基于無性繁殖過程的蝙蝠蛾擬青霉循環(huán)發(fā)酵方法
Sayed-Hassan-Raza Shah,陸震鳴,李碩碩,李華祥,羅志珊,張曉娟,史勁松,許正宏*
(1.江南大學(xué) 藥學(xué)院,江蘇 無錫 214122;2.江南大學(xué) 生物工程學(xué)院 糧食發(fā)酵工藝與技術(shù)國家工程實驗室,江蘇 無錫 214122)
Ophiocordycepssinensis isawell-known medicinal fungus that has been used as a traditional Chinese medicine for thousand years.Nowadays,the wild O.sinensis is in great market demand due to the presence of potent bioactivities,but it is extremely expensive due to the rarity in nature,and the difficulty of artificial cultivation.Thus,several fermentation products produced from the fungal isolates are used to substitute the wild O.sinensis[1-2].In previous studies,more than 10 fungal specieswereisolated from natural O.sinensis.Among these species,Hirsutella sinensis is an adopted anamorph of O.sinensis[3-6].Other fungal species isolated from O.sinensis,such as Paecilomyces hepiali,Synnematum sinensis,Cephalosporum sinensis,Gliocladium roseum,and Mortierellahepiali,have also been applied in large-scaleproduction and used ascommercial products[7].
The mycelial powder of P.hepiali has been comprehensively studied and it has been evolved into functional food in China for ages.Studies have showed that P.hepiali has various functions,such as restricting tumor proliferation,invasion,metastasis,and neovascularisation,inducing apoptosis,reversing drug resistance,enhancing immunity as well as protecting hepatic function[8].Adenosine in the mycelial powder of P.hepiali is believed as functional compounds which are beneficial to the human body[9].JinShuiBao capsule,the commercial product of P.hepiali Cs-4,hasbeen used in clinicsthroughout China.The annual output value of JinShuiBao capsulein 2014 ismorethan 2.2 billion RMB[6].
Submerged fermentation(SmF)isconsidered asthemost efficient way in industrial production of P.hepiali,to satisfy itslargeconsumption demand.However,the SmFof filamen-tous fungus is complex[10]due to many factors,such as strain,inoculum size,mycelium morphology,medium composition,and operation condition,which affect the biosynthesis of metabolites[11].Thus,it is necessary to continue with the ex cellent batch-repeatability,less time consuming,low cost,and lesslabor consuming fermentation method of P.hepiali.
Usually,mycelia grow on agar plate were used asinoculum for the SmFof P.hepiali.In thisstudy,an asexual reproduction-based repeated batch fermentation(RBF)of P.hepiali was performed with a modified inoculation method.A twenty-cycle RBFwas carried out in this study,and the biomass,sporulation,and metabolites calculation were examined in each batch.Themethodisdesigned tosavecostandimproveefficiency.
1 Materials and Methods
1.1 Materialsand Reagents
1.1.1 Fungal strains
P.hepiali were provided by theculture bank of National Engineering Laboratory for Cereal Fermentation Technology,Jiangnan University.Thestrainswerecultured on potato dextrose agar(PDA)medium.
1.1.2 Reagents
Yeastextractwaspurchased from Oxoid Ltd.(Basingstoke,UK).Other chemicalswere purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).Adenosine and oleanolic acid standardswere purchased from Sigma-Aldrich(MO,USA).All the chemicals used were either of analytical gradeor of thehighest purity commercially available.
1.1.3 Culture medium
PDA medium:200 g diced potatoes were boiled in 1∶1 of distilled water for 30 min and then were filtered with gauze with four layers;20 g glucose and 20 g agar were added into 1 L of filtrate.The medium wassterilized by autoclaving at 100 kPa121℃for 20 min.
Seed culture medium:30 g glucose,1 g yeast extract,0.5 g MgSO4,1 g KH2PO4,0.1 g vitamin B1,1 L distilled H2O,pH 6.5.
Basic sporulation medium:20 g glucose,0.5 g yeast extract,0.5 g MgSO4,3.0 g KH2PO4,1 L distilled H2O,pH 6.5.Themedium wassterilized by autoclaving at 100 kPa,121℃for 20 min.
Fermentation medium:20 g glucose,0.5 g yeast extract,0.5g MgSO4,3.0g KH2PO4,1Ldistilled H2O,pH 6.5.Thismedium wassterilized by autoclaving at 100 kPa121℃for 20 min.
1.2 Instrumentsand Equipments
BX60 Light microscope:Japan Olympus;SX-40 electron microscope,SX-40 Scanning electronmicroscope,HPLC chromaster:Japan Hitachi Group.
1.3 Methods
1.3.1 Inoculum preparation
The mycelial colony of P.hepiali was picked from the PDA slant to a500 ml Erlenmeyer flask containing 100 ml of seed culture medium.After inoculation,it was cultured by shake-flask cultivation at 150 r/min for 4 d in thecondition of 26℃.The asexual spores in the supernatant of fermentation broth were diluted in fermentation medium,counted using a hemacytometer under a light microscope,and adjusted to adequate concentrations.
1.3.2 Repeated batch fermentation
A total of 20 cycles of RBF were performed in 500 ml Erlenmeyer flaskscontaining100 ml of fermentation medium.P.hepiali sporesuspensionwasadded into fermentationmedium with the initial spore concentration of 1×105spores/ml,incubating for 4 d at 26℃with 150 r/min shaking speed.Fermentation broth(2 ml)in theflask wassampled every 24 h for 4 d.An appropriate portion of the supernatant(spore suspension)at 48 h in the cycle 1 wasinoculated into the cycle 2 with inoculum size of 1×105spores/ml.Subsequent cycleswere performed in the same manner asdescribed above.At least three timesparallel testswereperformed.
1.3.3 Microscopic observations
During the fermentation process of P.hepiali in flasks,the morphology of spores was observed under a light microscope.Suspensions(2 ml)containing spore aliquotswere taken out from shaking flask at daily intervals and were examined under a microscope.
(1)Specimenspreparation and microscopic observation
The fungal spores were produced in liquid culture after 1 d,and then centrifuged at a low speed and washed three times with 0.1 mol/L phosphate-buffered saline(PBS)buffer(adding 3.1 g of NaH2PO4·H2Oand 10.9 g of Na2HPO4to distilled H2O to make a volume of 1 L).Then fixed the acquisi tion in 3%glutaraldehydefor 2 h and in 1%osmic acid for 1 h respectively at 4℃.After washed four times in 0.1 mol/L PBSbuffer,the specimenswere dehydrated through a graded seriesof ethanol,immersed in 100%isoamyl acetate,dried by critical point drying,and coated with gold.Specimens were observed under ascanning electron microscope.
(2)Sporulation detection
Spore concentrationswerecalculated by hemacytometer counts.The fungal spores were determined by the sporulation number and the sporulation capacity was determined by the number of fungal sporesper gram of biomass.
1.3.4 Analytical methods
(1)Obtaining of mycelia:The fermentation broth was filtered in culture flask through a mesh(pore size,30μm),washed with distilled water.Then the mycelia sediment was obtained after centrifugation at 5 000 r/min for 10 min at 4℃.The mycelia were lyophilized and weighed to calculate the biomass,and stored at 4℃for further analysis[12].
(2)Determination of crude triterpenoids:Colorimetric method with vanillin-acetic acid system was used to analyze the crude triterpenoids production.The mycelia powders of P.hepiali(0.1 g)were extracted with methanol(5 ml)for 2 h at room temperature.The methanol extract wascentrifuged at 5 000 r/min for 10 min at 4℃,and then 0.2 ml of supernatant(with 0.2 ml methanol asthe blank)was transferred to a tube and heated withboilingwater until thesolventwascompletely evaporated.0.3 ml of 5%freshly prepared vanillin-acetic acid solution and 1.0 ml perchloric acid wereadded into theabove acquisition.The mixture was incubated at 60℃for 20 min,then immediately cooled with icewater.Acetic acid(10.0 ml)was added to each sample,and the absorbance was detected using a spectrophotometer at 550 nm[13].
Standard curve:Different concentrations of oleanolic acid wereused to obtain the standard curve[14](Y=4.83X-0.04,R2=0.996).X representsthemassof oleanolic acid(0.02-0.18mg),whereas Y representsoptical density at 550 nm.
(3)Determination of adenosine:Adenosine concentration was measured by HPLC,according to the procedure reported by LU et al.with some modification[15].The mycelia powdersof P.hepiali(0.5g)wereextractedfor2hwithmethanol(1.5 ml)at room temperature,and the supernatant was collected by centrifugation(4℃,5000 r/min,10 min).The extractions were concentrated to 1.0 ml through volatilization and filtration through a 0.22μm microporous membrane.Samplesolution(10μl)wasapplied to HPLCanalysis.Separation wasperformed in a Waters XBridge C18reversed-phase analysis column(4.6 mm×250 mm,5μm)at a flow rate of 1.0 ml/min,and the absorbance was determined at 254 nm.Solvent A(water,containing 0.5%acetic acid)and solvent B(100%acetonitrile)were used as the mobile phase.The gra dientconditionswere:0-20 min,5%-20%B;20-40 min,20%-50%B;40-70 min,50%-60%B;70-120 min,60%-80%B;120-150 min,80%-90%B;150-170 min,90%-100%B;170-171 min,100%-5%B;171-180 min,5%B.Content of adenosinewasdetermined by theexternal standard method.
Standard curve:Different concentrations of adenosine wereused to obtain thestandard curve(Y=65036X-10.5,R2=0.998).X representsthemassof adenosine(0.01-0.05 mg/ml),whereas Y representsoptical density at 254 nm.
1.3.5 Statistical analysis
The data in this study were plotted via Origin Pro 8.0 software(http://www.OriginLab.com).Thestatistic significant analysiswasanalyzed by SPSSPASWStatistics16.0 software(SPSSLab Corporation,http://www.spss.com),and the level of significancewasgiven asvaluesof Prob>Flessthan 0.05.
2 Results and analysis
2.1 Morphology of P.hepiali myceliaand spores
Filamentous hyphae of P.hepiali did not aggregate to form pelletsin shaking flask.Asexual sporulation began after 36 h of incubation,and a large number of spores were produced.Morphology of P.hepiali mycelia and spores in submerged culture were observed by scanning electron microscope(Fig.1).The reproduced sporeswere able to germinate when introduced into anutrient-rich environment.

Fig.1 Morphology of P.hepiali mycelia(A)and asexual spores(B)in submerged culture under scanning electron microscope
2.2 Timecourseof RBF
Fig.2 shows the time course of biomass,spore number,and metabolites production in the first three cycles of RBF.Thethreelinesrepresent thefirst three fermentation cyclesof RBF.
After inoculation,all of thesporesweregerminated within 6 h and filamentous mycelia was grew out of the spores.The biomassof P.hepiali increased quickly to 25 g/L within 2.5 d of incubation(Fig.2A).After 2 d of incubation,P.hepiali in the flasks sporulated asexually,and spore number in the fermentation broth increased to 0.6×108spores/ml(Fig.2B).The sporesin thefermentation broth on day 2 wereused astheinoculum for next batch of RBF.The production of adenosine and crudetriterpenoids(Fig.2Cand 2D)accumulated from day 0 to 4,although the biomass did not increase significantly from day 2.5.After incubated for 4 d,thesporeconcentration of 1.5×108spores/ml,biomassof(25.0±0.3)g/L,adenosine production of(0.93±0.5)mg/L,and crude triterpenoids production of(0.33±0.2)g/L in thefermentation broth wereobtained.

Fig.2 Time course of biomass,spore number,and metabolites production in the first three cycles of RBF
2.3 Stability of twenty-cycle RBFof P.hepialid
To check the stability of asexual sporulation and bioactivecompoundsformation for along-lasting operation,a total twenty-cycle RBFwere performed.Asshown in the Table 1,the biomass of P.hepialid on the 4 th day of fermentation changed between 25.5 g/L and 27.2 g/L,and no significant difference of biomass among different fermentation batches was observed(P>0.05).Spore numbers(0.8×108to 1.4×108spores/ml),adenosine production(49.5-62.3 mg/L),and crude triterpenoids production(126.3-142.7 mg/L)also remained constant throughout the RBFprocess(P>0.05).It indicated that this RBFprocess with P.hepiali exhibited good stability and sustainability.
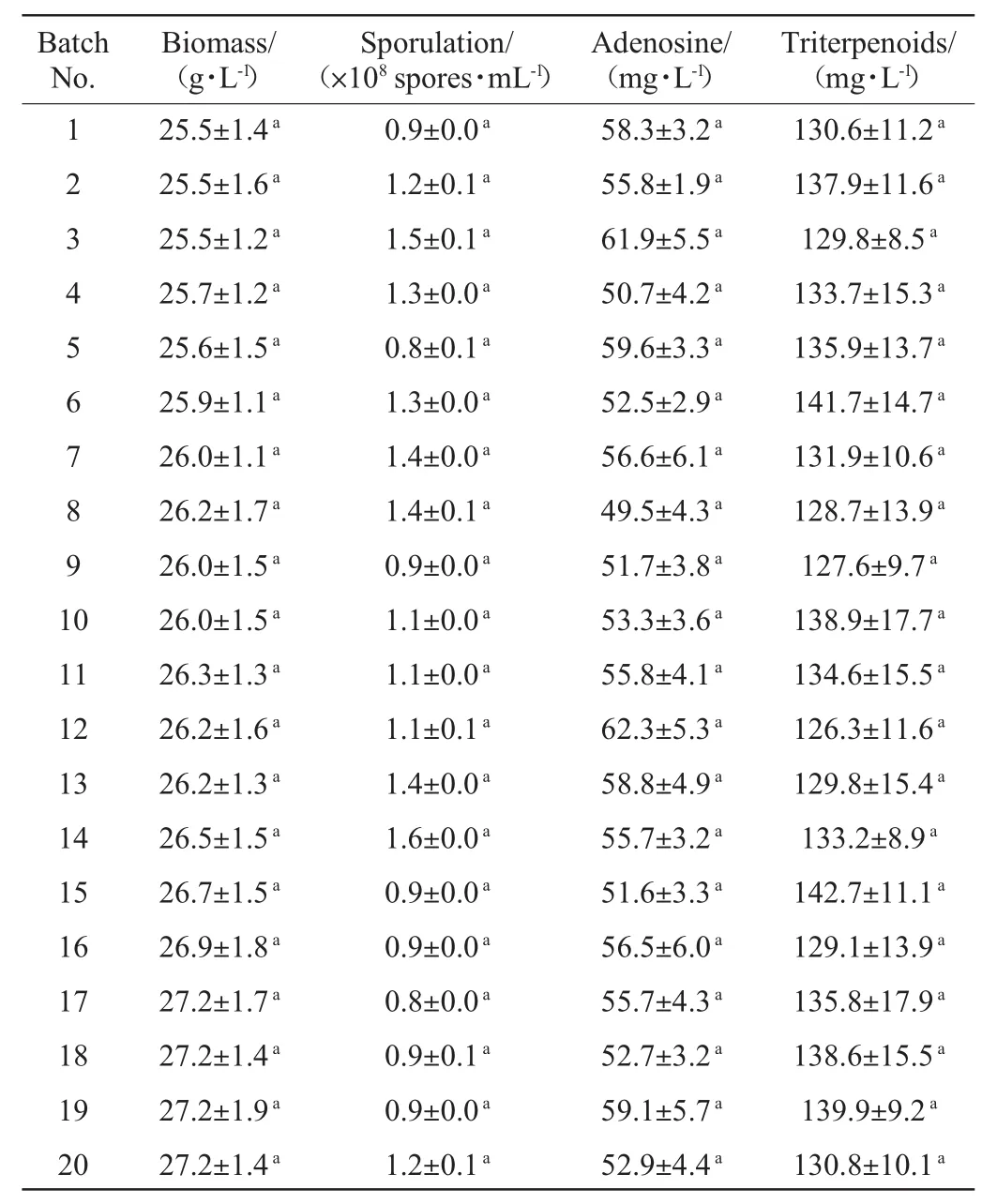
Table1 Biomass,sporulation,and triterpenoids and adenosine productions of P.hepiali during the 20 fermentation batches
3 Conclusion
In this study,twenty-cycle RBFof P.hepiali was established,by using an asexual spores-based inoculation method.Preparation of mycelial inoculum isnot needed during thefor initiating the fermentation process.The RBFprocess notably reduces cost and labor since it is omitting out inoculums preparation each time,which make the production more expensiveand extravagant.

