基于雙席夫堿配體的兩個錳バ配合物的合成、晶體結構和抑制脲酶活性
李運彤 董婧雯 蘆 瑤 谷易桐 商超男劉芙瑤 辛 雨 井長玲 由忠錄*,
(1遼寧師范大學化學化工學院,大連 116029)(2遼西育明高級中學,錦州 121000)
0 Introduction
Urease(EC 3.5.1.5;urea amidohydrolase)is a binuclear nickel-dependent hydrolase enzyme,which can be synthesized by numerous organisms,including plants,bacteria,algae,fungi,and invertebrates,and occurs widely in animal and soil[1-2].Urease enzyme catalyzes the decomposition of urea into ammonia and carbon dioxide in high efficiency[3],with the rate of catalyzed reaction 1014times higher than the noncatalyzed reaction[4].The enzyme possesses harmful effects on both human health and fertile soil.Bacterial urease is a virulent factor including the formation of infection stones,pyelonephritis,peptic ulceration,hepatic encephalopathy,and other diseases[5-6].High urease activity in soil leads to increase ammonia toxicity in air and economic problems[7-8].All these negative effects oblige us to explore effective urease inhibitors.And,in fact,urease inhibitors are now considered as the first line of treatment for infections caused by urease-producing microorganisms.Our research group has pioneered the study of Schiff base and their complexes as urease inhibitors[9-13].Although,a variety of urease inhibitors have been investigated in the past,such as quinolones[14],phosphoric triamides[15],and thioureas[16].Most of these compounds are banned from using in vivo because of their toxicity,instability,or severe side effects.So,it is crucial to synthesize new potent urease inhibitors with good stability,bioavailability,and low toxicity.Schiff bases have been reported as effective antibacterial agents[17-18],and show interesting urease inhibitory activity[19].Metal complexes have been proved to possess significant inhibitory activities on various enzymes[20-21].As a part of our ongoing research on urease inhibition with metal complexes,two manganeseバcomplexes were prepared and screened for their urease inhibition activity.The mechanism of urease inhibition was studied by using docking techniques.
Scheme 1 Structure of the Schiff base H2L
1 Experimental
1.1 General methods and materials
Starting materials,reagents and solvents were purchased from commercial suppliers with AR grade,and used without purification.Elemental analyses were performed on a Perkin-Elmer 240C elemental analyzer.IR spectra were recorded on a Jasco FT/IR-4000 spectrometer as KBr pellets in the 4 000~200 cm-1region.1H NMR spectrum for H2L was recorded on a Bruker spectrometer at 500 MHz.X-ray diffraction was carried out on a Bruker SMART 1000 CCD diffractometer.
1.2 Synthesis of H2L
3,5-Difluorosalicylaldehyde(3.16 g,0.02 mol)and propane-1,3-diamine(0.74 g,0.01 mol)were reacted in 50 mL methanol.The mixture was stirred at room temperature for 30 min to give a clear yellow solution.The solution was evaporated by distillation to give yellow solid product,which was recrystallized from ethanol to give H2L.Yield 87%.Anal.Calcd.for C17H14F4N2O2(%):C,57.6;H,4.0;N,7.9.Found(%):C,57.4;H,3.9;N,8.0.IR data(cm-1):1 637(s,C=N),1 487(s),1 402(w),1 266(m),1 123(w).1H NMR(500 MHz,CDCl3):δ 13.45(s,2H,OH),8.35(s,2H,CH=N),6.96(s,2H,ArH),6.80(s,2H,ArH),3.78(t,4H,CH2),2.18(m,2H,CH2).
1.3 Syntheses of complexes 1 and 2
A methanolic solution (30 mL)of manganese acetate tetrahydrate(0.25 g,1.0 mmol)was added to a methanolic solution(30 mL)of H2L(0.35 g,1.0 mmol)and ammonium thiocyanate (0.076 g,1.0 mmol)for 1 or sodium azide(0.065 g,1.0 mmol)for 2 with stirring.The mixture was stirred at room temperature for 30 min to give a brown solution.The resulting solution was allowed to stand in air for a few days until three quarter of the solvent was evaporated.Brown blockshaped crystals of the complex,suitable for X-ray single crystal diffraction were formed at the bottom of the vessel.The crystals were isolated by filtration,washed three times with cold methanol and dried in air.
Complex 1:Yield:37%.Anal.Calcd.for C18H14F4MnN3O3S(%):C,44.7;H,2.9;N,8.7.Found(%):C,44.9;H,3.0;N,8.6.IR data (cm-1):2 054 (s,NCS),1 615(s,C=N),1 564(m),1 458(s),1 348(m),1 295(m),1 273(m),1 127(m),995(w),828(m),645(w),550(w),440(w).
Complex 2:Yield:45%.Anal.Calcd.for C17H12F4MnN5O2(%):C,45.5;H,2.7;N,15.6.Found(%):C,45.4;H,2.7;N,15.8.IR data (cm-1):2 035 (s,N3),1 615(s,C=N),1 564(m),1 463(s),1 355(m),1 292(m),1 267(m),1 131(m),1 058(w),995(w),828(s),746(w),640(w),540(w),435(w).
1.4 X-ray crystallography
Diffraction intensities for the complexes were collected at 298(2)K using a Bruker SMART 1000 CCD area-detector diffractometer with Mo Kα radiation(λ=0.071 073 nm).The collected data were reduced with SAINT[22],and multi-scan absorption correction was performed using SADABS[23].Structures of the complexes were solved by direct methods and refined against F2by full-matrix least-squares method using SHELXTL[24].All of the non-hydrogen atoms were refined anisotropically.The hydrogen atoms attached to water ligand of complex 1 were located from a difference Fourier map and refined isotropically,with O-H and H…H distances restrained to 0.085(1)and 0.137(2)nm,respectively.The remaining hydrogen atoms were placed in calculated positions and constrained to ride on their parent atoms.Crystallographic data for the complexes are summarized in Table 1.Selected bond lengths and angles are given in Table 2.
CCDC:1817812,1;1817813,2.
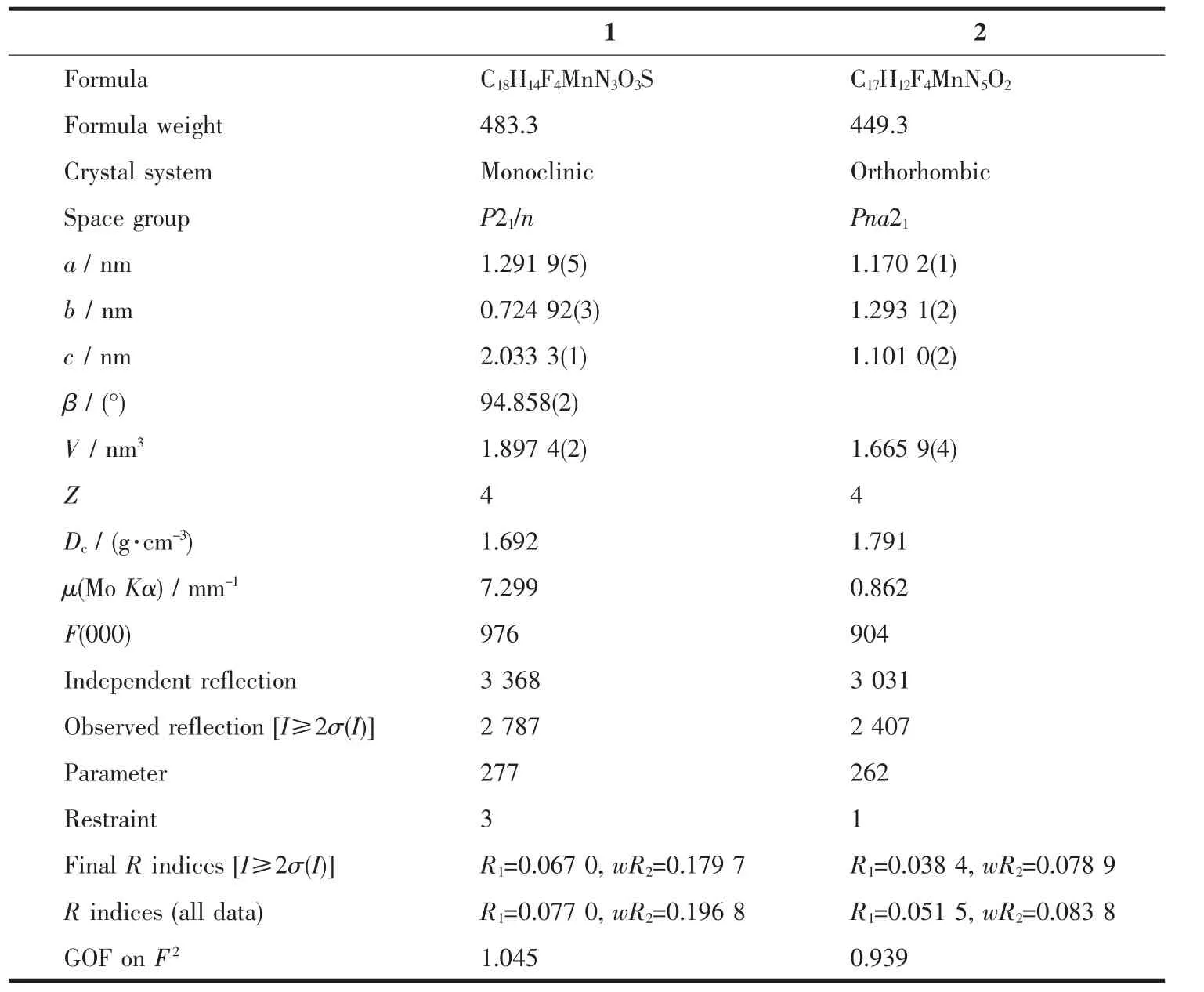
Table 1 Crystallographic and refinement parameters for the complexes
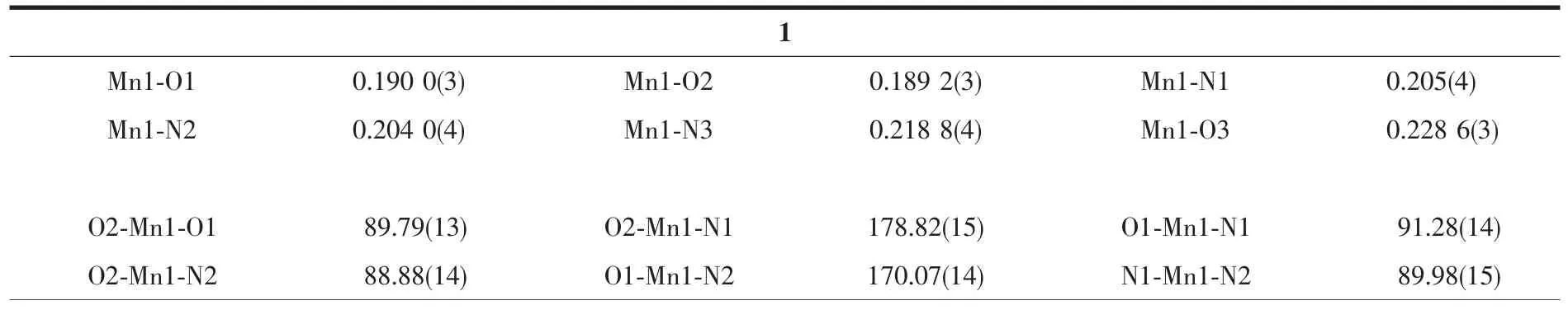
Table 2 Selected bond lengths (nm)and angles (°)for the complexes
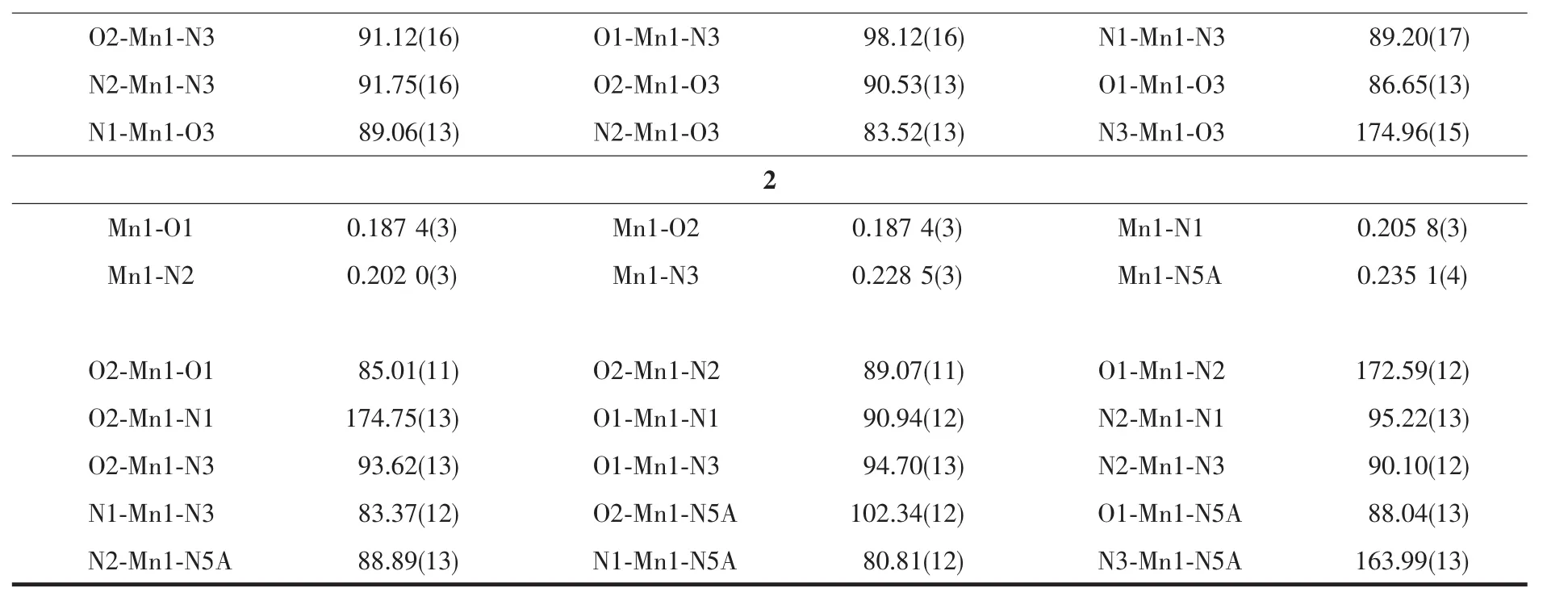
Continued Table 2
1.5 Urease inhibitory activity assay
The measurement of urease inhibitory activity was carried out according to the literature method[25].The assay mixture containing 75 μL of Jack bean urease and 75 μL of tested compounds with various concentrations(dissolved in DMSO)was pre-incubated for 15 min on a 96-well assay plate.Acetohydroxamic acid was used as a reference.Then 75 μL of phosphate buffer at pH 6.8 containing phenol red(0.18 mmol·L-1)and urea (400 mmol·L-1)were added and incubated at room temperature.The reaction time required for enough ammonium carbonate to form to raise the pH value of phosphate buffer from 6.8 to 7.7 was measured by micro-plate reader (560 nm)with end-point being determined by the color change of phenol-red indicator.
1.6 Molecular docking study
Molecular docking study of the complexes into the 3D X-ray structure of the Jack bean urease(entry 4UBP in the Protein Data Bank)was carried out by using the AutoDock 4.0 software as implemented through the graphical user interface AutoDockTools(ADT 1.5.2).The graphical user interface Auto Dock Tools was employed to setup the enzymes:all hydrogens were added,Gasteiger charges were calculated and nonpolar hydrogens were merged to carbon atoms.The Ni initial parameters are set as r=0.117 0 nm,q=+2.0,and van der Waals well depth of 0.419 kJ·mol-1[26].The 3D structure of the ligand molecule was saved in pdb format with the aid of the program Mercury.The resulting files were saved as pdbqt format.
The AutoDockTools was used to generate the docking input files.In the docking,grid box size of 70×60×60 for complex 1 points in x,y,and z directions was built,the maps were centered on the original ligand molecule (HAE)in the catalytic site of the protein.A grid spacing of 0.037 5 nm and a distancesdependent function of the dielectric constant were used for the calculation of the energetic map.100 runs were generated by using Lamarckian genetic algorithm searches.Default settings were used with an initial population of 50 randomly placed individuals,a maximum number of 2.5×106energy evaluations,and a maximum number of 2.7×104generations.A mutation rate of 0.02 and a crossover rate of 0.8 were chosen.The results of the most favorable free energy of binding were selected as the resultant complex structures.
2 Results and discussion
2.1 Structure description of complex 1
Molecular structure of complex 1 is shown in Fig.1.The Schiff base ligand coordinates to the Mn atom through the phenolate O and imino N atoms.The Mn atom is in an octahedral coordination,with the four donor atoms of the Schiff base ligand defining the equatorial plane,and with the water O atom and thiocyanate N atom occupying the axial positions.The coordination bond lengths in the complex are comparable to those observed in manganeseバcomplexes with Schiff base ligands[27-28].The displacement of the Mn atom from the equatorial plane towards the axial thiocyanate ligand is 0.008 8(2)nm.The dihedral angle between the two benzene rings of the Schiff base ligand is 19.2(5)°.In the crystal structure of the complex,molecules are linked through intermolecular O-H…O and O-H…F hydrogen bonds to form dimers(Fig.2).
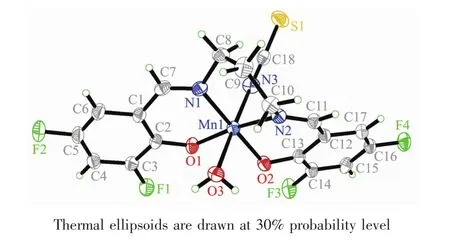
Fig.1 Perspective view of the molecular structure of 1 with the atom labeling scheme
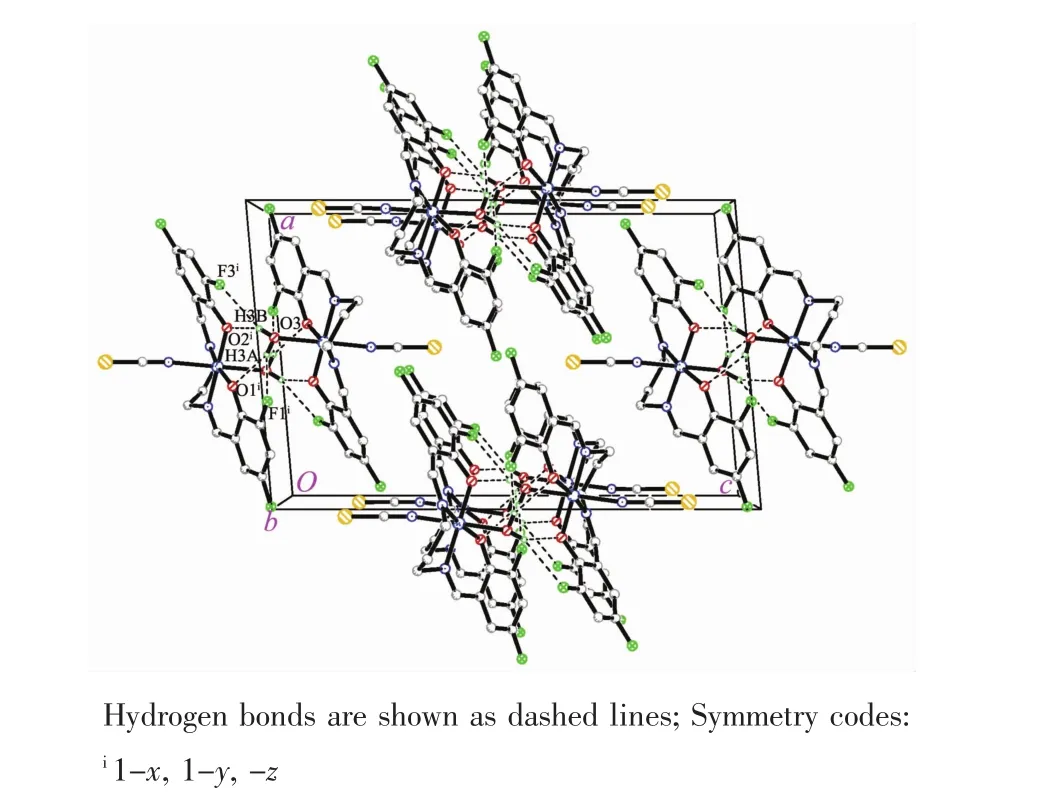
Fig.2 Molecular packing diagram of 1
2.2 Structure description of complex 2
Molecular structure of complex 2 is shown in Fig.3.The complex is an end-to-end azido-bridged polymeric manganeseバcompound.The Schiff base ligand coordinates to the Mn atom through the phenolate O and imino N atoms.The Mn atom is in an octahedral coordination,with the four donor atoms of the Schiff base ligand defining the equatorial plane,and with two azido N atoms occupying the axial positions.The coordination bond lengths in the complex are similar to complex 1,and also comparable to those observed in manganeseバcomplexes with Schiff base ligands[27-28].The displacement of the Mn atom from the equatorial plane is 0.000 9(2)nm.The dihedral angle between the two benzene rings of the Schiff base ligand is 35.5(4)°.In the crystal structure of the complex,molecules are linked through end-to-end azido ligands,to form 1D chains along c-axis direction(Fig.4).
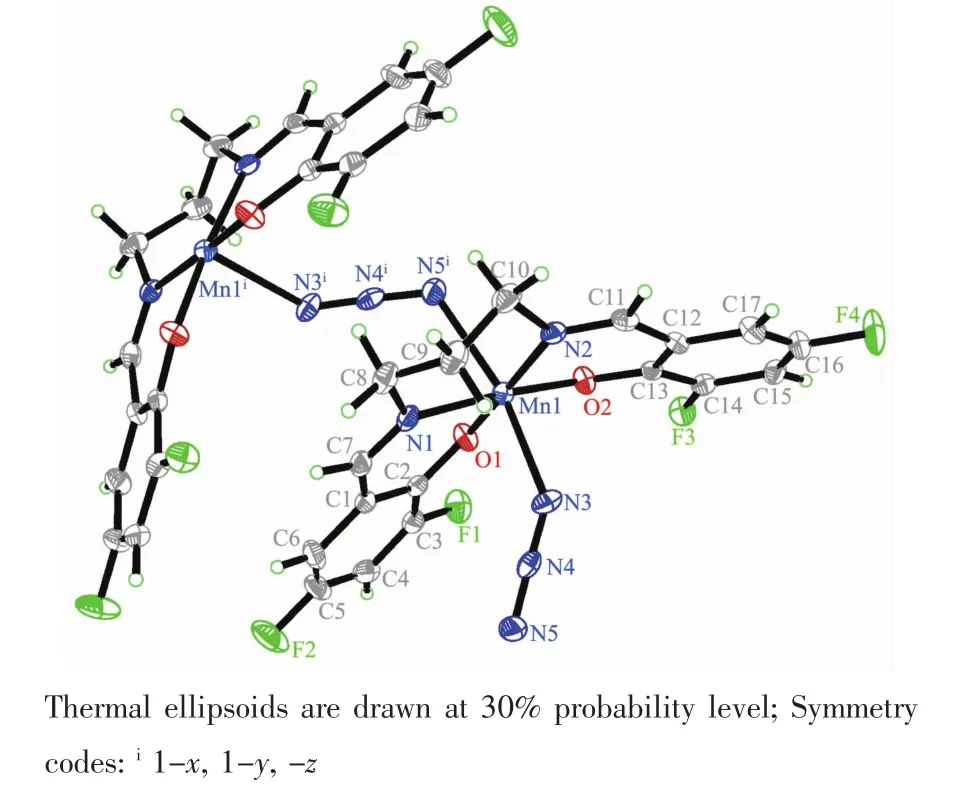
Fig.3 Perspective view of the molecular structure of 2 with the atom labeling scheme
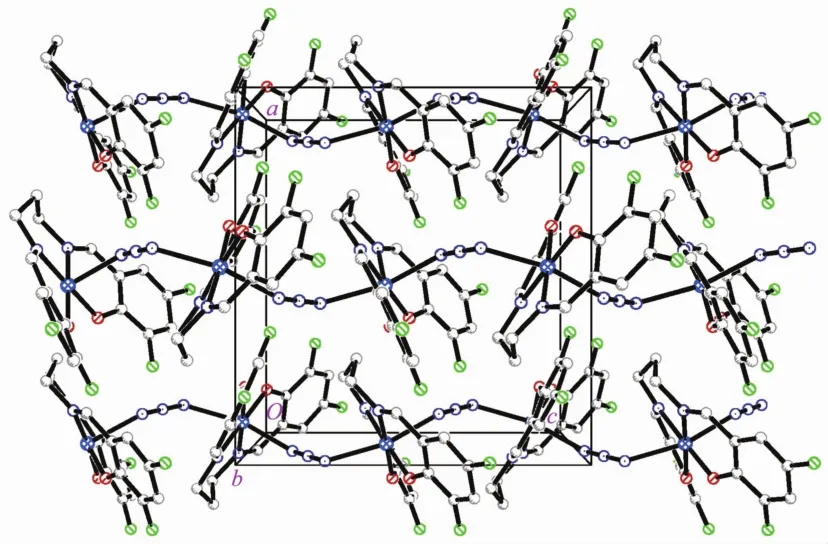
Fig.4 Molecular packing diagram of 2
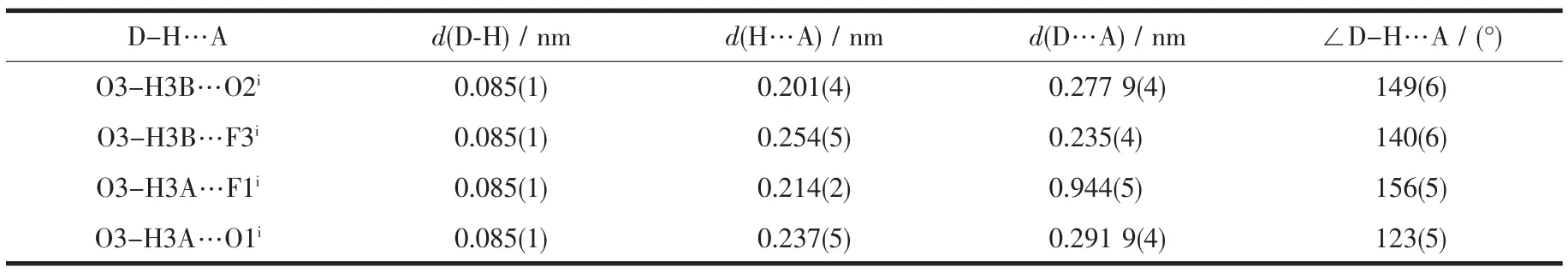
Table 3 Hydrogen bond parameters for complex 1
2.2 IR spectra
The weak and broad absorptions in the range of 3 300~3 500 cm-1in the spectra of H2L and complex 1 substantiate the presence of O-H groups,which are absent in complex 2.The strong absorption band at 1 637 cm-1for H2L is assigned to the azomethine ν(C=N),which is shifted to lower wavenumber in the spectra of the complexes,1 615 cm-1.This indicates the coordination through the two imino N atoms.The typical intense absorptions at 2 054 cm-1for complex 1 and 2 035 cm-1for complex 2 are assigned to the thiocyanate and azide groups,respectively.
2.3 Pharmacology
Results of the urease inhibition are summarized in Table 4.Acetohydroxamic acid and manganese acetate were used as references.The free Schiff base and complex 2 showed poor inhibition,while complex 1 showed effective inhibition,with IC50value of(81.0±3.7)μmol·L-1.The urease inhibition of the manganese complex is weaker than Schiff base copperギcomplexes,but higher than Schiff base zincギcomplexes reported previously[10,13,29-30].
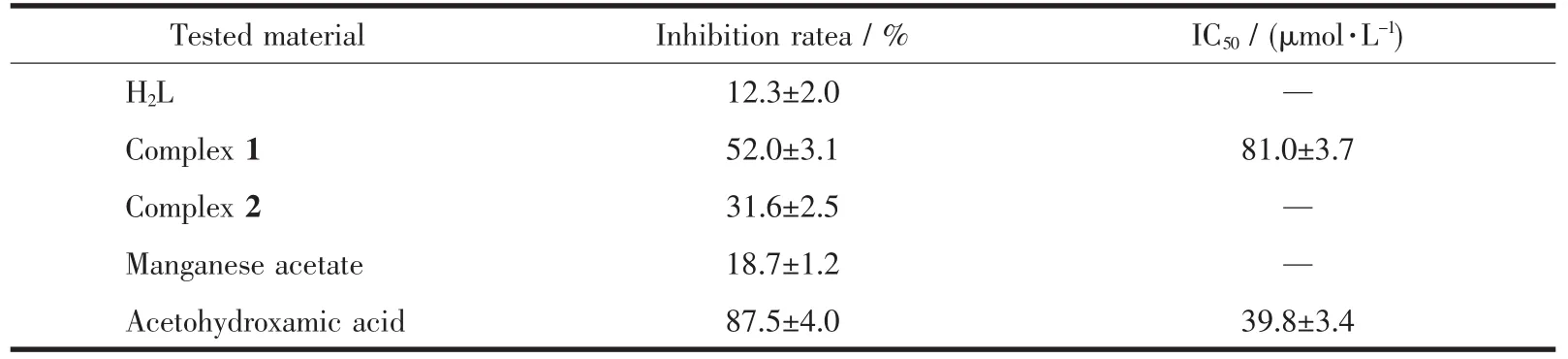
Table 4 Inhibition of urease by the tested materials
2.4 Molecular docking study
Moleculardocking study was performed to investigate the binding effects between the molecule of complex 1 and the active site of the Jack bean urease.The binding model is depicted in Fig.5.The results revealed that the molecule of complex 1 fit well with the active pocket of the urease.Additional interactions have been established in a variety of conformations because of the flexibility of the complex molecule and the amino acid residues of the enzyme.The optimized clusters (100 occurrences each)were ranked by energy level in the best conformation of inhibitor-urease modeled structures,where the docking score is-6.24.The negative value indicates that the molecule bind well with the urease.As a comparison,the docking score for the AHA inhibited model is-5.01.
The mechanism of urease inhibition was considered to be blockage of the entrance of the urease active pocket and the interaction of residues.The results endorse that complex 1 may serve as a structural template for the design and development of novel urease inhibitors.
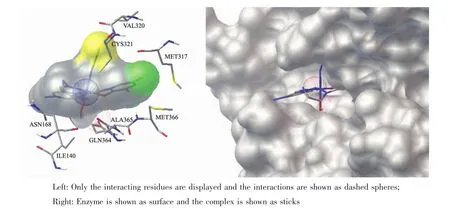
Fig.5 Binding mode of complex 1 with the urease
3 Conclusions
The present study reports syntheses,characterization and crystal structures of two Schiff base manganeseバcomplexes with bis-Schiff bases.The Schiff base ligand coordinates to the Mn atoms through the phenolate oxygen and imino nitrogen.The other sites of the octahedral coordination are occupied by water,thiocyanate or azide ligands.The thiocyanatocoordinated mononuclear complex has effective inhibitory activity against Jack bean urease,while the azido-bridged polymericcomplexhasnoactivity.Considering that Schiff base manganese complexes have interesting biologicalactivities,complex 1 described here may be used as a precursor for the design of novel urease inhibitors.
[1]Myrach T,Zhu A T,Witte C P.J.Biol.Chem.,2017,292:14556-14565
[2]Arshad T,Khan K M,Rasool N,et al.Bioorg.Chem.,2017,72:21-31
[3]Mira A B,Cantarella H,Souza-Netto G J M,et al.Agric.Ecosyst.Environ.,2017,248:105-112
[4]Dempsey R J,Slaton N A,Norman R J,et al.Agron.J.,2017,109:363-377
[5]Taha M,Ismail N H,Imran S,et al.J.Bioorg.Med.Chem.,2015,23:7211-7218
[6]Amtul Z,Siddiqui R A,Choudhary M I.Curr.Med.Chem.,2002,9:1323-1348
[7]Aslam M A S,Mahmood S U,Shahid M,et al.Eur.J.Med.Chem.,2011,46:5473-5479
[8]Saeed A,Khan M S,Rafique H.Bioorg.Chem.,2014,52:1-7
[9]Jing C L,Wang C F,Yan K,et al.Bioorg.Med.Chem.,2016,24:270-276
[10]You Z L,Liu M Y,Wang C F,et al.RSC Adv.,2016,6:16679-16690
[11]Qu D,Niu F,Zhao X L,et al.Bioorg.Med.Chem.,2015,23:1944-1949
[12]You Z L,Yu H Y,Zheng B Y,et al.Inorg.Chim.Acta,2018,469:44-50
[13]Pan L,Wang C F,Yan K,et al.J.Inorg.Biochem.,2016,159:22-28
[14]Abdullah M A A,Abuo-Rahma G E A A,Abdelhafez E M N,et al.Bioorg.Chem.,2017,70:1-11
[15]Mazzei L,Cianci M,Contaldo U,et al.Biochemistry,2017,56:5391-5404
[16]Saeed A,Ur-Rehman S,Channar P A,et al.Drug Res.,2017,67:596-605
[17]XIE Qing-Fan(解慶范),GUO Miao-Li(郭妙莉),CHEN Yan-Min(陳延民).Chinese J.Inorg.Chem.(無機化學學報),2018,34:309-316
[18]SHI Juan(史娟),LI Jiang(李江),GE Hong-Guang(葛紅光).Chinese J.Inorg.Chem.(無機化學學報),2017,33:463-470
[19]Khan K M,Rahim F,Khan A,et al.J.Chem.Soc.Pak.,2015,37:479-483
[20]Qin Q P,Meng T,Tan M X,et al.Eur.J.Med.Chem.,2018,143:1597-1603
[21]Oliveri V,Lanza V,Milardi D,et al.Mettallomics,2017,9:1439-1446
[22]SMART Ver.5.628 and SAINT Ver.6.02,Bruker AXS,Madison,Wisconsin,USA,1998.
[23]Sheldrick G M.SADABS,Program for Empirical Absorption Correction ofArea Detector,University ofG?ttingen,Germany,1996.
[24]Sheldrick G M.Acta Crystallogr.Sect.A:Found.Crystallogr.,2008,A64:112-122
[25]Mao W J,Lv P C,Shi L,et al.Bioorg.Med.Chem.,2009,17:7531-7536
[26]Krajewska B,Zaborska W.Bioorg.Chem.,2007,35:355-365[27]You Z L,Liu T,Zhang N,et al.Inorg.Chem.Commun.,2012,19:47-50
[28]Zhang N,Huang C Y,Shi D H,et al.Inorg.Chem.Commun.,2011,14:1636-1639
[29]Wang J,Qu D,Lei J X,et al.J.Coord.Chem.,2017,70:544-555
[30]Lu Y,Shi D H,You Z L,et al.J.Coord.Chem.,2012,65:339-352

