK,Yoshida Y,et al. Multiscale heart rate dynamics detected by phaserectified signal averaging predicts mortality after acute myocardial infarction[J]. Europace,2017,15(3):437-443.
[6] Liu Y,Syed Z,Scirica BM,et al. ECG morphological variability in beat space for risk stratification after acute coronarysyndrome[J]. J Am Heart Assoc,2016,3(3):135-143.
[7] Gao L,Chen YD,Shi YJ,et al. Prediction value of deceleration capacity of rate and GRACE risk score on major adverse cardiac events in patients with acute myocardial infarction[J]. Zhonghua Xin Xue Guan Bing Za Zhi,2016, 44(7):583-587.
[8] Zuern CS,Rizas KD,Eick C,et al. Severe autonomic failure as apredictor of mortality in aortic valve stenosis[J].Int J Cardiol,2018,176(3):782-787.
[9] Zuern CS,Eick C,Rizas KD,et al. Severe autonomic failure in moderate to severe aortic stenosis:Prevalence and association with hemodynamics and biomarkers[J]. Clin Res Cardiol,2017,101(7):565-572.
[10] Schaeffer BN,Rybczynski M,Sheikhzadeh S,et al. Heart rate turbulence and deceleration capacity for risk prediction of serious arrhythmic events in Marfan syndrome[J]. Clin Res Cardiol,2016,104(12):1054-1063.
[11] Guzik P,Piskorski J,Barthel P,et al. Heart rate deceleration runs for postinfarction risk prediction[J]. J Electrocardiol,2017,45(1):70-76.
[12] Eick C,Rizas KD,Meyer-Zürn CS,et al. Autonomic nervous system activity as risk predictor in the medical emergency department:A prospective cohort study[J]. Crit Care Med,2016,43(5):1079-1086.
[13] Demming T,Sandrock S,Kuhn C,et al. Deceleration capacity:A novel predictor for total mortality in patients with nonischemic dilated cardiomyopathy[J]. Int J Cardiol,2016,22(1):289-293.
[14] Bas R,VallverdúM,Valencia JF,et al. Evaluation of acceleration and deceleration cardiac processes using phaserectified signal averaging in healthy and idiopathic dilated cardiomyopathy subjects[J]. Med Eng Phys,2018, 37(2):195-202.
[15] Zou C,Dong H,Wang F,et al. Heart acceleration and deceleration capacities associated with dilated cardiomyopathy[J]. Eur J Clin Invest,2018,46(4):312-320.
[16] Arsenos P,Manis G,Gatzoulis KA,et al. Deceleration capacity of heart rate predicts arrhythmic and total mortality inheart failure patients[J]. Ann Noninvasive Electrocardiol,2016,21(5):508-518.
(收稿日期:2019-09-30)

