A Selective Luminescent Acetone Sensing Coordination Polymer Constructed from Zinc(II) Ions and Imidazolyl-bearing Ligands①
DONG Sheng-Ju HU Jin-Song CHENG Ting-Ting HE Zhi-Wei
?
A Selective Luminescent Acetone Sensing Coordination Polymer Constructed from Zinc(II) Ions and Imidazolyl-bearing Ligands①
DONG Sheng-Ju HU Jin-Song②CHENG Ting-Ting HE Zhi-Wei
(School of Chemical Engineering, Anhui University of Science and Technology, Huainan 232001, China)
Solvothermal reaction of imidazolyl-based synthon with deprotonated adipic acid produced a new coordination complex {[Zn(BIDPE)(hdc)]·2H2O}(1). The structure was characterized by single-crystal X-ray crystallography. It crystallizes in monoclinic, space group2/, at 293 K:= 12.005(2),= 17.956(3),= 12.039(2) ?,= 113.089(3)°,= 2387.3(7) ?3,= 4,= 1.524 g·cm-3,(Mo) = 1.082 mm–1and(000) = 1136. 5942 reflections were measured and 2180 independent reflections (int= 0.0679) were used in further refinement. Complex 1reveals 1D double-stranded chains containing [Zn2(BIDPE)2] and [Zn2(hdc)2] metallocyclic rings. The adjacent chains are linked by H-bonding and···interactions to form a 3D network. Thermal stability and luminescence property were studied. Interestingly, the luminescent emission of 1 can be quenched by the addition of trace amount of acetone, demonstrating its potential application for sensitive detecting acetone.
luminescence, coordination polymer, fluorescence quenching, acetone detection;
1 INTRODUCTION
Synthesis of CP (Coordination Polymer) materials has drawn more and more attention for their intri- guing aesthetic structures[1]and gigantic potential applications in fluorescence[2], molecular magne- tism[3], heterogeneous catalysis[4], and molecular sorption[5]. The factors influencing the construction of CPs are complicated, in particular, the organic ligands have significant influence on the desirable CPs because of the different spacer lengths[6], flexibility[7], steric hindrance effects[8], and so on. Thus, systematic studies of diversified organic ligands leading to different structures and properties are important and interest.
In recent years, more and more coordination poly- mers with two or three ligands have been reported[9], revealing that the mixed-ligands can result in greater tunability of structural frameworks than single ligands. Among various organic ligands, N-donor ligands were widely employed to construct coordina- tion polymers with fascinating architectures and interesting properties[10, 11]. Among N-donor ligands, multidentate ligands with imidazole or pyridine groups have attracted great interest[12, 13]. We recently have reported 4,4?-bis(imidazol-1-yl) diphenyl ether, which can be regarded as a V-shaped semirigid ligand. It has excellent coordination ability and can freely rotate the imidazole ring to meet the requirement of metal ions in the assembly process[14, 15]. In this paper, BIDPE and hexanedioic acid (H2hdc) were selected to solvothermally synthesize a new coordination polymer with intriguing structure,namely,{[Zn(BIDPE)(hdc)]·2H2O}(1), which was characterized by elemental analysis, IR spectrum and X-ray crystallography. The crystal structure and its thermal property have been studied. Interestingly, luminescent emission of 1 can be quenched by the addition of trace amount of acetone.
2 EXPERIMENTAL
2. 1 Materials and instruments
All reagents and solvents were commercially purchased and used without further purification. Powder X-ray diffraction (PXRD) data were recor- ded on a Bruker D8 Advance X-ray diffractometer using Curadiation (= 1.5418 ?), in which the X-ray tube was operated at 40 kV and 40 mA. Elemental analyses (EA) of C, H, and N were performed on a Perkin-Elmer 240C elemental analy- zer. FT-IR spectrum was carried out with KBr pellets (5 mg of sample in 500 mg of KBr) in the 400~4000 cm-1region. The as-synthesized sample was characterized by thermogravimetric analysis (TGA) on a Perkin Elmer thermogravimetric analyzer Pyris 1 TGA up to 1023 K at a heating rate of 10 K·min-1under N2atmosphere. The luminescent spectra were recorded on a Hitachi F-4600 spectrometer.
2. 2 Synthesis and characterization of complex 1
General procedure for the preparation of {[Zn(BIDPE)(hdc)]·2H2O}(1) A mixture of Zn(NO3)2·4H2O (0.1 mmol, 30 mg), H2hdc (0.1 mmol, 17 mg) and BIDPE (0.1 mmol, 28 mg) was dissolved in 9 mL of DMF/H2O (1:2, v/v). The final mixture was placed in a Parr Teflon-lined stainless- steel vessel (15 mL) under autogenous pressure and heated at 110 °C for 3 days. A large quantity of colorless-plank crystals were obtained, which were washed with mother liquid, and dried under ambient conditions (yield: 57% based on BIDPE). Anal. Calcd. for C24H26O7N4Zn: C, 52.61, H 4.78, N, 10.23. Found: C, 52.73; H, 4.71; N, 10.05. IR (KBr, cm-1): 3420(m), 3138(m), 2933(m), 1597(s), 1512(s), 1395(s), 1298(m), 1233(s), 1124(m), 1063(s), 965(m), 839(m), 739(w), 650 (s), 521(m).
2. 3 Crystal structure determination
X-ray crystallographic data of 1 were collected at room temperature using epoxy-coated crystals mounted on glass fiber. All measurements were made on a Bruker Apex Smart CCD diffractometer with graphite-monochromated Moradiation (= 0.71073 ?). The structure was solved by direct methods, and the non-hydrogen atoms were located from the trial structure and then refined anisotro- pically with SHELXL-97 (Sheldrick, 2008)using a full-matrix least-squares procedure based on2[16]. The positions of hydrogen atoms were fixed geo- metrically at the calculated distances and allowed to ride on the parent atoms. The selected bond lengths and bond angles are listed in Table 1, and the crystal data are listed in Table 2.
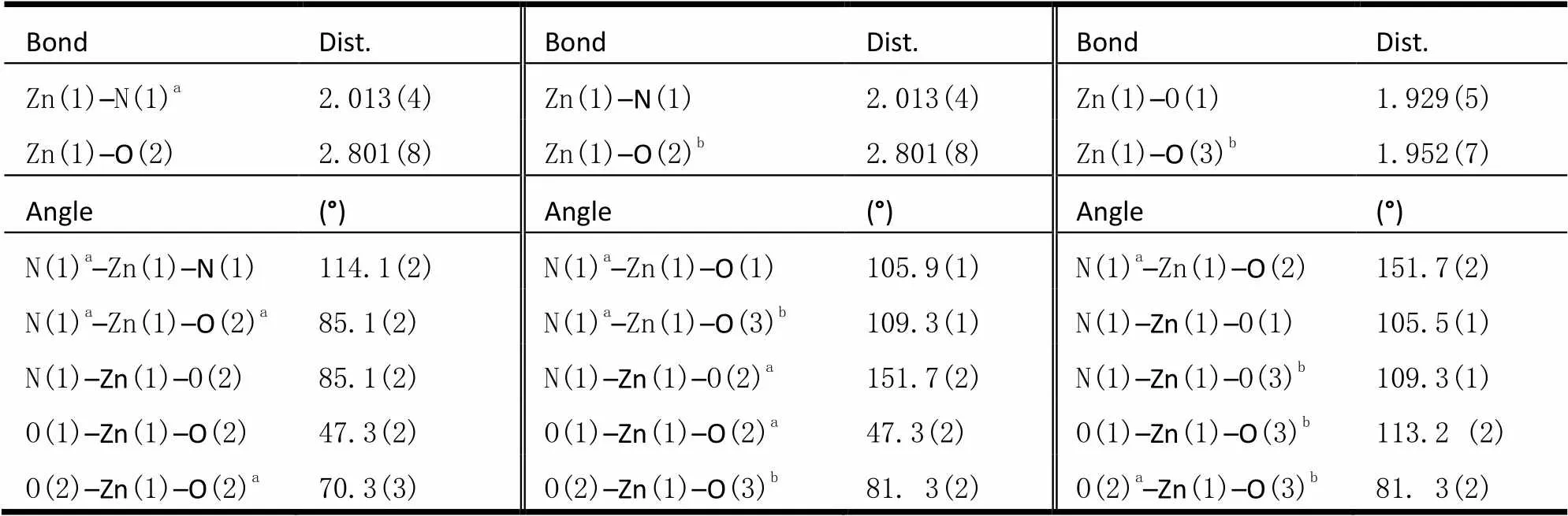
Table 1. Selected Bond Lengths (?) and Bond Angles (°) for Complex 1
Symmetry codes: (a), –,; (b) 1–,, 1–
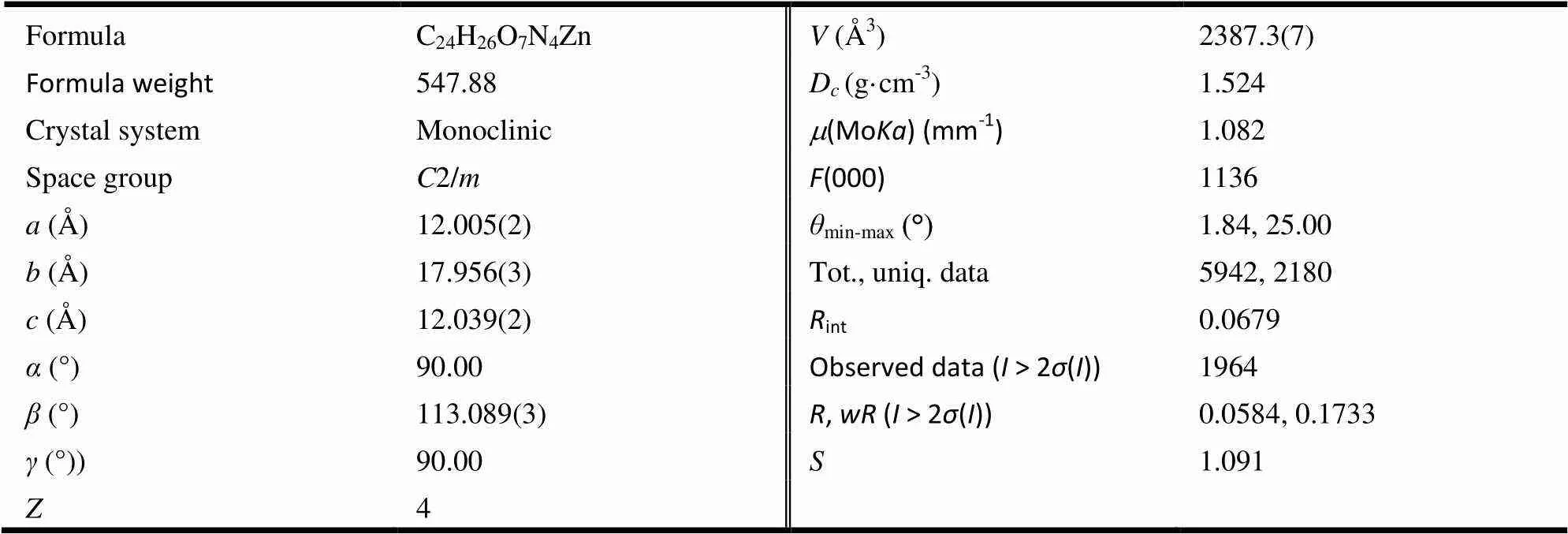
Table 2.Crystallographic Data and Structure Refinement Details for Complex 1
3 RESULTS AND DISCUSSION
3. 1 Crystal structure of {[Zn(BIDPE)(hdc)]·2H2O}n (1)
The fundamental building unitcontains one Zn(II) cation (half occupancy),half hdc2-anion, half BIDPE, and two types of lattic water molecules. As shown in Fig. 1, each Zn(II) atom is four-coordinated by two N atoms from two BIDPE ligands and two O atoms from hdc2-anions. The Zn–O lengths are 1.952(5) and 1.929(5) ?, and the Zn–N length is 2.013(4) ?, which are similar to those found in other Zn(II) complexes[17, 18].
Fig. 1. Coordination environment of the Zn(II) ion in 1. The hydrogen atoms are omitted for clarity
The neighboring two Zn(II) ions are linked by two BIDPE to achieve a 32-membered [Zn2(BIDPE)2] metallocyclic ring exhibiting maximum dimensions of ~15.4 × 11.0 ?2(Zn···Zn and O···O distances). Similarly, the neighboring two Zn(II) ions are linked by two hdc2-anions to form an 18-membered [Zn2(hdc)2] metallocyclic ring. These two types of rings are further linked alternately to give an infinitely double-stranded chain (Fig. 2). One lattice water molecule locates at the center of the [Zn2(hdc)2] ring, linking four oxygen atoms by H-bonding, while another locates on the edge of [Zn2(hdc)2] ring, linking two uncoordinated carboxylic oxygen atoms from adjacent chains by H-bonding (Table 3). These H-bonding interactions extend the structure into a 2D sheet (Fig. 3). Further inspection shows that the adjacent two groups of sheets are actually contacted with each other byinteractions (the center distances of 3.976 and 3.550 ?). Theinterac- tions extend the structure into a 3D network (Fig. 4). Obviously, these H-bonding andinteractions increase the stability of the whole crystal structure. This indicates that latticed water and aromatic rings play important roles in changing the structure and sustaining the stability. As we know from CCDC database, the [Zn2(hdc)2] ring systems are rarely reported[19].
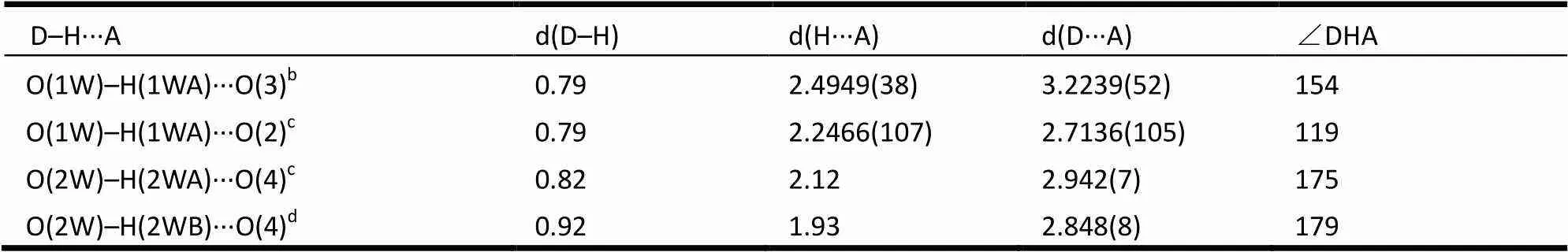
Table 3. Hydrogen Bond Lengths (?) and Bond Angles (°) for Complex 1
Symmetry codes: (a) 1–,, 1–; (b) 1–,, 1–; (c) –1/2+,1/2+, –1+; (d) 3/2–,1/2+,1–
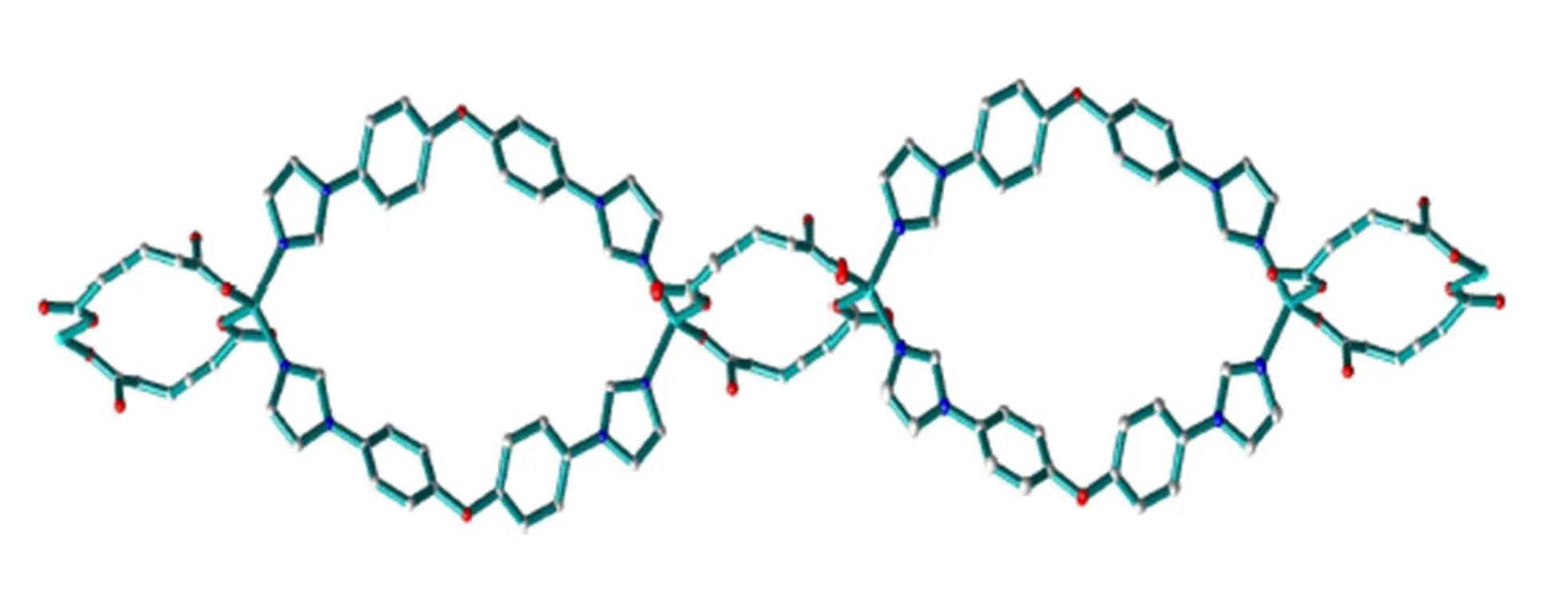
Fig. 2. A view of 1D double-stranded chain constructed by [Zn2(BIDPE)2] and [Zn2(hdc)2] rings
Fig. 3. 1D chains and free water linked with each other by H-bonding, extending into a 2D sheet
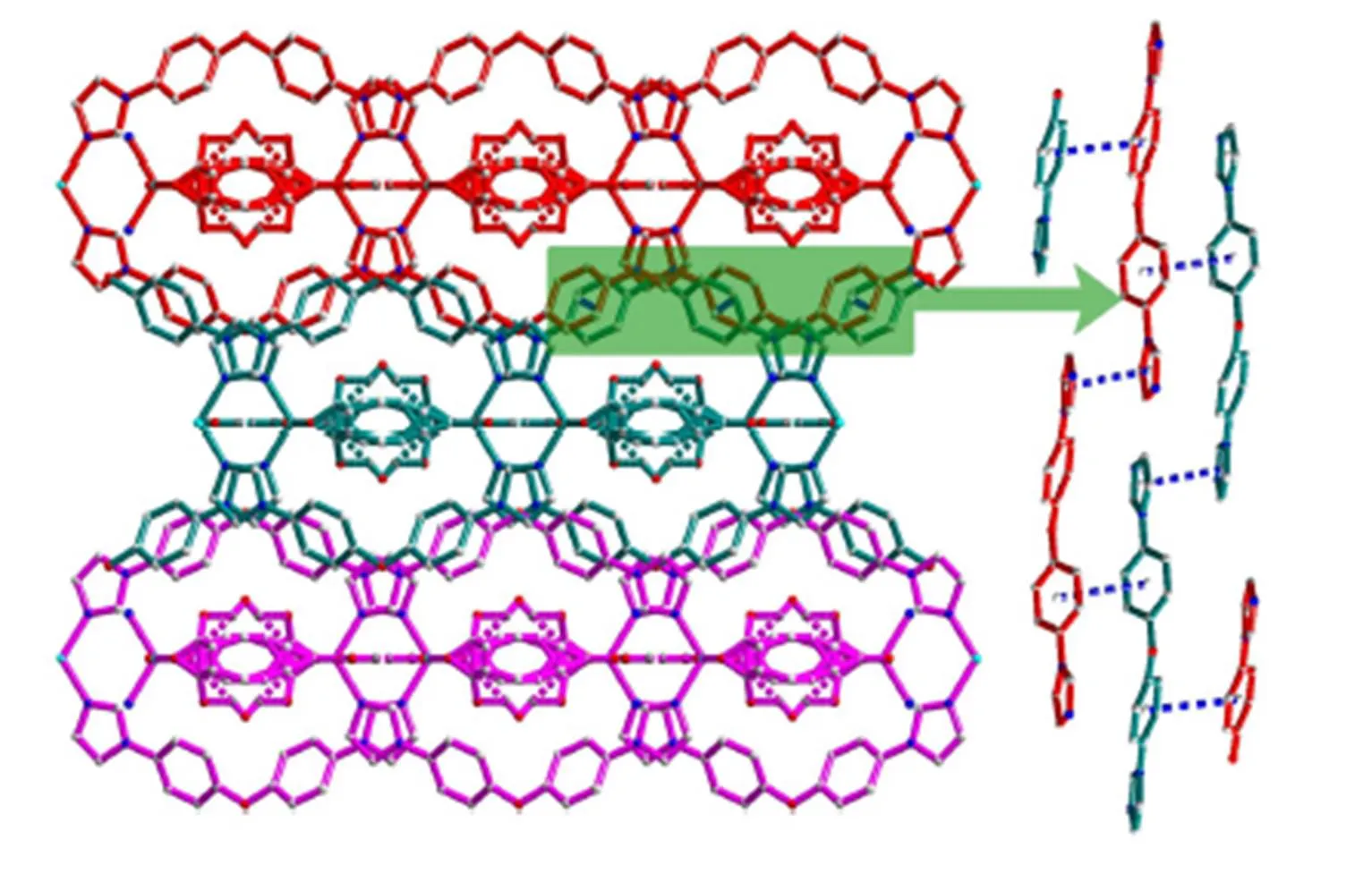
Fig. 4. View of the 3D network constructed by H-bonding and···interactions
3. 2 Thermal analysis and XRD results
To characterize the thermal stability of complex 1, the thermal behavior was studied by TGA (complex 1 of freshly prepared, after 15 days, and after sensing experiment) (Fig. 5). The result shows that a weight loss is observed from 40 to 120 °C, which is attributed to the release of lattice water (5.50%), and the network collapses at 320 °C. The thermal beha- vior shows complex 1 has good stability. To confirm whether the crystal structure is truly representative of the bulk material, PXRD experiment was carried out for 1. The experimental and computer-simulated PXRD patterns show that the bulk synthesized materials and the measured single crystals are the same (Fig. 6).
3. 3 Selective detection of acetone based on complex 1
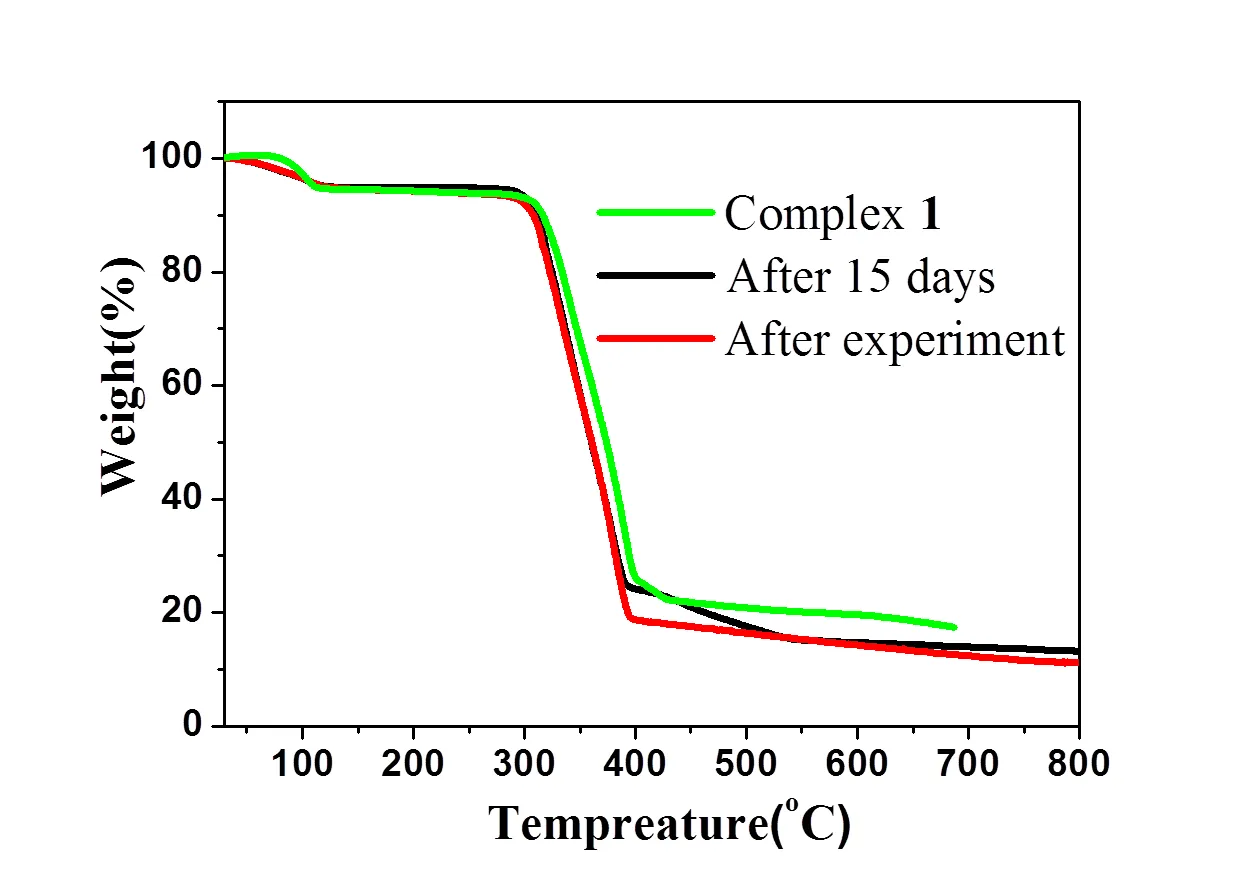
Fig. 5. Various TGA patterns of complex 1: freshly prepared, after 15 days, and after sensing experiment
Fig. 6. Powder X-ray diffraction pattern of complex 1
As a result of uncoordinated carboxyl oxygen and free water molecules, complex 1 was dispersed to various pure polar and non-polar solvents as to observe the interactions by liquid luminescent emission spectra. 3 mg of 1 was ground finely and then immersed in 3 mL of different organic solvents. After treated by ultrasonication for 30 mins, the mixture was aged for 24 h so as to form stable suspensions before measurements. As shown in Fig. 7a and b, complex 1 displays strong guest- dependent luminescence properties for most solvents (CHCl3, MeOH, EtOH, toluene, THF, CH3CN, CH2Cl2), but shows distinct quenching effect toward acetone, implying complex 1 might be a functional sensor material for selective detecting acetone. To better explore the quenching effect of acetone, as shown in Fig. 7c, a gradual decrease of the luminescence intensity was investigated upon the addition of acetone to the CH2Cl2emulsion of complex 1. The luminescence intensity of 1 versus the volume ratio of acetone could be confirmed to the first-order exponential decay function at low concentrations. When the acetone content is below 5.44 vol%, the decay meets the linearity relationship well (Fig. 7d). The acetone exhibits a wide absorption range from 225 to 325 nm, which leads to an overlap with the excitation spectrum of complex 1 centered at ~300 nm (Fig. 8). According to previous and our studies, the distinct luminescence quenching effect could be assigned to the energy transfers between acetone and complex 1 upon excitation.
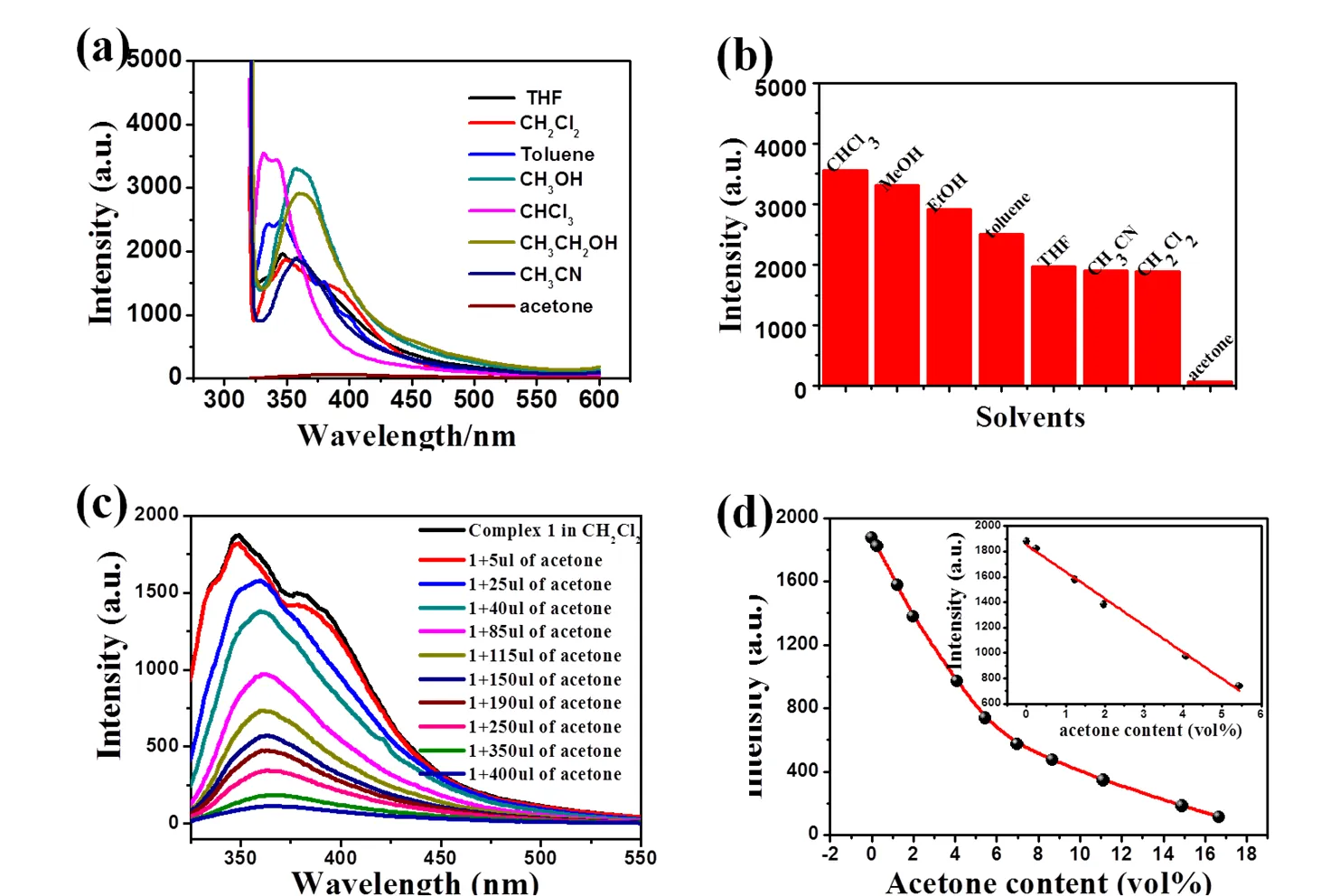
Fig. 7. (a) Emission spectra of complex 1 introduced to various pure solvents underex= 311 nm; (b) PL intensities of complex 1 introduced to various pure solvents underex= 311 nm; (c) Emission spectra of complex 1 at different acetone in CH2Cl2; (d) Luminescent intensities of 1 as a function of acetone content in CH2Cl2. The inset shows the emission quenching linearity relationship below 5.44 vol% (= –210.8523+ 1850.3923,= 0.9971)
Fig. 8. UV-vis spectra of differentsolvents and the excitation spectra of complex 1
4 CONCLUSION
In summary, complex 1 was synthesized and characterized by the self-assembly of BIDPE with adipic acid under solvothermal condition. It is found that this semirigid ligand can adjust the N···Ocore···N angles to meet the requirement of coordination. Complex 1 displays distinct quenching effect toward acetone, implying it might be a functional sensor material for selective detecting acetone. It is antici- pated that more coordination polymers containing semirigid ligands with various interesting network as well as properties will be synthesized.
(1) Jin, K.; Hu, J. S.; Lv, C. N.; Liu, J.; Li, S. Y.; Cheng, T. T. A n-tripodal ligand Cu-based coordination polymer with good catalytic activity for the reduction of 4-nitrophenol.. 2017, 36, 1361–1367.
(2) Hu, J. S.; Zhou, C. H.; Wang, Y. M.; Xu, K. P.; Li, Y. M. A new 2D → 3D network with the coexistence of inclined polythreading constructed by an N-centered tripodal linker.2015, 25, 3211–3213.
(3) Zhang, S. Y.; Liang, F. Y.; Wen, H. R.; Liu, S. J.; Lu, X. N.; Du, Z. Y. 2-4MOFs based on naphthalene-1,4,5,8-tetracarboxylate with magnetocaloric effect and slow magnetic relaxation properties.2017, 132, 123–129.
(4) Zhu, L.; Liu, X. Q.; Jiang, H. L.; Sun, L. B. Metal-organic frameworks for heterogeneous basic catalysis.. 2017, 117, 8129–8176.
(5) (a) Wang, R. W.; Zhang, M. H.; Wang, W.; Xu, Y. W.; Wang, Z. Y.; Dai, F. N.; Zhang, L. L.; Sun, D. F. Interpenetration of three 2D In-MOFs with (6,3) topology: syntheses, structures and fluorescent properties.2016, 35, 1714–1722; (b) Yang, H.; Jiang, Y.; Xu, B.; Li, C. C. Synthesis, crystal structure and luminescent property of a new indium(Ⅲ) coordination polymer.2016, 35, 1723–1727.
(6) Semitut, E. Y.; Sukhikh, T. S.; Filatov, E. Y.; Anosoval, G. A.; Ryadum, A. A.; Kovalenko, K. A.; Potapov, A. S. Synthesis, crystal structure, and luminescent properties of novel zinc metal-organic frameworks based on 1,3-bis(1,2,4-triazol-1-yl)propane.. 2017, 17, 5559–5567.
(7) Li, Y. L.; Zhao, Y.; Wang, P.; Kang, Y. S.; Liu, Q.; Zhang, X. D.; Sun, W. Y. Multifunctional metal-organic frameworks with fluorescent sensing and selective adsorption properties.. 2016, 55, 11821–11830.
(8) Jiang, J.; Huang, W.; Xu, J.; Pan, F. F.; Wu, D. Y. Influence of steric hindrance of N-heterocyclic ancillary ligands on the structure and magnetic properties of manganese(II) coordination polymers.2016, 642, 197–202.
(9) Yan, J. Z.; Lu, L. P. Crystal structure and theoretical studies of tetrahytrate (1-H-1,2,4-triazole-3,5-dicarboxylicacid)nickel.. 2017, 36, 1334–1340.
(10) Shanmugaraju, S.; Dabadie, C.; Byrne, K.; Savyasachi, A. J.; Umadevi, D.; Schmitt, W.; Kitchenc, J. A.; Gunnlaugsson, T. A supramolecular Tr?ger's base derived coordination zinc polymer for ?uorescent sensing of phenolic-nitroaromatic explosives in water.2017, 8, 1535–1546.
(11) Xue, J. R.; He, Z.; Zhang, S. F.; Liang, Y.; Zhang, X.; Jing, L. H.; Qin, D. B. Syntheses, structures, luminescence and magnetics properties of three new metal-organic frameworks based on rigid carbazole ligang.2016, 35, 1574–1581.
(12) Zhou, C. H.; Wang, Y. M.; Jin, K.; Ma, J. J.; Hu, J. S. An unprecedented acid/base stable Cu-based metal-organic framework with good catalytic activity for the reduction of 4-nitrophenol.2015, 62, 81–84.
(13) Wang, Q. H.; Wu, Y. W.; Song, L. Fluorescence quenching of a novel Eu(Ⅲ) coordination compound for the detection of trace amounts of water: synthesis, structure and photoluminescent property.. 2017, 36, 1341–1347.
(14) Hu, J. S.; Yao, X. Q.; Zhang, M. D.; Qin, L.; Li, Y. Z.; Guo, Z. J.; Zheng, H. G.; Xue, Z. L. Syntheses, structures, and characteristics of four new metal-organic frameworks based on flexible tetrapyridines and aromatic polycarboxylate acids.2012, 12, 3426–3435.
(15) Hu, J. S.; Zhou, X.; Liu, X. H.; Xing, H. L.; He, J.; Zheng, H. G. Two stacked 2D chiral/2D chiral → 2D achiral sheets based on V-shaped imidazolyl ligand and flexible aliphatic acids..2013, 33, 15–18.
(16) (a) Sheldrick, G. M.University of Go?ttingen: Germany 1997;
(b) Sheldrick, G. M.. University of Go?ttingen: Germany 1997.
(17) Yadagiri, R.; Bhavesh, P.; Kamal, K. B.; Eringathodi, S. Multiresponsive adenine-based luminescent Zn(Ⅱ) coordination polymer for detection of Hg2+and trinitrophenol in aqueous media.. 2017, 17, 1363–1372.
(18) Deng, Y.; Chen, N. J.; Li, Q. Y.; Wu, X. J.; Huang, X. L.; Lin, Z. H. Highly fluorescent metal-organic frameworks based on a benzene-cored tetraphenylethene derivative with the ability to detect 2,4,6-trinitrophenol in water.. 2017, 17, 3170–3177.
(19) Blatov, V. A.; Carlucci, C.; Ciani, G.; Proserpio, D. M. Interpenetrating metal-organic and inorganic 3D networks: a computer-aided systematic investigation. Part I. Analysis of the Cambridge structural database.2004, 6, 378–395.
9 November 2017;
30 October 2018 (CCDC 933977)
①This work was supported by the National Natural Science Foundation of China (No. 21671004, 51404006)
.Hu Jin-Song, associate professor. E-mail: jshu@aust.edu.cn
10.14102/j.cnki.0254-5861.2011-1885
- 結構化學的其它文章
- Two Cd(II) Coordination Polymers Based on a Flexible Tricarboxylate Ligand: Syntheses, Structures, and Photoluminescence and Catalytic Properties①
- Blue-emissions Modulated by Packing Forces in Alkaline-earth Metal Organic Frameworks Based on Thiophene-2,5-dicarboxylic: Structures and Theoretical Calculations①
- Syntheses, Crystal Structures and DNA-binding Properties of Zn(II), Ni(II) and Co(II) Compounds Containing Thiazole Derivatives①
- A New Luminescent Cd(II) Coordination Polymer Constructed with 2-(Carboxymethoxy)benzoic Acid①
- Synthesis, Structure, and Optical Limiting Properties of an Axial Substituted Bis(8-oxide quinoline)zirconium Phthalocyanine①
- Synthesis and Characterization of Compounds Containing 1,2,3-Triazole via Click Reaction and Ag(I) Complex①

