基于8-羥基喹啉與d10金屬原位合成的雙核配合物的合成、晶體結構及其光致發光性質
高 翔 徐 微 吳昌麗 朱錫淼 區泳聰 吳建中
(華南師范大學化學與環境學院,廣州市能源轉化與儲能材料重點實驗室,廣州 510006)
In situ ligand synthesis involved in the research of coordination complexes is a special method to design and synthesis of intriguing organic ligands and coordinated structures with good potential applications,such as magnetism,luminescence and gas sorption[1-4].In recent years,a large number of in situ synthetic ligands have been discovered such as 5-substituted 1H-tetrazoles,[2+2]cycloaddition,[3+3]cycloaddition[5-9]Among such enormous cases,the in situ reaction for 8-hydroxyquinoline,which has been few reported yet[10-12],gave out an excellent ligand,7,7′-(ethane-1,1-diyl)diquinolin-8-ol))(H2L),with rich binding sites and transformable dihedral angle for two quinoline rings.Up to now,the in situ synthetic ligands H2L have mostly emerged in the structures involving heterometallic multinuclear units,such as transition metal ions(3d)-lanthanide ions (4f)[11],alkaline metal ions(3s)-3d[13].Because of the flexibility and symmetry of the quinoline rings which like a pair of oar in H2L ligand,homometallic multinuclear units could be constructed.Recently,we have reported a green in situ synthetic method for this H2L ligand in a homometallic dinuclear Znギcomplex[14],which structure are documented at the same time[12].
The research on the influence factor in constructing coordination structures is one of the most attractive areas throughout the development of chemistry.The guest molecules including solvents molecules and/or counter ions could affect dramatically the lattice structures[15-16].Furthermore,plenty of noncovalent interactions such as hydrogen bonds and/or π-π stacking between H2L ligands could make coordination complexes stable and diverse spatial structures,which is worth to explore the relationship between structures and properties[17-18].
Herein,we report three novel d10zinc and cadmium coordination complexes based on H2L ligand formed by 8-hydroxyquinoline in situ,[M2(HL)3]NO3·H2O(M=Cd(1),Zn(2,3)).Complexes 1~3 contain trilobed wheel-like positive charged dinuclear units[M2(HL)3]+which are rare in the structure of complexes[19-21],composed of three partial deprotonated HL-ligands and two metal ions.Meanwhile,it is interesting that the guest molecules,one NO3-counterion and one free water molecule,are crystallized in different places for the complexes and make the lattice structures different through different noncovalent interactions.It is intriguing for us to explore the influence between space structure and properties.Further,the thermal stabilities and the luminescent properties of complexes and the in situ synthetic ligands have been studied.The fluorescence of complex 1~3 show green emission,which are obviously bathochromic shift comparing to that of organic ligands H2L which are firstly extracted from complexes.
1 Experimental
1.1 Materials and physical measurements
All reagents and solvents were commercially available and used as received withoutfurther purification.Power X-ray diffraction (PXRD)data for microcrystalline samples of 1~3 were recorded at 293 K on a Bruker D8 Advance X-ray powder diffractometer with Cu Kα radiation(λ=0.154 184 nm)at 40 kV and 40 mA.The scans were run from 5°to 50°.Simulated PXRD patterns of 1~3 were generated by Mercury software.Infrared spectra were recorded in KBr tablets in the range of 4 000~400 cm-1on a Nicolet FT-IR-170SX spectrophotometer.Thermogravimetric analysis(TGA)was performed on a NETZSCH TG209F3 thermogravimetric analyzer at a heating rate of 10℃·min-1in N2atmosphere.The emission and excitation spectra of solid samples of 1~3 were measured on a Hitachi F-4600 Spectrophotometer at room temperature with a xenon arc lamp as the light source.
1.2 Syntheses of the complexes 1~3 and ligand
1.2.1 Synthesis of[Cd2(HL)3]NO3·H2O(1)
Complex 1 was successfully synthesized by solvothermal reaction.A mixture of Cd(NO3)2·6H2O(151.7 mg,0.5 mmol),8-hydroxyquinoline(72.5 mg,0.5 mmol)and 0.25 mol·L-1NaOH(1 mL)in C2H5OH/H2O(4 and 8 mL,respectively)solution was heated in a stainlesssteel reactor with a Teflon liner(23 mL)at 160℃for 72 h and cooled to ambient temperature for 48 h.Brown polyhedral crystals were obtained in 62.8%yield based on 8-hydroxyquinoline ligand.Elemental analysis Calcd.for C60H47N7O10Cd2(%):C,57.61;H,3.79;N,7.84.Found(%):C,57.31;H,3.80;N,7.23.1.2.2 Synthesis of[Zn2(HL)3]NO3·H2O(2)
Complex 2 was obtained by a similar method as described for 1 using Zn(NO3)2·6H2O(152.5 mg,0.5 mmol)in place of Cd(NO3)2·6H2O,and without NaOH in the system.Yellow block crystals were obtained in 58.9% yield based on 8-hydroxyquinoline ligand.Elemental analysis Calcd.for C60H47N7O10Zn2(%):C,62.29;H,4.10;N,8.48.Found(%):C,62.38;H,4.21;N,8.27.
1.2.3 Synthesis of[Zn2(HL)3]NO3·H2O(3)
Complex 3 was synthesized by a mixture of Zn(NO3)2·6H2O(151.5 mg,0.5 mmol),8-hydroxyquinoline(73.5 mg,0.5 mmol)in C2H5OH/H2O(8 and 2 mL,respectively)solution was heated in a stainlesssteel reactor with a Teflon liner(23 mL)at 120℃for 72 h and cooled to ambient temperature for 18 h.Yellow block crystals were obtained in 49%yield based on 8-hydroxyquinoline ligand.Elemental analysis Calcd.for C60H47N7O10Zn2(%):C,62.29;H,4.10;N,8.48.Found(%):C,62.22;H,4.16;N,8.34.
1.2.4 H2L ligand extraction
H2L ligand has been extracted by dissolving the crystals of complex 1(12.6 mg)in 15 mL CH2Cl2,and gradually add Na2S aqueous solution.After centrifugation and filtration.The mother liquid was moved to a clean small beaker,with the volatilization of solvent,the brownish yellow precipitate was collected,(7.3 mg,Yield:76.1%).The FT-IR spectra,1H NMR,and mass spectra of ligand H2L are shown in Fig.S4~S6.
1.3 X-ray crystallography
The single-crystal diffraction data of complexes 1~3 were collected on Oxford Diffraction Xcalibur Nova with Cu Kα radiation(λ=0.154 184 nm)at 150(2)K.The absorption corrections were applied by SADABS[22].The structure were solved by direct method,and all non-hydrogen atoms were refined anisotropically by full-matrix least-squares techniques using SHELXTL[23].Hydrogen atoms connected to carbon atoms were idealized positions and riding model was used for their refinement.Further details of the crystal data and structure refinements for complexes 1~3 are summarized in Table 1.The bond lengths,angles and Hydrogen bonds are represented in Table S1 and S2,respectively.The presence of-CH-CH3in H2L ligand have been confirmed by single crystal X-ray diffraction data and FT-IR spectra analysis (Fig.S3)with the characteristic peaking at 2 972 cm-1(νas),2 929 cm-1(νs),2 893 cm-1(ν)and 1 247 cm-1(δs).
CCDC:1842916,1;1842917,2;1842918,3.

Table 1 Crystal data and structure refinements for 1~3
2 Results and discussion
2.1 Synthesis
As mentioned above,synthesis is the heart of chemistry.The development of green synthetic methods is of great benefit to the environment and chemistry.While building the complexes,we found that NaN3,a toxic substance for living beings used in the previous literatures,was not necessary in the in situ synthesis of 8-hydroxyquionline and can be replaced by other bases,such as NaOH,Na2CO3and NaHCO3,even withoutanyadditionalbase.However,the environment of in situ synthesis should not be acid because no crystals could be obtained when tiny Lewis acids are mixed in the system.Furthermore,for zinc complexes,different temperatures could affect the crystallization of structures.To improve the product yields of complex 3,different ratio for the ethanol and H2O are explored and show that the volume ratio of ethanol/H2O,equal to 8∶2,is the best ratio.For cadmium complex,highertemperaturescould improve the yields,and unfortunately could not play a part in the formation of another structure which has not been obtained yet even.This moderate in situ reaction conditions give suggestive and significant ways to explore greener synthetic methods.
2.2 Structural description
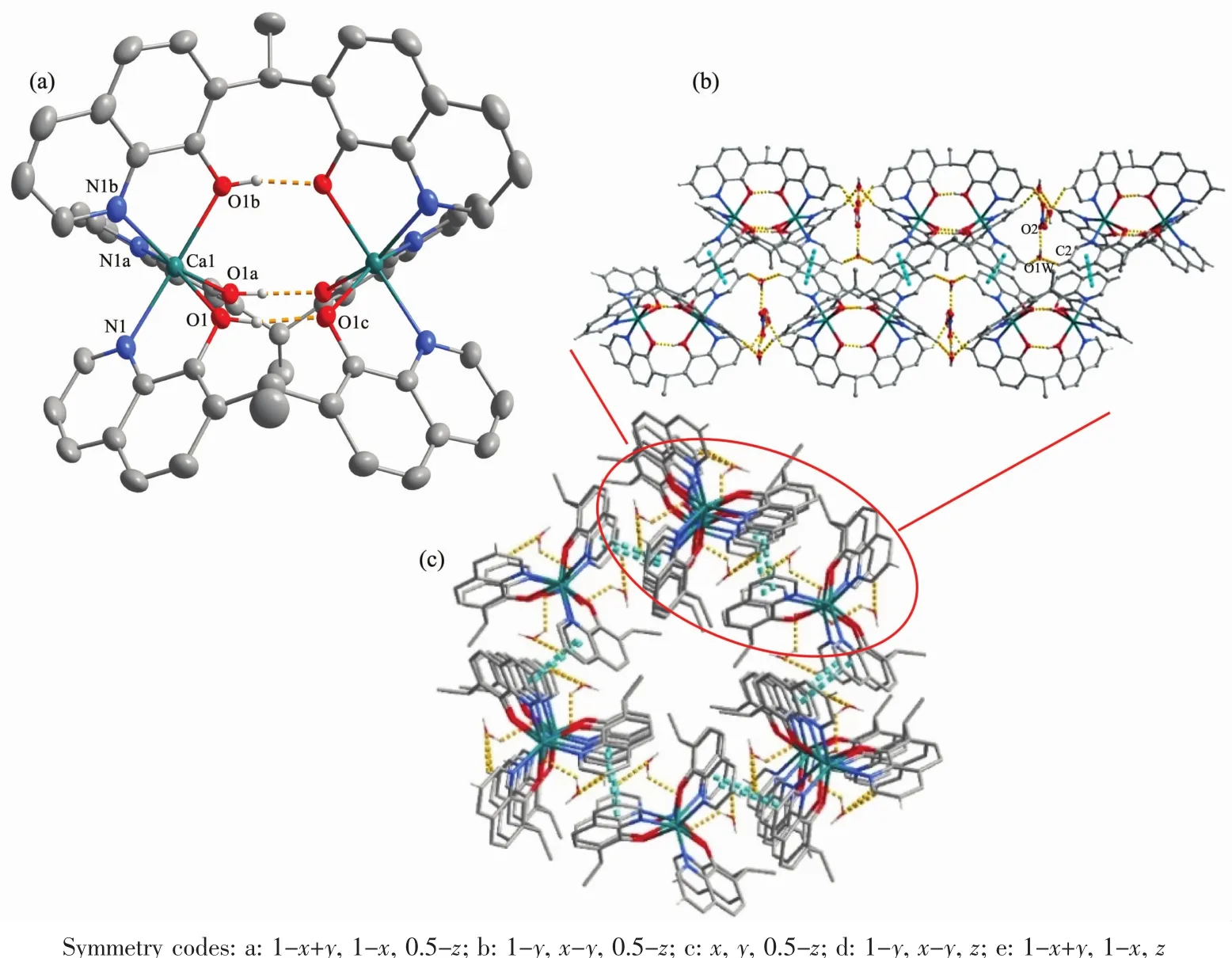
Fig.1 (a)Coordination structure for the[Cd2(HL)3]+unit in 1 with thermal ellipsoids shown at 50%probability;(b)View of 1D supramolecular chain;(c)View along c axis of 3D supramolecular framework extended by the intermolecular hydrogen bonding and π-π stacking interactions
Single-crystal structure analysis reveals that the structures of complex 1 and 3 crystallize in the same space group P63/m,and the 63screw axis is along c axis.Therefore,we just describe the structure of complex 1 in detail.The asymmetric unit of 1 consists of one third of crystallographic independent Cd2+cation,half of a HL-ligand,one-sixth of lattice water molecule and NO3-.As shown in Fig.1a,Cd2+ion is surrounded by three oxygen atoms and three nitrogen atoms from 8-quinolinol units of HL-ligands in a distort octahedral geometry.Two Cd2+cations are connected by three HL-ligands to form a trilobed wheel-like positive charged cadmium dinuclear unit[Cd2(HL)3]+(Cd-O 0.231 32(16)nm,Cd-N 0.230 0(2)nm).The partially deprotonated HL-ligand exhibit a particular intramolecular hydrogen bond(O(1)…O(1c)0.243 9(3)nm),which play an importantrole in structuralstability and charge balance.Meanwhile,the configuration of HL-ligand is V-shaped with the dihedral angle of 61.56(14)°,which differ from the dihedral angle of 56.05(2)°,89.6°and 83.1°in literature[11,13].As shown in Fig.1b,the C2 atoms in 8-quinolinol rings are connected by the weak hydrogen bonds with the free water molecules O1w(C(2)…O(1W)0.309 2(9)nm),resulting in the formation of 1D supramolecular chains.Furthermore,intermolecular hydrogen bonding interactions between O1w atoms and the O2 atoms from NO3-(O(1W)…O(2)0.256 1(12)nm),and π-π stacking interactions between the pyridine rings(Cg4…Cg4a 0.346 8(2)nm;Cg4 is the centroid of the N1-C1-C2-C3-C4-C9 ring;Symmetry codes:a:y,-x+y,-z)expand the structure into 3D supramolecular network(Fig.1c).
For complex 2,the coordination unit[M2(HL)3]+is similar to that of 1,but the lattice structures are different.Complex 2 crystallizes in a space group R3.The rotation axis is the link of N atoms in nitrate ions along c axis too.As shown in Fig.2a,weak hydrogen bonds between the C5,C3,C12 from HL-ligands and O3 atoms from NO3-ions extend the[Zn2(HL)3]+units into 2D supramolecular layers(C(5)…O(3g)0.342 0(4)nm,C(3)…O(3g)0.357 3(4)nm,C(12)…O(3d)0.369 7(4)nm).Further,another hydrogen bonding interactions(C(10)…O(3)0.334 8(4)nm)connect the layers into 3D supramolecular network(Fig.2b).The aqua molecules are disorderly situated in the relative hydrophobic environment.Comparing to the two kinds of lattice structures,different interactions between guest molecules(NO3-groups and aqua molecules)and[M2(HL)3]+units are the key role.
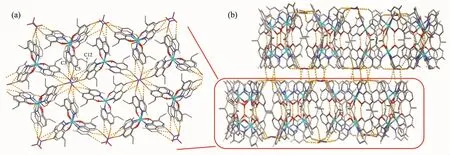
Fig.2 (a)View along c axis of 2D supramolecular layer;(b)3D supramolecular network connected via weak hydrogen bonds between H atoms of carbon and O atoms of NO3-ions
2.3 Thermogravimetric analysis
The thermal stabilities of complexes 1 and 2 were determined by TG in the temperature range of 30~800 ℃ under nitrogen gas(Fig.3).The pure phase of complexes 1~3 were confirmed by the PXRD(Fig.S1 and S2).Complex 1 gradually lost a weight of 1.81%from room temperature to 310℃,releasing one lattice water (Calcd.1.44%).Meanwhile,complex 2 gradually lost one lattice water(Calcd.1.56%,Obsd.1.89%),from room temperature to 350℃.And then forboth complexes,alargesudden weightloss occured.It indicates the cation units in the structures are both highly thermal stable,but complex 2 have a better thermal stability,due to the difference in space stack interactions caused by different locations of guest molecules H2O and NO3-.Interestingly,the weight loss of the two complexes is obviously different.In 1,the weight loss should correspond to the decomposi-tion of one HL-ligand(Calcd.25.2%);while in 2,that might be due to the loss of one HL-ligand and one NO3-counter ion (Calcd.32.6%).From structural analysis,nitrate groups in 2 form hydrogen bonds to the cation units[M2(HL)3]+much more than those groups in 1.Therefore,while the HL-ligand break away from the structure,the NO3-group in 2 might be more fragile.
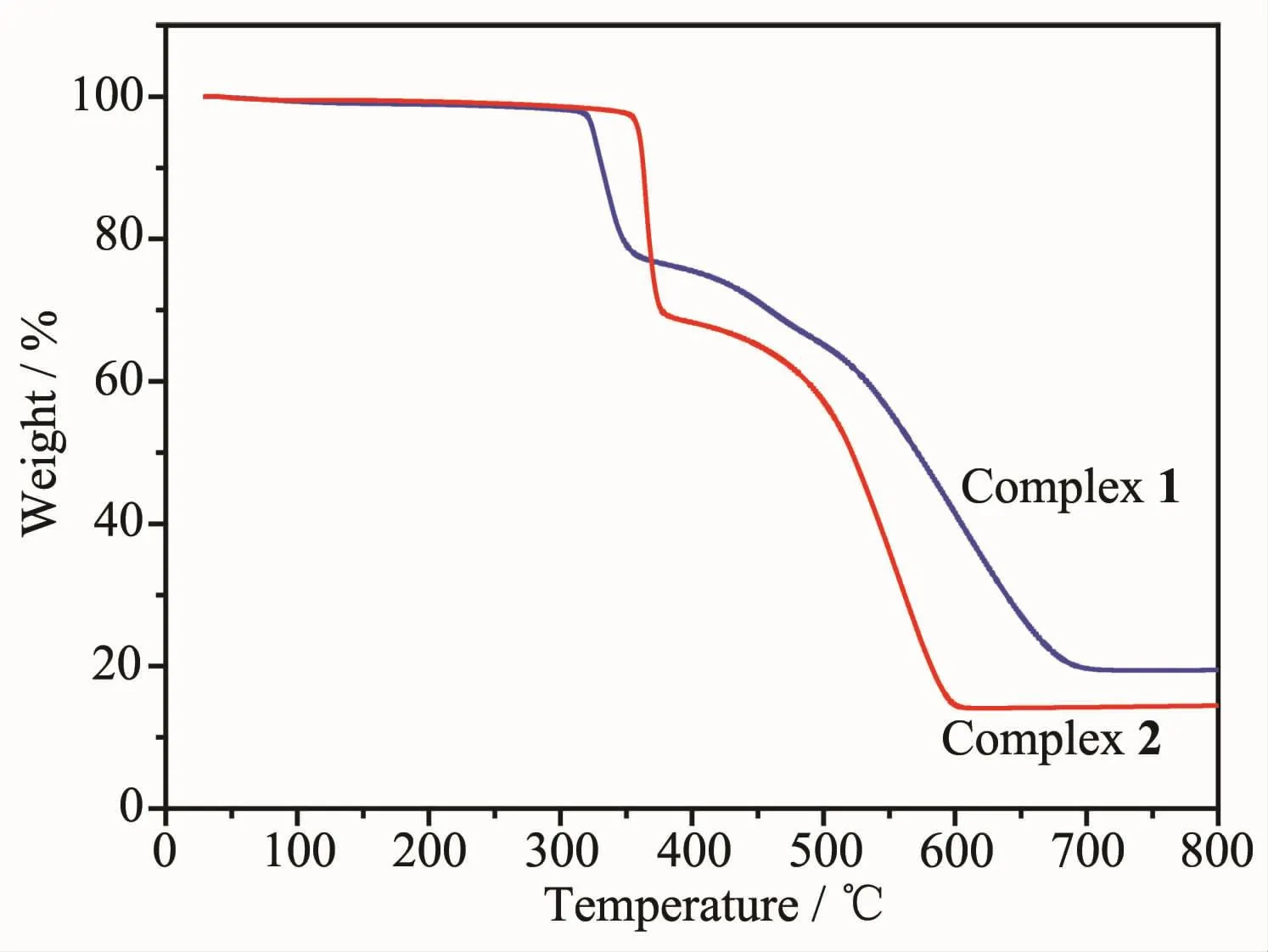
Fig.3 TG curves of complexes 1 and 2
2.4 Luminescent properties
Although there are several structures about the in situ synthetic ligand H2L,it is not reported about the luminescent properties of the ligand.As the molecular structure of ligand H2L contains two 8-hydroxyquinoline units which connected by an ethyl group,it is possible to exhibit aggregation-induced emission(AIE)effect[24],so we extracted it from the complex 1 and explored its fluorescence properties.The solid-state fluorescence shows that the emission peak is 484 nm,excited at 367 nm(Fig.4a).While dissolved in acetone,and deionized water is added dropwise to the solution,unfortunately,the results show the ligand H2L has no AIE characteristics.The solid-state luminescent properties of the as-synthesized samples 1~3 are also investigated at room temperature(Fig.4b).The fluorescence of complexes 1 and 3 showed green emission peaks at 518 nm (λex=430 nm)and 503 nm (λex=375 nm),respectively.However,the fluorescence of complex 2 exhibited a yellow-green emission peaks at 551 nm(λex=425 nm),which had a similar curve and showed an obvious bathochromic-shifted emission by 48 nm comparing to the fluorescence of complex 3.The result might be related to the different lattice structures of two complexes,which composed of different noncovalent interactions of guest molecules H2O and NO3-.This observation is meaningful in deeply understanding the relationship between structure and fluorescence performance.Furthermore,the lifetimes for complexes 1 and 2 were ca.7.8 and 2.83 ns,respectively[25-26].The short lifetime suggests that luminescence should be attributed to the intraligands fluorescent emission rather than the LMCT(ligand-to-metal charge transfer)(Fig.S8 and S9),the difference of lifetime may be the result of different noncovalent interactions.
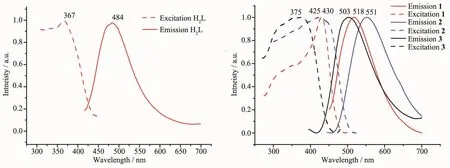
Fig.4 Luminescent spectra of H2L ligand and complexes 1~3
3 Conclusions
In summary,we have synthesized and characterized three novel d10zinc and cadmium complexes 1~3,and the ligand H2L has been extracted in the first time from complex 1.Complexes 1~3 contain novel positively charged dinuclear unit,[M2(HL)3]+,which is unique in the structure of complexes and exhibit green light emissions.H2L ligand show a blue light emission.Different locations of guest molecules in structures result in different noncovalent interactions between guest molecules and positively charged dinuclear units,and the obvious bathochromic-shifted emission in three complexes.It is meaningful in deeply understanding the relationship between lattice structures and fluorescence performance.
Supporting information is available at http://www.wjhxxb.cn

