Conceptual design of an extractive distillation process for the separation of azeotropic mixture of n-butanol-isobutanol-water☆
Hengjun Gai,Kaiqiang Lin,Yirong Feng,Meng Xiao,Kai Guo,Hongbing Song*
College of Chemical Engineering,Qingdao University of Science and Technology,Qingdao 266042,China
Keywords:Extractive distillation Triple azeotropic system Conceptual design Butanol dehydration
ABSTRACT In many chemical processes,large amounts of wastewater containing butanol and isobutanol are produced.Given that n-butanol-isobutanol-water can form triple azeotrope,high-purity butanol cannot be recovered from the wastewater by ordinary distillation.To economically and effectively recover butanol from this kind of wastewater,1,4-butanediol is selected as an extractant to break the formation of the azeotropes,and a doubleeffect extractive distillation process is proposed.The conceptual design of the proposed process is accomplished based on process simulation.With the proposed process,the purity of recovered butanol and water is greater than 99.99 wt%.In comparison with the conventional azeotropic distillation process,economic analysis shows that the operating cost of the proposed process is lower:when the capacity of wastewater treatment is 100 t·h-1,the total operating cost decreases by 5.385 ×106 USD per year,and the total annual cost of the new process decreases by 5.249×106 USD per year.In addition,in the extractive distillation system,variable effects on separation purities and cost are more complex than those in the ordinary distillation system.The method and steps to optimize the key variables of the extractive distillation system are also discussed in this paper and can provide reference for similar studies.
1.Introduction
In many chemical processes,such as butanol production[1],octanol production,biological pharmacy process[2],and dye production[3],large amounts of wastewater containing n-butanol and/or isobutanol are produced.Butanolis one of the major raw materials for organic synthesis and a high-energy contentfuel[4,5],thus,the recovery ofbutanol from these kinds of wastewater is economically and environmentally friendly.Unfortunately,n-butanol-water and isobutanol-water can form minimum-boiling heterogeneous azeotropes.Moreover,n-butanol-isobutanol-water can form triple azeotropic systems.Therefore,high-purity butanol cannot be recovered from the wastewater by ordinary distillation.
At present,to separate water from butanol,several methods,including azeotropic distillation,adsorption,freeze crystallization,gas stripping,liquid-liquid extraction,reverse osmosis,membrane distillation,vacuum pervaporation,and extractive distillation,are mentioned in literature[5-12].Among them,azeotropic distillation is the most widely used.For example,William L.Luyben designed a two-column process to separate n-butanol and water[13].Each column is a stripper with its individual reboiler.The bottom of the first column and the second column are 99.9 mol%water and 99.9 mol%n-butanol,respectively.To separate isobutanol and water,Víctor Hugo,Grisales Díaz,and Gerard Olivar Tostproposed two new processes[14].One is a two-column process combined with vapor compression distillation,and the other is a double-effect process,which employs three columns.Isobutanol with a purity of 99.7 wt%can be obtained using these two processes.
Extractive distillation is another feasible way to separate azeotropic systems using a suitable solvent.Xueli Huang proposed an extractive distillation using 1,4-butanediol(BDO)as solventto separate isobutanol and water[15].This process demonstrates that the heaviestcomponent BDO can break the formation of isobutanol-water azeotrope.
When butanol is produced by propylene carbonyl synthesis method,wastewater containing approximately 30%n-butanol and 30%isobutanol is produced.Because ofthe high demand and outputofbutanol,the amount of wastewater produced is quite large.Before the biochemical treatment of wastewater,the separation of n-butanol and isobutanol from wastewater can further increase economic bene fits.However,the studies mentioned above involve only the separation of the two kinds of binary systems(n-butanol-water or isobutanolwater).Studies on the separation of triple system(n-butanolisobutanol-water)have rarely been reported.Therefore,it is of great practical significance to develop the process and technology that can separate the triple system.In this paper,a double-effect extractive distillation process for the separation of n-butanol-isobutanol-water is proposed and a concept is designed.
2.Process Design and Objective
2.1.Separation processes for n-butanol-isobutanol-water azeotropes
Component data of butanol wastewater were collected from a butanolplantin Shandong Province,China,and are listed in Table 1.The flow rate of the wastewater was 100 t·h-1.

Table 1 The component data of butanol wastewater
The mixture of water,isobutanol,and n-butanol can form three kinds of azeotrope systems.The properties of these azeotrope systems can be viewed by using the Aspen Conceptual Design tool,as shown in Table 2.In Table 2,isobutanol and water,as well as n-butanol and water,can form minimum-boiling heterogeneous azeotrope at 0.1 MPa with azeotropic temperatures of 90.33 °C and 92.61 °C and azeotropic composition of 34 wt%and 42 wt%water,respectively.The azeotropic temperature of the triple azeotrope systems was 90.59°C.Thus,achieving the concentration target by simple distillation was impossible.

Table 2 Reports of the various azeotropic mixtures
According to the mixture properties of n-butanol,isobutanol,and water,two separation processes based on azeotropic distillation and extractive distillation are shown as follows:
(1)Azeotropic distillation
The wastewater was first fed into the decanter(D01)to separate the two phases(i.e.,organic phase and aqueous phase).Then,the organic and aqueous phases were fed into the top of the butanol column and water column for stripping,respectively.The top streams ofthe two columns were fed into the decanter(D02)after cooling down to approximately 40°C.Correspondingly,the bottom streams of the two columns were butanol and water,both with purity greater than 99.99 wt%(Fig.1).
(2)Extractive distillation
Fig.2 shows the proposed extractive distillation process.The water content in the feed flow is 30 wt%and is higher than the solubility of water in butanol.Hence,a pre-dewatering device,consisting of a decanter(D01)and the pre-concentration column(T01),was setup to remove dissolved water.
With regard to improvement over traditional extractive distillation processes[15],the double effect distillation technique was adopted to reduce energy consumption.Specifically speaking,the overhead vapor of the solvent recovery column(T03)was used to heat the reboiler of the extractive column(T02).In cases when the latent heat was insuf ficient,T02 was equipped with an auxiliary reboiler heated with fresh steam.
The feed flowed into the decanter(D01)and was split into aqueous and organic phases.The aqueous phase with 81.8 wt%water flowed into the first stage of T01.With T01,the aqueous phase was separated into 99.99 wt%water as the bottom stream and azeotropic mixture as the top stream.The top stream of T01 was fed into D01 again.The organic phase from D01,mainly composed of butanol,was fed into the middle of the extractive distillation column(T02).Meanwhile,BDO,which was used as solvent,was fed to T02 from a slightly higher position.
The solvent used can change the intermolecular forces.That is,it changes the activity coefficient of the component to be separated.Chunxia Kou,et al.[16]have used theoretical models to predictsolvents suitability for water-isobutanol systems.The study found thatthe selection ofcompounds,more similarto the heavy-key components in polarity,as solventcan effectively reduce the degree ofvolatility ofheavy-key components.A ternary system is composed of a water-isobutanol system with 1,3-propanediol,1,2-propanediol,1,3-butanediol and 1,4-butanediol,respectively.By using the modified UNIFAC model and selective calculation Eq.(1),the above solvents were found to have varying degrees of selectivity.Among them,1,4-butanediol has the highest selectivity.At the same time,it has a high boiling pointand is easy to recycle.

T02 consisted of a rectifying section,an extractive section,and a stripping section.The top product was water with purity greater than 99.99%,and the bottom of T02 was a mixture of butanol and BDO.The bottom stream was fed into the recovery column(T03)to separate BDO from water.Regenerated BDO recycled back to T02 with a little fresh makeup.Butanol,which is a mixture of n-butanol and isobutanol,was distilled from the top of T03.If necessary,butanol and butanediol can be further separated by distillation.The separation methods of nbutanol and isobutanol have been discussed in some studies[17,18];thus,these methods will not be studied in this paper.
2.2.Conceptual design based on simulation
The optimization design of azeotropic distillation process is relatively simple,and many studies about similar systems have been reported.Therefore,only the conceptual design steps for the extractive distillation process is described in this paper,which excludes the azeotropic distillation process.
All process simulations were performed using Aspen Plus V7.2.RadFrac module was used to represent the distillation column.To design and optimize all distillation process accurately,choosing apposite thermodynamic model to describe the phase equilibrium[vapor-liquid equilibrium(VLE)and liquid-liquid equilibrium(LLE)]of four components is necessary.For the VLE,the thermodynamics models can be correctly represented using NRTL,NRTL-RK and UNIQUAC[19].However,for the LLE,according to the study of Marcela C,the phase composition of system was correctly described by the UNIQUAC model[20].So,for this four-componentsystem,the VLE and LLE were calculated by the interaction parameters in the UNIQUAC model in this paper.For this fourcomponent system,the VLE and LLE were calculated by the interaction parameters in the UNIQUAC model.
In the wastewater,the binary interaction parameters of the UNIQUAC between water,n-butanol,isobutanol and BDO are collected and incorporated into the UNIQUAC property method.They can be easily viewed in Aspen Plus and here showed in Table 3.
2.3.Design specification
After treatment,butanol and water purities should exceed 99.99 wt%.

Fig.1.Flowchart of the azeotropic distillation process:C01 and C02:condensers;D01 and D02:decanters;H01 and H02:reboilers.
The solventwas recycled.With high solventpurity,reducing the impurities in T02 overhead water is more conducive;however,this process will increase the heat duty of T03.Some studies showed thatachieving a high purity solvent is easy[21].Moreover,our calculations showed that the purity of recovered solvent can be easily increased to more than 99.99%when the latent heat of the top stream of T03 was less than the heat demand of the reboiler at bottom of T02.Hence,the purity of BDO from the bottom of T03 was set to above 99.99 wt%.

Table 3 Binary interaction parameters of four components
2.4.Optimization method for design variables
As mentioned in many papers[22-25],a large number of the design variables are to be optimized,with the objective of minimizing the total annual cost(TAC),such as the temperature of solvent feed,the mass flow ofsolventin this paper(S),totalnumberofstages ofextractive distillation column(NT),upper feed and lower feed stages(NUand NL),and the reflux ratio(RR).TAC,which consisted of annual capital investment cost and annual operating cost,was calculated as follows:

Capital investment cost consisted of the column shell,trays,reboilers,and condensers and is estimated by the equation in Appendix A,which were directed by a book[26].Tray costs were calculated to cost 600 USD·m-2.A plant lifetime of 10 years was used in the TAC calculation.Moreover,the costs considered for various utilities are as follows:0.03 USD·t-1cooling water,15 USD·t-1lower pressure steam(LPS,0.6 MPa,160 °C),and 20 USD·t-1high pressure steam(HPS,4.2 MPa,254°C).The heating medium used in two processes was listed in Table 4.

Table 4The heating medium used in two processes
In the extractive distillation system,variable effects on separation purities and TAC are more complex than that in an ordinary distillation system.For example,the purity ofthe T02's top product(water)is in fluenced by not only RRbut also S.Figs.3-5 show the relationship of the T02's top product and RRwith different mass flows of solvent.

Fig.3.Relationship of mass fraction of water and R R with different mass flows of solvent.
In Fig.3,results reveal that water purity increases with the increase of solvent mass flow.Unlike conventional distillation,purity did not monotonically increase with the increase of reflux ratio,and excessive reflux ratio will have an adverse effect on the purity of the top production.The reason for this may be that the reflux flow was too large,and more water was returned to the column to dilute the solvent;thus,S became relatively inadequate and more butanol entered the top of the tower,as shown in Fig.4.In Fig.5,if the reflux flow is too small,then more solvent enters the top of the column;this finding reduces the purity of the overhead product.With a great extraction dose,the distillation column will have high size and energy consumption.An appropriate amountof solvent needs to be calculated when the production meets the separation requirements.

Fig.4.Relationship of RR and mass fraction ofbutanol with different mass flows ofsolvent.
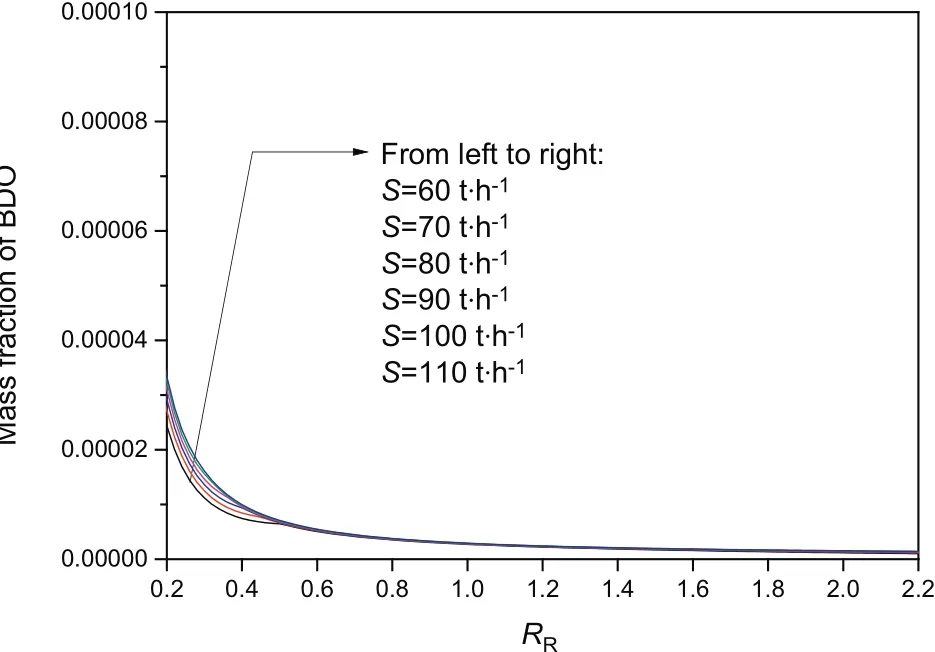
Fig.5.Relationship of R R and mass fraction of BDO with different mass flows of solvent.
(1)Considering the complexity of the relationship,the methods and steps of optimizing parameters for extractive distillation column(T02)are as follows:
(2)Using Design Spec/Vary function in Aspen Plus to specify the value of reflux ratio and distillate rate as adjustment variables,specify the mass fraction of water in top and bottom production as control variables.Water on the top and at the bottom production reaches 99.99 wt%and 0.001 wt%by manipulating the adjustment variables,respectively.
(3)Assign a value to the total number of stage(NT),
(4)Calculate the mass flow of solvent(S),
(5)Adjust the NUand NLto find the minimum duty of T02 reboiler,
(6)Go back to Step(2)until the duty of T02 reboiler is minimized,
(7)Go back to Step(1)until the duty of T02 reboiler is minimized.
The heatload of the recovery column(T03)is mainly affected by the S variable.As for the T03,the reflux ratio is set1.2 times ofminimum reflux ratio,seeking the minimum duty of the reboiler by optimizing the number of stages.To simplify the calculation,we compared the TAC,exceptthatofT01 ofeach case thata mass flow ofsolventstands fora case.
The procedure for optimal design is shown in Fig.6.
3.Case Study

Fig.6.Program for seeking optimal design.
Based on the simulation results,the conceptual design of the new process for the triple azeotrope system separation is accomplished.The basic operation conditions for the two kinds of processes are determined,and results for the extractive process are shown in Table 5.
The sensitivity analysis shows the in fluence of operating conditions on the performance of the process.
3.1.The operating pressure and temperature
As for the determination of operating pressure,given that the separation process is facilitated by low pressure[16],the operating pressure should be as low as possible.However,a low pressure means a low tower top temperature,and if the temperature goes below 50°C,cooling water will no longer be suitable as a cooling medium and a high price cooling medium must be used.
When the purity of the water at the top of T02 is 99.99%,in accordance with the relationship between top temperature and the operatingpressure shown in Fig.7,the operating pressure of T02 is set to approximately 0.013 MPa.Accordingly,the bottomtemperature ofT02 is 89°C.
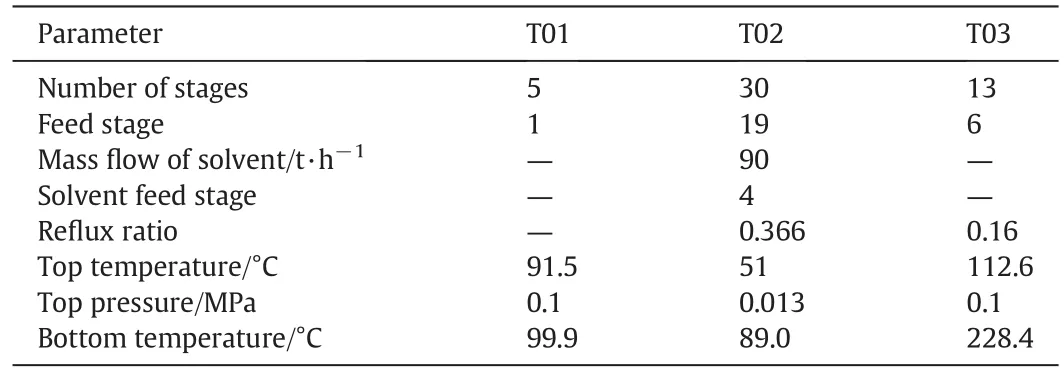
Table 5 Optimal designing results for the extractive process

Fig.7.Relationship between top temperature of T02 and operating pressure of T02.
To meet the demand that the overhead vapor of T03 heats the reboiler of T02,the top temperature of T03 should be more than 109°C,which is 20 °C higher than the bottom temperature of T02.When T03 is operated at atmospheric pressure,the top temperature is 112.6°C,which meets the requirements.
The upper feed temperature is set at 5 °C to 10 °C below the boiling temperature of the extractive distillation column top production for an extractive distillation[27,28].In this case,the upperfeed temperature is set at 45°C in the following work.
3.2.The theoretical plate numbers and the mass flow of solvent(S)
The effects of the totalnumber of stages of extractive distillation column(NT),upper feed and lower feed stage(NUand NL),and mass flow of solvent on the TAC are shown in Figs.8-11.

Fig.8.Effect of N T on duty of reboiler.
In Fig.8,TAC increases with the NTmore or less than 30.As for the NU,ifthe upperfeed stage is above the fourth,then meeting the requirement of separation is impossible,given that the number of stage in rectifying section is not enough.
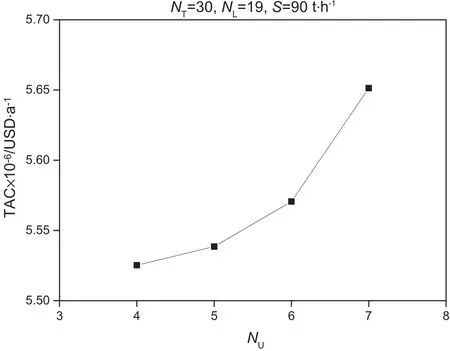
Fig.9.Effect of N U on duty of reboiler.

Fig.10.Effect of N L on duty of reboiler.

Fig.11.Effect of mass flow of solvent on TAC.
The effect of NLon TAC is shown in Fig.9.If the lower feed stage is close to the bottom,the stages in the stripping section are not enough,which leads to high difficulty in extracting water from butanol in this section.Meanwhile,the lower feed stage is far from the bottom that the extractive section cannot supply sufficient contact time for the solvent with the mixture.That is,the reflux ratio is needed to increase the insufficient number of stages in the extractive section.
After all the optimal parameters are determined under the condition of each mass flow of solvent(S),Fig.11 presents the relationship between TAC and mass flow solvent(S).TAC is lowest at S=90 t·h-1.We count the cost of solvent out from TAC,as the cost of about 1-2 kg·h-1fresh makeup solventturns out to be negligible in comparison with millions of TAC.When S=90 t·h-1,1.495 kg·h-1fresh solvent needs to be added.
Temperature and liquid-phase composition in T02 are plotted in Fig.12.The change ofcomposition profile is in line with the temperature.As shown in Fig.12(a),temperature decline greatly occurred on NLbecause of great deal of wasted water.To meet the requirement of separation,only water concentrated in the rectifying section is observed;in the extractive section,due to the solvent BDO added,the relative volatility between water and butanol is changed;water flows up toward the top of T02 and butanol,and BDO flows down toward the bottom of T02;in the stripping section,waterbarely existed atthe bottom ofT02,which isthe best result of separation.

Fig.12.Temperature(a)and liquid-phase composition profiles(b)in the extractive column.
3.3.Comparison between the azeotropic distillation process and the extractive distillation process
After treatment with the two processes,the mass fraction of the products is presented in Table 6.The two processes are efficient for treatment of the wastewater,and the purity of all kinds of products meets the requirements.
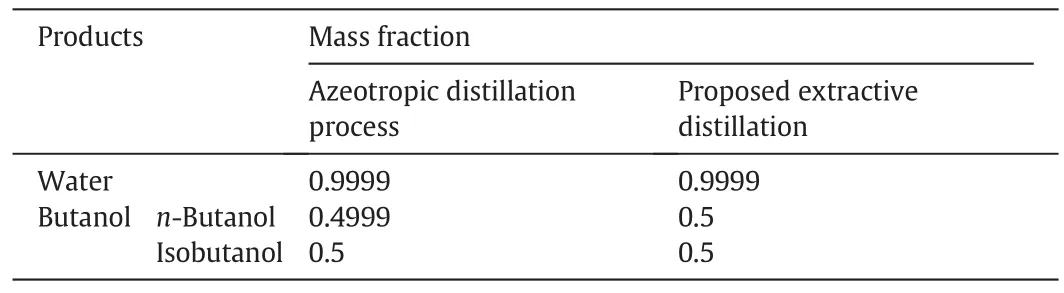
Table 6 Mass fraction of products from the two processes
The capital costs of the columns are affected seriously by reflux ratios,recycle flow rates,and solventusages in the distillation cases.Additionally,these parameters affect directly the heat duties of the process and the quality of the final products.The cost comparison between the two processes,including investments of equipment and costs of utilities,is listed in detail in Table 7.If the new process is adopted to replace the azeotropic distillation process,the capital investment cost increases by 0.522×106USD as a set of pre-concentration system and more heat-exchanging areas are required,and the annual capital cost increases by 0.0522×106USD.However,with less energy required by the proposed extractive distillation process,a great deal of steam and cooling water can be saved.As a result,TAC of the proposed process is 4.942×106USD per year lower than that of the azeotropic distillation process.As a result,the TAC of the new process has an obvious decrease and the new process has a great energy-saving potential.

Table 7 Cost comparison between the two processes
4.Conclusions
Anew solvent(1,4-butanediol)forseparating n-butanol-isobutanolwater system is found and a novel double-effect extractive with 1,4-butanediol as a solvent is proposed to realize energy conservation in this paper.The process and the column configurations are investigated by using simulation software Aspen Plus.TAC is used as an object of function to economically evaluate the azeotropic distillation process and novel process.The in fluence of the most important operational parameters are studied through sensitivity analysis.
Although the new process is a bit complicated,which leads to more operating difficulties.However,with the increasing shortage of energy,the noveldouble-effectextractive distillation has greateconomic potential compared with the azeotropic distillation process.The total annual cost of the novel process decreases by 5.249×106USD per year.It is obvious that the new process has great research value.
At present,there are few studies on this system.This paper can play a reference role in the study of this system.Secondly,the system is very common in chemical production,the research in this paper can have a certain guiding significance for chemical production process.
Nomenclature
D diameter of column,m
H height of column,m
HPS medium pressure steam
LMTD the log-mean temperature different
LPS low pressure steam
NLlower feed stage
NUsolvent feed stage
NTnumber of stage
p* saturated vapor pressure of component i or j
QCheat duty of the condenser,Gcal·h-1
QEheat duty of the heat exchanger,Gcal·h-1
QRheat duty of the reboiler,Gcal·h-1
RRreflux ratio
S the mass flow of solvent,kg·h-1
Sijsolvent selectivity
TBbase temperature,°C
TRDreflux-drum temperature,°C
TLtemperature of lower feed,°C
TSsteam temperature,°C
TUtemperature of upper feed,°C
ΔTCtemperature difference of condenser,°C
ΔTRtemperature difference of reboiler,°C
UCheat-transfer coefficient of the condenser, 0.852 kW·K-1·m-2
URheat-transfer coefficient of the reboiler,0.568 kW·K-1·m-2
VMAXthe max of vapor volume flow,ft3·s-1
(αij)Srelative volatility of component i to j in the presence of a certain amount of solvent
(αij)Brelative volatility of component i to component j in the absence of solvent
γ activity coefficient of component i or j
ρVthe density of the max vapor volume flow,lb./ft3
Appendix
The cost of column shell(MM$):

The area of condenser(m2):

The area of reboiler(m2):

The area of heat exchanger(m2):

The cost of heat exchanger(including condenser,reboiler and heat exchanger)(MM$):

 Chinese Journal of Chemical Engineering2018年10期
Chinese Journal of Chemical Engineering2018年10期
- Chinese Journal of Chemical Engineering的其它文章
- Recent progress in the green synthesis of rare-earth doped upconversion nanophosphors for optical bioimaging from cells to animals☆
- One-step synthesis of hydrophobic magnesium hydroxide nanoparticles and their application in flame-retardant polypropylene composites☆
- Controllable preparation of ZnO porous flower through a membrane dispersion reactor and their photocatalytic properties☆
- Distribution and degradation kinetics of cyhalodiamide in Chinese rice field environment☆
- Co-pyrolysis characteristics of typical components of waste plastics in a falling film pyrolysis reactor☆
- Reliable,environmentally friendly method for the recycling of spent Ag/α-Al2O3 catalysts using(NH4)2Ce(NO3)6☆
