The OncoPPi network: cancer-associated protein-protein interaction networks for therapeutic target discovery
FU Haian
(Department of Pharmacology, Emory University School of Medicine, Atlanta, Georgia 30322, USA)
Abstract:As genomics advances reveal the cancer gene landscape, a daunting task is to translate these enormous volumes of information into efficacious genomics-based treatments for cancer patients. To address this challenge, the NCI Cancer Target Discovery and Development (CTD2) Network was created in the US. As a member of the CTD2 Network, the Emory CTD2 Center focuses specifically on the functional dimension of the cancer genome to establish re-wired protein-protein interaction (PPI) networks in cancer for therapeutic target discovery. To achieve this goal, high throughput screening technologies were utilized along with high throughput informatics approach. We aim to rapidly map molecular interactions among cancer associated proteins, leading to the generation of a cancer-associated PPI (OncoPPi) network. OncoPPi is a focused PPI resource that links cancer genes into a signaling network for discovery of PPI targets and network-implicated tumor vulnerabilities. This resource is accessible from the Emory OncoPPi portal. Emory’s approach is an integral component of the CTD2 network, which allows timely data sharing and close collaboration. Together, we aim to develop the next generation of pathway-perturbation agents to selectively disrupt re-wired oncogenic signaling networks for genomics-directed precision medicine.
Keywords: genomics; cancer; protein-protein interaction; target; network
0 Introduction
Comprehensive molecular characterization of human cancers driven by large-scale genomics initiatives, such as Therapeutically Applicable Research to Generate Effective Treatment (TARGET), Cancer Genome Characterization Initiative (CGCI), and The Cancer Genome Atlas (TCGA) Research Network, has led to the generation of vast amounts of tumor-derived sequencing data. Integrated analysis of such data has revealed driver oncogenes and inactivated tumor suppressor genes that are essential for the development and progression of cancer, and has re-defined the molecular subtypes of cancers. However, it remains a daunting challenge to determine how these genomic changes can be exploited to rapidly develop safe therapies. The NCI Cancer Target Discovery and Development (CTD2) Network in the US (https://ocg.cancer.gov/programs/ctd2) (Fig.1) aims to accelerate the translation of these enormous volumes of information into efficacious genomics-based medicine for the treatment of patients with cancer. As one of twelve centers in the CTD2Network, the Emory CTD2Center focuses specifically on the functional dimension of the cancer genome by interrogating re-wired protein-protein interaction (PPI) networks for therapeutic target discovery.
Fig.1 Emory CTD2 Center is one of 12 national centers in the NCI CTD2 Network in the U.S. Other centers include teams from Harvard/MIT’s Broad Institute, Columbia University, Dana Farber Cancer Institute, Fred Hutchinson Cancer Research Center, Johns Hopkins University, Oregon Health & Science University, Stanford University, University of California San Diego, University of California San Francisco, and University of Texas MD Anderson Cancer Center
1 Translating genomics data into PPI networks for therapeutic manipulation
As genomics advances have revealed critical chromosomal abnormalities, it has become clear that the impact of a specific mutation is not restricted to the activity of the particular gene product that carries the mutation. Intra- and intercellular proteins exert their functions primarily through interactions with other cellular components through well-orchestrated signaling networks. As such, the impact of an oncogenic mutation can spread along a signaling network to alter the activity of normal effector proteins, propagating the altered biological information to induce a pathological phenotype in tumors. For example, activating mutations in EGFR often lead to persistent stimulation of AKT, ERK and STAT to drive uncontrolled cell survival, proliferation, and tumor growth. Thus, disruption of PPIs at the junction of signaling networks critical for growth control may impair oncogenic signal transduction and attenuate tumorigenesis. Thus, understanding how cancer driver mutations are integrated within growth control signaling networks may lead to new opportunities for pathway perturbation and novel therapeutic discovery.
Such a network concept is particularly relevant in light of the fact that a large number of cancer driver mutations have been found in tumor suppressor genes, although most current anticancer drugs target oncogenes. Traditionally, pharmacological manipulation of the loss of function of a tumor suppressor to restore its activity has been considered a difficult task, but network behavior should allow the restoration of tumor suppressor function through pathway perturbation. For example, dissociation of MDM2 and p53 may stabilize the tumor suppressor function of p53. Tumors with loss of CDKN2A often have upregulated CDK4 leading to increased CDK4/RB interaction. Inhibition of CDK4 or disruption of the functional association of CDK4 and RB may lead to reduced cell growth advantage in these tumors. Therefore, linking tumor suppressors to growth signaling networks has significant implications in revealing potential strategies to target tumor vulnerability in a patient population with a particular tumor suppressor alteration.
Another major revelation of the emerging cancer genomics landscape is that the majority of identified cancer driver genes (>70%) encode proteins with no enzymatic activity. Clinical successes from genomics-based targeted cancer therapies primarily revolve around oncogenic protein kinases, such as erlotinib for EGFR/L858R-baring cancer patients and crizotinib for patients with ALK-fusions. While we celebrate such impressive medical accomplishments, albeit only short-term remission in most cases, we recognize major hurdles with patients having tumors that carry non-enzyme-coding driver genes. These gene products participate in protein complexes that are involved in diverse cellular functions, ranging from cell cycle control, to epigenomic modulation, to RNA processing. Thus, it is essential to place these driver oncogene products in the context of protein-protein interaction networks in order to uncover their mechanisms of action and potential options for therapeutic intervention.
To address these daunting challenges and to leverage the tremendous genomics advances for accelerated therapeutic discovery, one approach is to define the functional dimension of the cancer genome by linking the actions of oncogenes and tumor suppressors to signaling pathways and PPI networks associated with acquired hallmarks of cancer and tumor vulnerabilities. We hypothesize that tumor-associated genomic changes transmit signals through PPI nodes and hubs that integrate tumorigenic pathways and networks to exert transformation and invasive phenotypes. Therefore, pathway perturbation through the disruption of vital oncogenic PPIs that constitute the basic units of these nodes and hubs would permit the functional annotation of these interactions in tumorigenesis and progression and allow for the discovery of new molecular targets for genomics-based precision therapies.
2 Discovering cancer targets in re-wired PPI networks using a high-throughput approach
As the initial step toward functional annotation of protein interactions, we used data mining to establish a collection of gene libraries that primarily include cancer drivers and associated genes(Fig.2). These driver genes are frequently mutated, deleted, or amplified in patient tumors as revealed by various genomics initiatives. To examine their interconnectivity, these candidate genes are subcloned and expressed in multiple technological platforms to map protein-protein interactions. These platforms include Time-resolved F?rster Resonance Energy Transfer (TR-FRET) technology that monitors direct protein-protein association due to stringent distance requirements (<100 ?) for signal generation. For efficient mapping of PPI networks, this study is powered by robot-driven high throughput screening technologies in a miniaturized high density plate format (384-/1 536-well). Significant PPIs are identified based on statistical parameters and used to build PPI networks with a systems biology approach. With this high throughput technology, our current work has not only confirmed known PPIs, but also identified a large number of novel PPIs among driver gene products and their associated proteins. Such a PPI network provides a framework for new avenues to interrogate the function of key driver oncogenes and tumor suppressor genes for therapeutic intervention. Our initial PPI screening has led to the generation of the first cancer-associated protein-protein interaction network that we termed OncoPPi network[1].
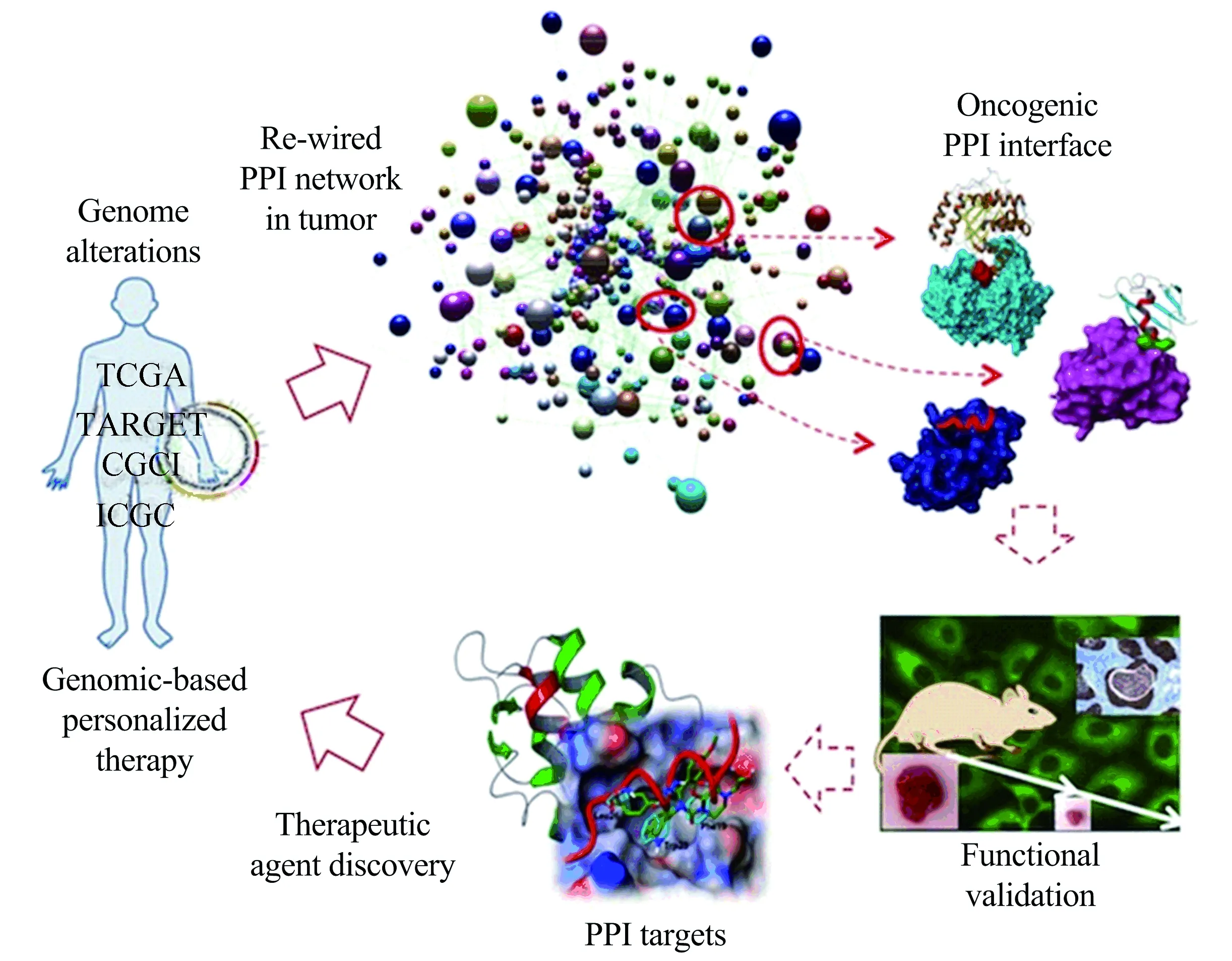
Fig.2 The Emory Center’s approach to accelerating genomics-based cancer target and therapeutic discovery
To directly associate oncogenic forms of cancer driver genes with potential therapeutic targeting strategies, we have taken a systematic screening approach to scan and compare the interaction profiles of wild-type driver genes with that of mutant counterparts. Our goal is to discover driver gene mutation-dependent re-wired PPI networks and specific mutant PPIs as oncogenic PPI targets. Associated with this goal, parallel screenings are being performed in cells treated with therapeutic agents to reveal therapeutic-induced PPI signatures.
To uncover the potential therapeutic significance of the identified oncogenic PPIs, computational and experimental approaches are combined to rapidly define minimal PPI interfaces and to discover minimal fragments (peptides) that are necessary for binding and sufficient to disrupt the intended PPIs. Such antagonist peptides are used to determine the requirement of selected PPIs for tumorigenesis and progression in cancer cell lines and mouse models. Supporting evidence from such studies along with patient-derived information will be used to advance the definition of a particular PPI as a potential drug target and/or biomarker for certain tumors.
Functional peptide antagonists may be further developed as therapeutic agents through advanced technologies such as peptide stapling and theranostic nanoparticles. In addition, the defined antagonist peptide structures and PPI interfaces can be used as pharmacophores forinsilicoscreening and provide the basis for novel assay design for HTS to discover small molecule modulators(Fig.2). Targeting mutant oncogene-specific PPIs in such a way is expected to develop therapeutic agents with much improved tumor selectivity, thus safety profiles.
3 Integrating PPI discoveries with the CTD2 network and the OncoPPi portal for data sharing
It is important to note that our genomics-based PPI network interrogation approach is an integral component of the CTD2Network, which allows timely data sharing and close collaboration(Fig.1). For example, powerful informatics tools developed in member centers are being implemented collaboratively to integrate our PPI results into large sets of cancer cell response profiling data from genome-wide RNAi/CRISPR screening and pharmacological agent screening to identify molecular signatures for tumor dependency. Innovative tumor models developed in the CTD2Network will be employed for oncogenic PPI validation studies. Together, integrated analysis of data sets from multiple platforms, including RNAi/CRISPR, chemical agents, and PPI screens, will accelerate the discovery and advancement of novel cancer targets for therapeutic development. For rapid data sharing with the scientific community, we have established the OncoPPi portal that integrates our experimental results with genomics, pharmacological agents, and clinical data sets (http://oncoppi.emory.edu/)[2]. Our data are freely available to scientific community through this interactive portal.
Placing cancer driver genes, both oncogenes and tumor suppressor genes, into the context of growth signaling PPI networks reveals new opportunities to design promising therapeutic strategies for protein targets with or without enzymatic activities. Such opportunities heavily rely on vigorous functional validation in relevant cancer models and linking of PPI node and hub functions to patient outcomes in a team science setting. Together, we can reach the once un-reachable, the PPI targets, to develop the next generation of pathway-perturbation agents to selectively disrupt re-wired oncogenic signaling networks for genomic alteration-directed precision medicine.
