Influence of dehydrating agents on the oxidative carbonylation of methanol for dimethyl carbonate synthesis over a Cu/Y-zeolite catalyst☆
Dong-Ho Lee ,Jiin You ,Je-Min Woo ,Jung Yoon Seo ,Young Cheol Park ,Jong-Seop Lee ,Hyunuk Kim ,Jong-Ho Moon ,*,Seung Bin Park *
1 Greenhouse Gas Laboratory,Korea Institute of Energy Research,152 Gajeong-ro,Yuseong-gu,Daejeon,34129,Korea
2 Department of Chemical and Biomolecular Engineering,Korea Advanced Institute of Science and Technology,291 Daehak-ro,Yuseong-gu,Daejeon,34141,Korea
3 Energy Material Laboratory,Korea Institute of Energy Research,152 Gajeong-ro,Yuseong-gu,Daejeon,34129,Korea
1.Introduction
Dimethyl carbonate(DMC)is a versatile chemical widely used in industry as a methylating agent,oxygenated fuel additive,and secondary battery electrolyte because ofits low toxicity and rapid biodegradability[1-4].In particular,among various applications,DMC has been recently identified as a promising oxygenated fuel additive to replace methyltert-butyl ether(MTBE)in gasoline or diesel oil due to its high oxygen content and good blending octane[5].
DMC is easily produced by reaction of phosgene with methanol,but the chemical process has several problems due to the toxicity of phosgene[6,7].Therefore,new DMC processes have been developed that do notuse phosgene,including the oxidative carbonylation of methanol in liquid phase(Enichem,Italy),the methyl nitrite process(Ube,Japan),the ester exchange process(Bayer,Germany),the vapor-phase oxidative carbonylation of methanol(Dow,USA),and the direct synthesis of methanol and carbon dioxide[8-12].
The oxidative carbonylation of methanol using copper based catalysts has been considered as one of the safest pathways for producing DMC,without the usage of phosgene,the high toxicity of material[13-16].Recently,many studies have focused on increasing the MeOH conversion(XMeOH)and the DMC selectivity(SDMC)of the oxidative carbonylation reaction.Copper catalysts with various supports such as zeolites and activated carbon have been used in oxidative carbonylation reactions[15,17],and CuCland CuCl2have exhibited excellent activity and selectivity as catalyst precursors in the synthesis of DMC.However,when CuCl and CuCl2are used,the acidity increases due to HCl formation during the oxidative carbonylation process,resulting in the corrosion of the reactor[7].Thus,it is important to reduce the Cl content of the catalyst.
King[16,18]prepared Cu/Y catalyst by the solid-state ion exchange(SSIE)of CuCl and HY zeolite.The Cu/Y catalyst was active and stable in oxidative carbonylation of methanol.And,Andersonet al.[13,19]prepared Cu/Xand Cu/ZSM-5 catalysts by the SSIE of CuCl and HXor HZSM-5 zeolites.The Cu-O species formed on these zeolites were proposed as the active species in oxidative carbonylation of methanol[20-22].After oxidative carbonylation of methanol,DMC was the primary product over Cu/Y catalyst,whereas byproduct,dimethoxy methane(DMM),was the major product generated over Cu/ZSM-5 catalyst and Cu/MOR catalyst.The higher catalytic activity and DMC selectivity of the Cu/Y catalyst were attributed to the weaker adsorption of CO onto the Cu+cations in Y zeolite[23].
The overall reaction pathway for the oxidative carbonylation reaction during DMC synthesis from carbon monoxide,oxygen,and methanol is shown below.As the equation indicates,this reaction produces H2O as a by-product.The removal of H2O during the reaction should thus accelerate the forward reaction,as per Le Chatelier's principle.

This simple hypothesis motivated us to study the influence of dehydration on such an oxidative carbonylation reaction so as to improve the MeOH conversion.Herein,we report the influence of different metal oxides(ZnO,MgO,and CaO)on the oxidative carbonylation of methanol.The dehydrating agents were characterized by thermogravimetric and differential thermogravimetric(TG/DTG)analysis and X-ray diffraction(XRD)before and after the reaction to con firm that the dehydration reaction occurredviathe metal oxide.
2.Experimental
2.1.Preparation of the catalyst
The Cu/Y-zeolite catalyst was prepared by the ion exchange method from CuCl2·2H2O(99.0%,Junsei,Japan)and commercial Y-zeolite in its NH4form(Aldrich).When preparing the catalyst,13.5 g of CuCl2·2H2O(99.0%,Junsei,Japan)was first dissolved in 30 ml of distilled water.Then,10.0 g of NH4Y-zeolite was added and stirred at 298 K.The product was subsequently dried at353 K for 12 h.The physically adhered Cu and Cl on the catalyst surface were removed by washing with distilled water several times.Finally,the residual water was removed by drying again at 353 K for 12 h.The obtained powder was calcined in a tube furnace under N2at 873 K for 24 h.
2.2.Characterization of the catalyst
X-ray fluorescence(XRF)tests were carried out to evaluate the Cu and Cl contents of the catalyst using a ZSX Primus instrument(Rigaku,Japan).The specific surface area and pore size of the catalyst were determined by nitrogen adsorption experiments(Autosorb-1,Quantachrome Instruments,USA).The samples were degassed at 473 K for 6 h prior to the measurements.XRD patterns were obtained on a Rigaku D/Max-2500(Japan)diffractometer with CuKα(40 kV,100 mA)radiation in the diffraction angle(2θ)range of 5-90°at a rate of 5(°)·min-1.Temperature-programmed desorption of ammonia(NH3-TPD)experiments were performed to determine the acidity of the catalyst on a BELCAT-M instrument(BEL,Japan).As a pretreatment,100 mg of the sample was degassed at 573 K under helium flow for 1 h,after which the temperature of the sample was maintained at 373 K.Then,5%NH3/He was introduced in the sample cell for ammonia adsorption.After adsorption,the temperature of the sample was raised from 373 to 1073 K at a rate of 10 K·min-1.Thermogravimetric and differential thermogravimetric(TG/DTG)analyses were carried out on an SDT Q600 instrument(TA Instruments Co.,USA).The sample was heated from 298 K to 1100 K at a heating rate of 10 K·min-1under nitrogen at a flow rate of 100 ml·min-1.
2.3.Catalytic activity
The stainless autoclave reactor containing a 50 ml Teflon lining was used for the oxidative carbonylation reaction tests.First,0.2 g of the catalyst and 0.1 g of the dehydrating agent(ZnO,MgO,or CaO)were introduced in the reactor with 10 ml of methanol(Acros,99.9%)under 3.67 MPa of carbon monoxide(99.99%)and 1.83 MPa of oxygen(99.99%).The reaction tests were carried out at 403 K and 5.50 MPa(total pressure)for 18 h.The MeOH,CO,and O2with a 1.00:1.72:0.86 molar ratio was used for the DMC synthesis.After the reaction,the reactor was cooled down to 273 K to prevent the evaporation of light products such as methylformate(MF,b.p 305 K)and dimethoxymethane(DMM,b.p.315 K).All the condensed products were filtered through a syringe filter(PTFE,0.45 μm)and analyzed by gas chromatography(6890A,Agilent Co.,USA,FID detector with DB-WAX).Since all the experiments of this paper were carried out using batch reactors,it was not easy to analyze gas composition such as CO2or CO.The MeOH conversion and DMC selectivity were calculated as follows:

Since the main purpose of this study is con firming the influence of dehydrating agents during the oxidative carbonylation reaction,only fresh catalysts were used.
2.4.Dehydration reaction
The reactions involved in the oxidative carbonylation process for DMC synthesis without and with a dehydrating agentare shown below:Oxidative carbonylation(without dehydration):2CH3OH+CO

If water(the by-product)is removed during the reaction,the reverse reaction is suppressed as per Le Chatelier's principle.As a result,the MeOH conversion could be increased due to the predominance of the forward reaction[24-27].The influence of three dehydrating agents(ZnO,MgO,and CaO)on this reaction was thus examined with the aim of increasing the MeOH conversion and DMC selectivity.In this study,the catalytic reaction experiments with or without dehydrating agent were carried out for 18 h.Although it is not possible to reach equilibrium in 18 h,it is sufficient time to compare the MeOH conversion depending on the effect of the dehydrating agents.
3.Results and Discussion
3.1.Characterization of the Cu/Y-zeolite catalyst
After the catalyst was prepared,a washing step with distilled water was carried out to reduce the Cl content that could potentially cause corrosion in the reactor and reduce the catalytic activity.To evaluate the effect of the washing step in the reduction of the Cl content,NH4-zeolite and the Cu/Y-zeolite catalyst were analyzed by XRF,and the results are presented in Table 1.After preparation of the catalyst,the Cu content of the Cu/Y-zeolite catalyst was 24.44 wt%,while the Cl content was only 1.31%after one washing step.

Table 1 XRF results for NH4Y-zeolite and Cu/Y-zeolite catalyst(wt%)
The specific surface area of NH4Y-zeolite and the Cu/Y-zeolite catalyst has been measured.Before the exchange of Cu ions,NH4Y-zeolite had a specific surface area of880 m2·g-1while,after exchange,the specific area decreased to 697 m2·g-1.
The XRD patterns of NH4Y-zeolite and the Cu/Y-zeolite catalyst are compared in Fig.1,where it can be seen that both patterns are similar and that no peaks corresponding to Cu and CuO species are present.This indicates that Cu was well dispersed on the surface of Y-zeolite during the preparation of the Cu/Y-zeolite catalyst.In addition,this result also suggests that the preparation of the catalyst does not have a significant effect on the framework structure of the zeolite,as the diffraction peak intensity is only slightly weakened[7,28].
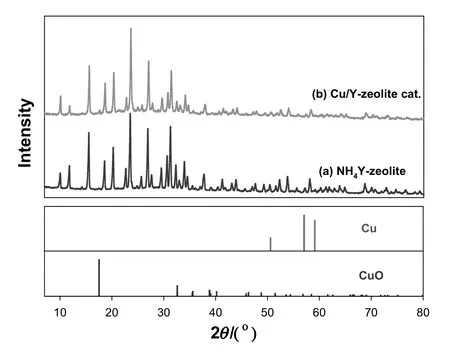
Fig.1.XRD patterns of(a)NH4Y-zeolite,(b)Cu/Y-zeolite catalyst.
The NH3-TPD results for the fresh(before the DMC synthesis reaction)and used Cu/Y-zeolite(after the reaction)catalysts are shown in Fig.2.The overall peak size of the used Cu/Y-zeolite catalyst sample is slightly reduced compared to that of the fresh Cu/Y-zeolite catalyst,while the position and size of the peak remain almost unchanged.This con firms that,in terms of the acid strength and acid center,there are almost no differences between the fresh and used Cu/Y-zeolite catalysts.
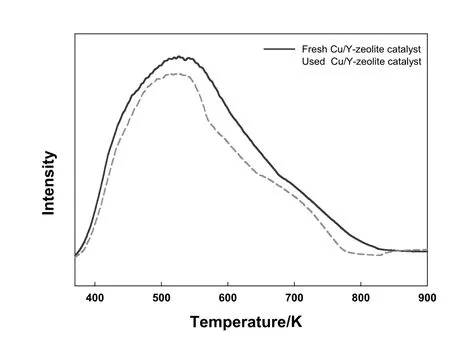
Fig.2.NH3-TPD profile of fresh and used Cu/Y-zeolite catalysts.
3.2.Influence of the metal oxide additive on the oxidative carbonylation of methanol
Three metal oxides(ZnO,MgO,and CaO)were added to the oxidative carbonylation reaction and the resulting MeOH conversion and DMC selectivity were compared.The metal oxide additives react with H2O to form metal hydroxides,as per the following reaction equations:

Fig.3 showed results of metal oxide additives influence on the MeOH conversion and DMC selectivity,and Table 2 showed composition of organic products.As shown in Fig.3,in the presence of only the Cu/Y-zeolite catalyst,the MeOH conversion(XMeOH)was 8.7%and the DMC selectivity was 39.0%.When CaO was added,the MeOH conversion(XMeOH)reached 14.6%,the highest value obtained for all the dehydrating agents used in this work.CaO reacts with H2O to form Ca(OH)2,thus removing the formed H2O.As a result,the MeOH conversion increases form 8.7%to 14.6%and DMC selectivity increases 39.0%to 53.1%.The reaction tests were carried out in the autoclave(batch reactor)for 18 h at 403 K and 5.5 MPa.As shown in Table 2,when MgO,which exhibits good dehydrating ability,was used,the DMC selectivity became higher than that of CaO,but the MeOH conversion became lower than that without a dehydrating agent(i.e.,using only the catalyst).Therefore,CaO was concluded to be the most effective dehydrating agent since,when used as additive,both the methanol conversion and DMC selectivity are superior to those without a dehydrating agent.

Fig.3.Effect of metal oxide additives on the oxidative carbonylation of methanol.

Table 2 Composition of the organic products
Table 3 shows the melting points of the metal oxides and the decomposition temperature of the metal hydroxides.Since all the melting points of the metallic species are above 1703 K(that is,beyond the temperature range of the TG/DTG instrument),they could not be determined by TG/DTG analysis.However,if the metal oxide reacts with H2O forming the corresponding metal hydroxide during the oxidative carbonylationreaction,the solid residue(comprising the Cu/Y zeolite catalyst,metal oxide,and metal hydroxide)can be analyzed by TG/DTG analysis to confirm the presence of metal hydroxides.

Table 3 Melting points of metal oxides and decomposition temperatures of corresponding metal hydroxides
Fig.4 shows the results of the TG/DTG analysis of the solid residue collected after the reaction.The recovered sample is composed of the used Cu/Y zeolite catalyst,any unreacted metal oxide(dehydrating agent:ZnO,MgO,or CaO),and any formed metal hydroxide(Zn(OH)2,Mg(OH)2,or Ca(OH)2).As shown in Fig.4(a)and(b),the first largest peaks in the DTG graphs are observed at around 373 K(that is,below 473 K),which arise from the mass loss due to moisture evaporation.In Fig.4(a),at~398 K(see Table 3),a secondary small peak is observed due to the decomposition of Zn(OH)2.Considering the size of the secondary peak,a relatively small amount of Zn(OH)2was formed by reaction of ZnO with H2O during the reaction.In Fig.4(b),a secondary DTG peak is observed at~623 K(see Table 3),which is the decomposition temperature of Mg(OH)2.This also con firms that Mg(OH)2was formed upon reaction of MgO with H2O during the reaction.Finally,in Fig.4(c),a large peak is also observed in the DTG graph at ca.373 K due to moisture evaporation.However,the largest mass loss is observed between 853 K(the decomposition temperature of Ca(OH)2,see Table 3)and 973 K.This means that a large amount of Ca(OH)2was formed by reaction of CaO with H2O.Since the size of the secondary peak(decomposition of the metal hydroxide)is larger,this means that a larger amount of metal oxide reacted with H2O compared to the other dehydrating agents.As a result,H2O was efficiently removed during the reaction.Additionally,as shown in Fig.3,when CaO is used as the dehydrating agent,the MeOH conversion is greatly improved.In accordance with Figs.3 and 4,the MeOH conversion and metal hydroxide formation are consistent with the order CaO?MgO>ZnO.

Fig.4.TG/DTG analysis of the solid residue(used catalyst,metal oxide and metal hydroxide)recovered after reaction:(a)ZnO,(b)MgO,and(c)CaO.
Fig.5 shows the XRD analysis of the fresh Cu/Y zeolite catalyst and the solid residue recovered after the reaction.The patterns in Fig.5(a)and(b)exhibit XRD peaks corresponding to the used Cu/Y zeolite catalyst,metal oxides(ZnO and MgO),and metal hydroxides(Zn(OH)2and Mg(OH)2).The formation of metal hydroxide increases with the increasing reactivity of the metal oxide toward H2O.Since the reverse reaction of oxidative carbonylation is minimized by removing the H2O by-product,the MeOH conversion increases.On the contrary,as the reactivity of the metal oxide decreases,the formation of the metal hydroxide is also reduced.As a result,the MeOH conversion decreases.After the reaction with a dehydrating agent,as seen in Fig.5(a)and(b),large and sharp peaks are observed for the metal oxides(ZnO and MgO),while small and board peaks are seen for the metal hydroxides(Zn(OH)2and Mg(OH)2).Because ZnO and MgO are less reactive toward H2O than CaO[Figs.3 and 5(c)],only trace amounts of the metal hydroxides(Zn(OH)2and Mg(OH)2)are formed.These results mean that the application of ZnO and MgO as dehydrating agents is not suitable for DMC synthesisviaoxidative carbonylation.Fig.5(c)shows the XRD peaks of the used Cu/Y zeolite catalyst,CaO,and Ca(OH)2.In contrast to the ZnO and MgO cases,the peaks corresponding to the metal oxide(CaO)are very small.In addition,since the reactivity of CaO toward H2O is much higher than those of ZnO and MgO,larger and sharper XRD peaks for the metal hydroxide are observed.This result shows that CaO has excellent reactivity toward H2O and that a large amount of Ca(OH)2was formed.The results of Figs.3-5 indicate that CaO is the most reactive dehydrating agent among the three metal oxides tested based on the batch experiments and the TG/DTG and XRD analyses.Furthermore,the excellent reactivity of CaO toward H2O was able to increase the MeOH conversion(XMeOH)of the oxidative carbonylation reaction by removing the H2O by-product.

Fig.5.Comparison of the XRD patterns of the fresh Cu/Y zeolite catalyst(bottom)and the solid residues recovered after the reaction:(a)ZnO,(b)MgO,and(c)CaO.
Three metaloxides(ZnO,MgO,and CaO)have ability for the remove of H2O during the reaction.Among them,since CaO is more basic than ZnO and MgO[29],the basicity of CaO is presumed to promote activation CH3OH and production of methoxy species during reaction[30].As a result,the highest MeOH conversion was obtained when using CaO.Therefore,CaO was considered as the best dehydrating agent in the oxidative carbonylation reaction.
4.Conclusions
This study investigated the influence of dehydration on the oxidative carbonylation of methanol for DMC synthesis.First,a Cu/Yzeolite catalyst was prepared by the ion exchange method from CuCl2and NH4/Y-zeolite.The catalyst was characterized by XRF,N2adsorption(BET method),XRD,and NH3-TPD to determine its Cu and Cl content,surface area,structure,and acidity.Next,three metal oxides(ZnO,MgO,and CaO)were used as dehydrating agents to minimize the reverse reaction by removing the H2O by-product of the oxidative carbonylation reaction.Among them,CaO showed the largest yield of metal hydroxide formation(Ca(OH)2),con firming its superior dehydrating ability.When CaO was used as the dehydrating agent,the MeOH conversion increased form 8.7%to 14.6%and DMC selectivity increased from 39.0%to 53.1%.The MeOH conversion increased upon the addition of a metal oxide in the order CaO?MgO>ZnO,while the DMC selectivity(SDMC)increased following the order MgO>CaO>ZnO.Finally,the fresh Cu/Y zeolite catalyst and the solid residue recovered after the reaction(comprising the used Cu/Y zeolite catalyst,metal oxide,and metal hydroxide)were analyzed by TG/DTG and XRD to con firm that the dehydration reaction occurredviathe metal oxide(MO+H2O→M(OH)2).
From the viewpoint of the potential industrial applications of the present work,the use of Cu/Y-zeolite as a heterogeneous catalyst and CaO as a solid dehydrating agent will contribute to the development of a continuous process for the DMC synthesisviaoxidative carbonylation of methanol.
[1]Y.Ono,Catalysis in the production and reactions of dimethyl carbonate,an environmentally benign building block,Appl.Catal.A Gen.155(1997)133-166.
[2]Y.Ono,Dimethyl carbonate for environmentally benign reactions,Catal.Today35(1997)15-25.
[3]M.A.Pacheco,C.L.Marshall,Review of dimethyl carbonate(DMC)manufacture and its characteristics as a fuel additive,Energy Fuel11(1997)2-29.
[4]X.Zheng,A.T.Bell,A theoretical investigation of dimethyl carbonate synthesis on Cu-Y zeolite,J.Phys.Chem.112(2008)5043-5047.
[5]Z.Du,L.Xiong,Z.Lin,X.Li,Y.Ding,Y.Wu,Oxidative carbonylation of methanol to dimethyl carbonate over Cu(II)-1,10-phenanthroline bromide complexes,Chin.J.Chem.Eng.22(2014)1117-1121.
[6]U.Romano,R.Tesel,M.M.Mauri,P.Rebora,Synthesis of dimethyl carbonate from methanol,carbon monoxide,and oxygen catalyzed by copper compounds,Ind.Eng.Chem.Prod.Res.Dev.19(1980)396-403.
[7]H.Zheng,J.Ren,Y.Zhou,Y.Niu,Z.Li,Preparation of Cu+/SiO2-ZrO2 catalysts for the oxidative carbonylation of methanol to dimethyl carbonate,J.Fuel Chem.Technol.39(2011)282-286.
[8]U.Romano,R.Tesel,G.Cipriani,L.Micucci,U.S.Patent 4218391,to Enichem chemical company(1980).
[9]H.Miyazaki,Y.Shiomi,S.Fujitsu,K.Masunaga,U.S.Patent 4384133,to Ube chemical company(1983).
[10]G.L.Curnutt,U.S.Patent 4625044,to Dow chemical company(1986).
[11]J.Nam,M.J.Choi,D.H.Cho,J.K.Suh,S.B.Kim,The influence of support in the synthesis of dimethyl carbonate by Cu-based catalysts,J.Mol.Catal.A Chem.370(2013)7-13.
[12]J.K.Nam,D.H.Cho,J.K.Suh,S.B.K.Kim,Dimethyl carbonate synthesis by methanol oxidative carbonylation,Kor.Chem.Eng.Res.49(2011)530-534.
[13]S.A.Anderson,T.W.Root,Investigation of the effect of carbon monoxide on the oxidative carbonylation of methanol to dimethyl carbonate over Cu+X and Cu+ZSM-5 zeolites,J.Mol.Catal.A220(2004)247-255.
[14]Y.Wang,R.Jiang,X.Zhao,S.Wang,Synthesis of dimethyl carbonate by gas-phase oxidative carbonylation of methanol over activated carbon-supported copper catalysts,J.Nat.Gas Chem.9(2000)205-211.
[15]K.Tomishige,T.Sakaihori,S.-i.Sakai,K.Fujimoto,Dimethyl carbonate synthesis by oxidative carbonylation on activated carbon supported CuCl 2 catalysts:catalytic properties and structural change,Appl.Catal.A Gen.181(1999)95-102.
[16]S.King,Reaction mechanism of oxidative carbonylation of methanol to dimethyl carbonate in Cu-Y zeolite,J.Catal.161(1996)530-538.
[17]M.S.Han,B.G.Lee,B.S.Ahn,H.S.Kim,D.J.Moon,S.I.Hong,The role ofcopper chloride hydroxides in the oxidative carbonylation of methanol for dimethyl carbonate synthesis,J.Mol.Catal.A Chem.203(2003)137-143.
[18]S.King,Oxidative carbonylation of methanol to dimethyl carbonate by solid-state ion-exchanged CuY catalysts,Catal.Today33(1997)173-182.
[19]S.A.Anderson,T.W.Root,Kinetic studies of carbonylation of methanol to dimethyl carbonate over Cu+X zeolite catalyst,J.Catal.217(2003)396-405.
[20]I.J.Drake,K.L.Fujdala,A.T.Bell,T.D.Tilley,Dimethylcarbonate productionviathe oxidative carbonylation of methanol over Cu/SiO 2 catalysts preparedviamolecular precursor grafting and chemical vapor deposition approaches,J.Catal.230(2005)14-27.
[21]I.J.Drake,Y.Zhang,D.Briggs,B.Lim,T.Chau,A.T.Bell,The local environment of Cu+in Cu-Y zeolite and Its relationship to the synthesis of dimethyl carbonate,J.Phys.Chem.B110(2006)11654-11664.
[22]R.Wang,Z.Li,Surface reactions of CuCl 2 and HY zeolite during the preparation of CuY catalyst for the oxidative carbonylation of methanol,Chin.J.Catal.35(2014)134-139.
[23]Y.Zhang,D.N.Briggs,E.De Smit,A.T.Bell,Effects of zeolite structure and composition on the synthesis of dimethyl carbonate by oxidative carbonylation of methanol on Cu-exchanged Y,ZSM-5,and Mordenite,J.Catal.251(2007)443-452.
[24]J.I.You,J.M.Woo,H.U.Kim,Y.C.Park,J.H.Park,J.H.Moon,Effect of dehydration on DMC synthesis over ceria catalysts,Clean Technol.22(2016)196-202.
[25]M.Honda,M.Tamura,Y.Nakagawa,S.Sonehara,K.Suzuki,K.i.Fujimoto,K.Tomishige,Ceria-catalyzed conversion of carbon dioxide into dimethyl carbonate with 2-cyanopyridine,ChemSusChem6(2013)1341-1344.
[26]M.Honda,M.Tamura,Y.Nakagawa,K.Nakao,K.Suzuki,K.Tomishige,Organic carbonate synthesis from CO 2 and alcohol over CeO 2 with 2-cyanopyridine:scope and mechanistic studies,J.Catal.318(2014)95-107.
[27]A.Bansode,A.Urakawa,Continuous DMC synthesis from CO2 and methanol over a CeO2 catalyst in a fixed bed reactor in the presence of a dehydrating agent,ACS Catal.4(2014)3877-3880.
[28]Y.Wang,H.Zheng,L.Zhong,K.Xie,Investigation of the interaction between Cu(acac)2and NH4Y in the preparation of chlorine-free CuY catalysts for the oxidative carbonylation of methanol to a fuel additive,RSC Adv.5(2015)10232-102331.
[29]L.Ding,P.Rahimi,R.Hawkins,S.Bhatt,Y.Shi,Naphthenic acid removal from heavy oils on alkaline earth-metal oxides and ZnO catalysts,Appl.Catal.A Gen.371(2009)121-130.
[30]Y.Zhang,A.T.Bell,The mechanism of dimethyl carbonate synthesis on Cuexchanged zeolite Y,J.Catal.255(2008)153-161.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Controlling dispersion and morphology of MoS2 nanospheres by hydrothermal method using SiO2 as template☆
- Morphological,mechanical and thermal properties of cyanate ester/benzoxazine resin composites reinforced by silane treated natural hemp fibers☆
- Thermal conductivity of PVDF/PANI-nanofiber composite membrane aligned in an electric field☆
- A simple strategy to synthesize and characterization of zirconium modified PCs/γ-Al2O3☆
- Antioxidant activity of phytosynthesized biomatrix-loaded noble metallic nanoparticles
- Cr(III)removal from simulated solution using hydrous magnesium oxide coated fly ash:Optimization by response surface methodology(RSM)☆
