Crystal Structures, Thermal Behaviors and Biological Activities of Acylhydrazone Compounds Containing Pyrazine Rings and Halogen Atoms①
YANG Jie LIU Xiang-Rong YUE Wu-Si YANG Zai-Wen ZHAO Shun-Sheng YANG Zheng
?
Crystal Structures, Thermal Behaviors and Biological Activities of Acylhydrazone Compounds Containing Pyrazine Rings and Halogen Atoms①
YANG Jie LIU Xiang-Rong②YUE Wu-Si YANG Zai-Wen ZHAO Shun-Sheng YANG Zheng
(710054)
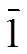
acylhydrazone, crystal structure, CT-DNA/BSA,antibacterial activities, anticancer;
1 INTRODUCTION
Acylhydrazones are special Schiff base including -CONHN=CH- and have been widely used in medi- cine and pesticide fields[1-3]. The nitrogen and oxygen donor atoms in acylhydrazone make it easily form hydrogen bonds resulting in the inhibi- tion of many physiological chemical processes in organism[4-6]. Moreover, acylhydrazones often serve as ligand to create novel metal organic complexes which possess more attractive structures and outstanding bioactivity[7, 8].
The synthesis of acylhydrazones is usually through the condensation reaction of hydrazine with aldehyde or ketone, so it is more flexible and has imaginary space to construct required acylhydra- zone molecules by not only selecting different hydrazine and aldehyde or ketone, but also depen-ding on the superposition effects of the two above groups.
We intend to design a new kind of acylhydrazone compounds possessing higher bioactivities by combining the pyrazine rings and halogen atoms, because pyrazine has excellent antitumor, antibac- terial and antituberculous activities[9-12]. Further- more, the halogen atoms in molecules can enhance the bioactivities obviously[13-15].
In this work, three acylhydrazone compounds were synthesized and their single crystals were all obtained. Among them, 1 and 3 arenovel com- pounds, while 2 was synthesized by Barbar Mil- czarska[16]but its single crystal structure has not been reported. The single-crystal structures of the three hydrazones were characterized by elemental analyses and single-crystal X-ray diffraction (XRD), and their thermal stabilities were studied by thermogravimetry.
The interactions of the three compounds with calf thymus DNA (CT-DNA) were investigated by several methods like UV-Vis spectrum, fluores- cence spectrum and viscosity measurement.The interactions of three compounds and bovine serum albumin (BSA) were explored by fluorescence spectra. The antibacterial activities were tested against,and. And anticancer activities were also evaluated against human lung cancer cells A549 by MTT experiment. Synthetic routes of the three compounds are shown in Scheme 1.
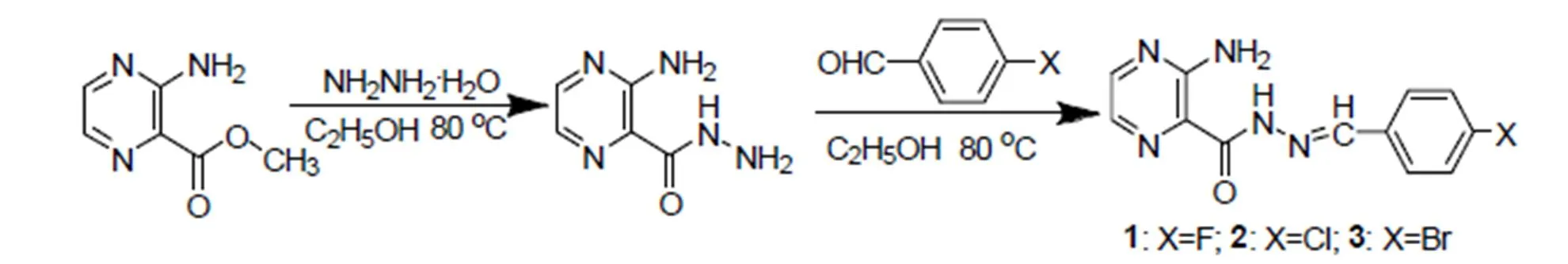
Scheme 1. Synthetic routes of the three compounds
2 EXPERIMENTAL
2. 1 Materials and instruments
Hydrazine hydrate (80%), acetic acid, ethanol, 4-fluorobenzaldehyde, 4-chlorobenzaldehyde, 4- bromobenzaldehyde, methylene blue,thiazole blue and 2-amino-3-pyrazine-carboxylate were all of analytical grade and used without further purify- cation. CT-DNA was biological reagent and pur- chased from American Sigma Company. BSA was purchased from J&K Scientific LTD.,andwere obtained from China General Microbiological Cul- ture Collection Center.
The melting points were measured on XT4-100B melting point apparatus (China). Elemental analyses (C, H, and N) were performed on a PE 2400-II elemental analyzer (America). Infrared spectra were recorded on a Bruker Tensor-II Fourier infrared spectrometer (Germany). Crystal structures were determined on a Bruker Apex-Ⅱ CCD diffract- tometer (Germany).1H NMR spectra were obtained on a Bruker Avance-400 MHz NMR spectrometer (Switzerland). UV-Vis absorption spectra were recorded on a TU-1900 ultraviolet visible spectro- photometer (China). Thermal decomposition pro- cesses were measured on a Mettler Toledo TG- DSC1 HT thermogravimetric analyzer (Swiss). Fluorescence spectra were recorded on a Perkin Elmer LS55 spectrofluorometer. Optical densities were measured using BAJIU SAF-680T microplate reader (China).
2. 2 Syntheses of 1~3
2. 2. 1 Synthesis of 2-amino-3-pyrazine-carbohydrazide(C5H7N5O)
A solution of methyl 2-amino-3-pyrazine-car- boxylate (2 mmol, 0.3063 g) in ethanol (15 mL) was added to 80% hydrazine hydrate (5 mL). The mixture was placed in a thermostat water bath and heated at 80 ℃to reflux under stirring for 4~5 h,then slowly cooled to room temperature over 48 h. Yellow needle crystals were obtained,then filtered, washed with cold ethanol and dried.
2. 2. 2 Syntheses of compounds 1~3
Synthesis of 4-fluorobenzaldehyde-2-amino-3- pyrazine hydrazone (1): 2-amino-3-pyrazine- carbo- hydrazide (0.3 mmol, 0.0459 g) and 4-fluorobenzal- dehyde (0.3 mmol, 32 μL) were dissolved in ethanol. The mixture was placed in a thermostat water bath and heated at 80 ℃ to reflux under stirring for 4 h. Then the resultant solution of 4-fluorobenzalde- hyde-2-amino-3-pyrazine hydrazone (1) was fil- tered and the product recrystallized from etha- nol/acetic acid (v(ethanol):v(acetic) = 4:1, 20.0 mL). After 3 days, bright yellow flaky crystals suitable for X-ray crystallographic analysis were obtained. 4-chlorobenzaldehyde-2-amino-3-pyrazine hydra- zone (2) and 4-bromobenzaldehyde-2-amino-3-py- razine hydrazone (3) were synthesized as the same method.
Compound 1: Yield: 70.38%. m.p. 198.48~198.68 ℃.Anal. Calcd. for C12H10FN5O·H2O (1, %): C, 55.55; H, 3.857; N, 27.00. Found (%): C, 52.98; H, 4.076; N, 24.00.IR (KBr, cm-1): 3260 (N–H), 3150 (N–H), 1680 (C=O), 1500 (C=N), 835 (C–F).1H NMR (400 MHz, DMSO-6): 12.09 (s, 1H, NH), 8.61 (s, 1H, CH), 8.30 (d,= 2.3 Hz, 1H, pyrazine-H), 7.91 (d,= 2.3 Hz, 1H, pyrazine-H), 7.82~7.72 (m, 2H, Ar–H), 7.64 (s, 2H, NH2), 7.37~7.26 (m, 2H, Ar–H).
Compound 2: Yield: 74.24%. m.p. 261.78~262.08 ℃. Anal. Calcd. for C12H10ClN5O·2CH3COOH (2, %): C, 52.23; H, 3.627; N, 21.86. Found (%): C, 52.53; H, 3.564; N, 23.47. IR (KBr, cm-1): 3256 (N–H), 3146 (N–H), 1674 (C=O), 1514 (C=N), 556 (C–Cl);1H NMR (400 MHz, DMSO-d6): 12.14 (s, 1H, NH), 8.60 (s, 1H, CH), 8.29 (d,= 2.3 Hz, 1H, pyrazine-H), 7.90 (d,= 2.3 Hz, 1H, pyrazine-H), 7.77~7.68 (m, 2H, Ar-H), 7.63 (s, 2H, NH2), 7.57~7.49 (m, 2H, Ar-H).
Compound 3: Yield: 76.56%. m.p. 272.14~272.44 ℃. Anal. Calcd. for C12H10BrN5O (3, %): C, 44.98; H, 3.123; N, 21.86. Found (%): C, 45.27; H, 3.088; N, 20.62. IR (KBr, cm–1): 3256 (N–H), 3148 (N–H), 1673 (C=O), 1511 (C=N), 573 (C–Br).1H NMR (400 MHz, DMSO-d6): 12.15 (s, 1H, NH), 8.58 (s, 1H, CH), 8.29 (d,= 2.3 Hz, 1H, pyrazine-H), 7.90 (d,= 2.3 Hz, 1H, pyrazine-H), 7.71~7.61 (m, 6H, 4×Ar–H, 2×NH2).
2. 3 Crystal structure determination
Three kinds of single crystals with dimensions of 0.35 × 0.28 × 0.23 mm3(1), 0.37 × 0.28 × 0.14 mm3(2) and 0.31 × 0.23 × 0.15 mm3(3) were put on a Bruker ApexⅡCCD diffractometer with a graphite- monochromatic Moradiation (= 0.71073 ?) at room temperature by using anscan mode. Absorption corrections were applied with the program SADABS[17]. The crystal structure was solved by direct methods using SHELXS-97 pro- gram[18]. Non-hydrogen atoms were refined by full-matrix least-squares using SHELXL-97 and the hydrogen atoms were placed in the calculated positions.
2. 4 Thermogravimetric experiments
5~10 mg compound was placed in the N2atmos- phere and the thermogravimetric experiment was carried out for each compound from room tempera- ture to 800 ℃ at heating rates of 5, 10 and 15.00 ℃·min-1, respectively.
2. 5 Interactions of compounds with CT-DNA
2. 5. 1 UV-Vis absorption spectra[19]
The compounds were dissolved in tris-HCl (0.01 mol·L-1pH = 7.90) buffer solution with con- centration of 1 × 10-5mol·L-1. 3.0 mLcompound solution was added into a cuvette and tris-HCl buffer solution was the reference solution. And 50 μL CT-DNA solution (100.0 mg·L-1) was then gradually added into the compound solution 5 times at 1 minute intervals by using micro inject- tor. The UV absorption spectrum was determined in the wavelength range of 250~500 nm.
2. 5. 2 Fluorescence spectra[20]
CT-DNA solution (100.0 mg·L-1) was mixed with methylene-blue liquid (1 × 103mol·L-1) and the concentration ratio of CT-DNA solution to methylene blue liquor was 10:1. Then 3.0 mL mixture was added into a cuvette. 50 μL compounds solution (1 × 10-5mol·L-1) were added into the cuvette 5 times at 1 minute interval by using micro injector. The fluorescence spectra were recorded at an excitation wavelength of 630 nm and cover a wavelength range of 650~900 nm. The widths of entrance and exit slits were 5 nm.
2. 5. 3 Viscosity measurements[21]
The viscosity measurements were carried out on an Ubbelodhe viscometer immersed in a thermostat water bath at 25 ℃. 10 mL Tris-HCl (0.01 mol·L-1pH = 7.90) buffer solution was added into the Ubbelodhe viscometer. The flow time0was measured by a digital stopwatch. Then CT-DNA concentration remained unchanged (10 mL 1.88 × 10-4mol·L-1) and the compound solutions (1.88 × 10-3mol·L-1) were gradually and continuously added into the CT-DNA for 6 times with an interval to be 30 min. Each sample was measured three times, and the average flow time was calculated. Relative viscosities were calculated from the following formula:
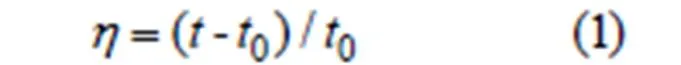
where0is the viscosity of CT-DNA alone andis the viscosity of mixed solution of compounds and CT-DNA. Data are presented as (/0)1/3versus(compound)/(CT-DNA).
2. 6 Interactions of compounds with BSA[22]
BSA was dissolved in Tris-NaCl (0.01 mol·L-1, pH = 7.20) buffer solution with concentration of 110-7mol·L-1. Then 3.0 mL solution was added into a cuvette. 30 μL compound solution (1 × 10-5mol·L-1) was added into the cuvette 6 times at 1 minute interval by using microinjector. The fluorescence spectra were recorded at an excitation wavelength of 280 nm over a wavelength range of 300~540 nm.The widths of entrance and exit slits were 5 nm.
2. 7 Antibacterial activity
Antibacterial activities of compounds were tested by microplate reader method[23]. Three strains were inoculated into 5 mL of Luria-Bertani (LB) medium at 37 ℃ for 3.5 h. Compound solutions were prepared with LB medium and diluted into various concentrations. Then 5 μL bacterial suspen- sions with concentration of 105colony forming units per mL were added into 5 mL compound solution. 250 μL mixture was put into a sterile 96-well plate. After 6 h incubation, optical densities (OD) were measured by microplate reader at 630 nm and inhibition rates were calculated as:
Inhibition rate = (1–ODsample/ODcontrol) × 100% (2)
And IC50was calculated by PASW Statistics software.
2. 8 Cytotoxic activity[24]
Cytotoxicities of compounds 1~3 against human lung cancer cells (A549) were determined by using the MTT (3-(4,5-dimethylthiazol-2yl)-2,5-diphe- nyl-tetrazolium bromide) assay. The logarithmic growth phase cells were plated in 96-well plate and incubated at 37 ℃ for 24 h to allow cell attachment. Then the A549 cells were treated with three compounds separately at the concentration of 0, 10, 20, 30, 40, 50 and 100 uM for 24 h, and then 90 uL of culture medium and 10 uL of MTT solution (5 mg/mL in PBS) were added to each well. After 4 h incubation, the medium was discarded and 100 uL of DMSO was added to each well for dissolving the formazan crystals. OD was also measured by a microplate reader at 570 nm and inhibition rates were calculated by formula (2).
3 RESULTS AND DISCUSSION
3. 1 Crystal structure

Fig. 1. Crystal structure of 1(Probability of ellipsoid is 30%)
Fig. 2. Crystal packing structure of 1
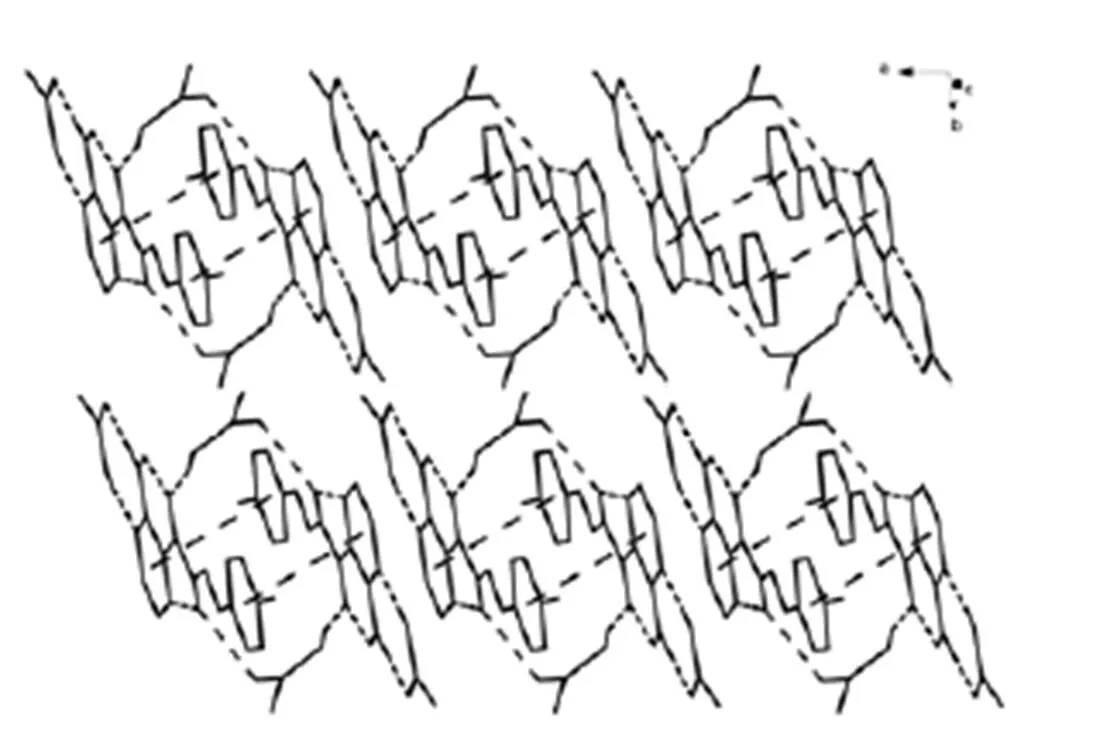
Fig. 3. Crystal packing structure of 2
Fig. 4. Crystal packing structure of 3

Table 1. Crystallographic Data for 1~3

Table 2. Selected Bond Distances (?) and Bond Angles (o) for 1~3
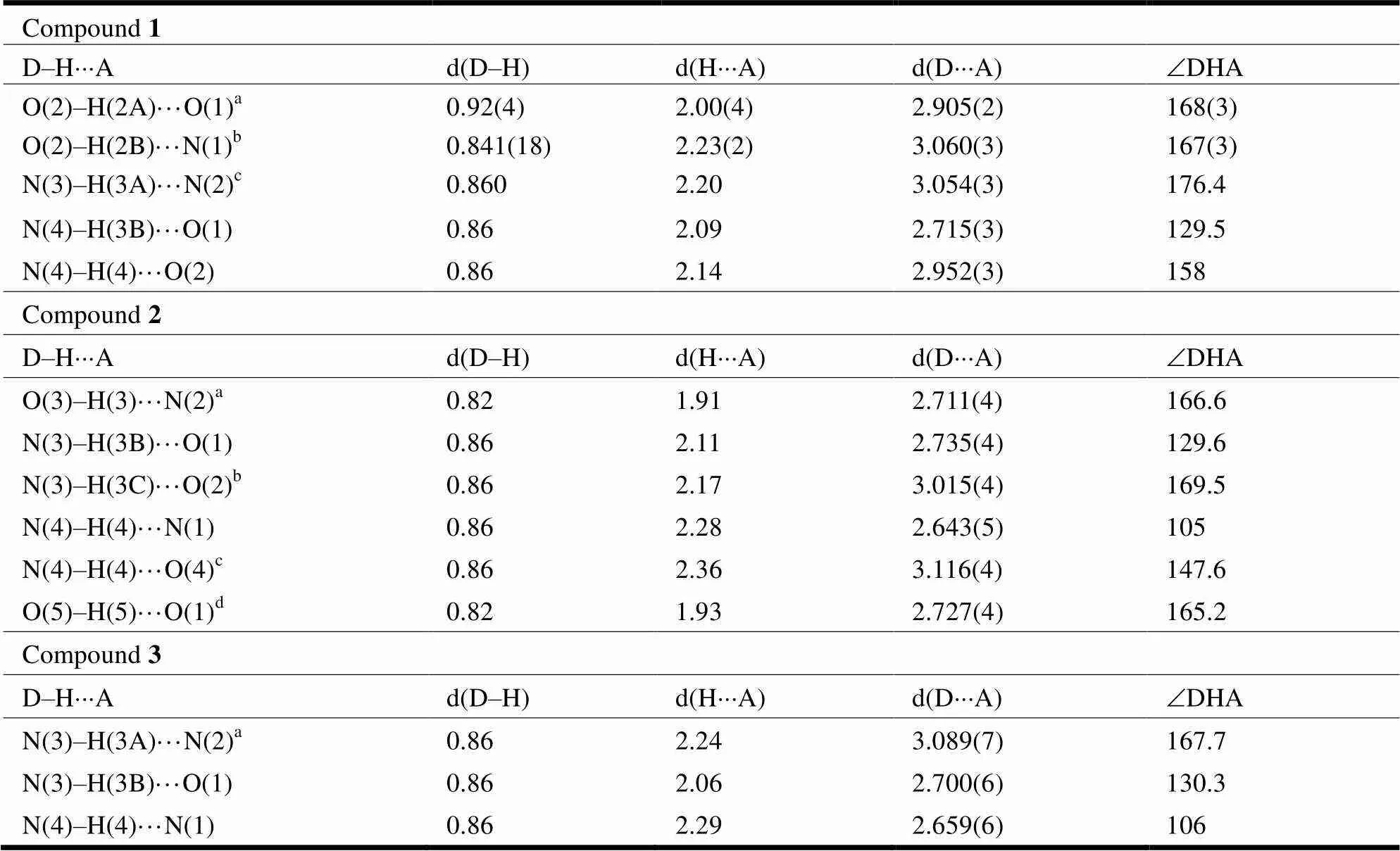
Table 3. Hydrogen Bond Distances (?) and Bond Angles (°) for 1~3
Symmetry codes: for 1 (a)1,,; (b)1,,;(c)–+2, –+1, –+1; for 2 (a),– 1,
; (b),+ 1,; (c) –+ 1, –+ 1, –+ 1; (d)– 1,,; for 3 (a) –, –+ 1, –+ 2

Table 4. Selected π-π Stacking Interactions of Compounds 2 and 3
As seen in Fig. 1 and Table 2,the dihedral angle of pyrazine and benzene rings in compound 1 is 8.18°, which indicates the two rings are not in the same plane. The torsion angles of C(8)–C(7)– C(6)–N(5) and C(12)–C(7)–C(6)–N(5) are 57.9(4)° and –174.3(2)°, also illustrating that C(6)–N(5) is obviously deviated from the benzene ring. The bond length of C(6)–N(5) (1.227(2) ?) is shorter than N(4)–C(5) (1.347(2) ?), attributed to a C=N double bond existing between C(6)–N(5)[25]. The C(5)– O(1) bond distance is 1.227(2) ?, which is the typical C=O double bond[26]. As shown in Fig. 2, there are three kinds of hydrogen bonds in 1: N–H···N, O–H···O and N–H···O.Through the hydrogen bonds between intermolecules, the 1D chains are further linked into a 2D network which stabilizes the structure.Hydrogen bond distances and anglesare given inTable 3.
The dihedral angles of pyrazine and benzene rings in compounds 2 and 3 are 1.47° and 4.69°. Thus compound 2 shows better coplanarity among 1~3.Moreover, interchain-stacking interac- tions are founded between the pyrazine and benzene rings in the crystals of 2 and 3. The parameters of-stacking interactions are shown in Table 4.-stacking interactions and hydrogen bonds extend the structure of compounds 2 and 3 into 1D double-chain structures. All the three compounds exist in a keto form.
3. 2 Thermal stabilities
The TG-DTG curve of compound 1is shown in Fig. 5. The TG curves at the heating rates of 10.00 and 15.00 ℃·min–1are similar to that of the 5.00 ℃·min–1. FromFig. 5, the thermal decom- position temperature of maximum weight loss for 1 is 282 °C at theheating rate of 5.00 °C·min–1. And the decomposition temperature of compounds 2 and 3 is alsomore than 280 ℃, showing that the three compounds possess good thermal stabilities. The thermal decomposition of 1can be divided into two stages and the weight loss percentage of the first stage is 5.01%, which might be the loss of water molecules. The mass loss of the second stage is 60.89%, which is assigned to the bond breaking of C(4)–C(5) (calcd. 63.71%). Compound 2 undergoes just one stage of decomposition and the temperature ofendothermic peak is 283 ℃. The weight loss at this stage is 69.47%, corresponding to the bond breaking of C(1)–N(3) and C(4)–C(5) (calcd. 71.64%). The decomposition process of compound 3 is also one stage with mass loss of 43.09% which is attributed to the bond breaking of N(4)–N(5) and C(10)–Br(calcd. 67.75%).
小學數學教師應當意識到學習評價的重要性,通過對評價環節的科學設置,將讓學生獲得數學思維發展的契機,同時亦可以讓數學教師獲取學生的學習情況反饋。具體而言,小學數學教師應當利用多媒體技術將班級學生所完成的計算習題進行課上展示,并組織班級學生共同進行查錯和改正,如此便達成了帶領學生共同進行學習評價的目的,這樣將讓整體的數學課堂教學效果更為理想。
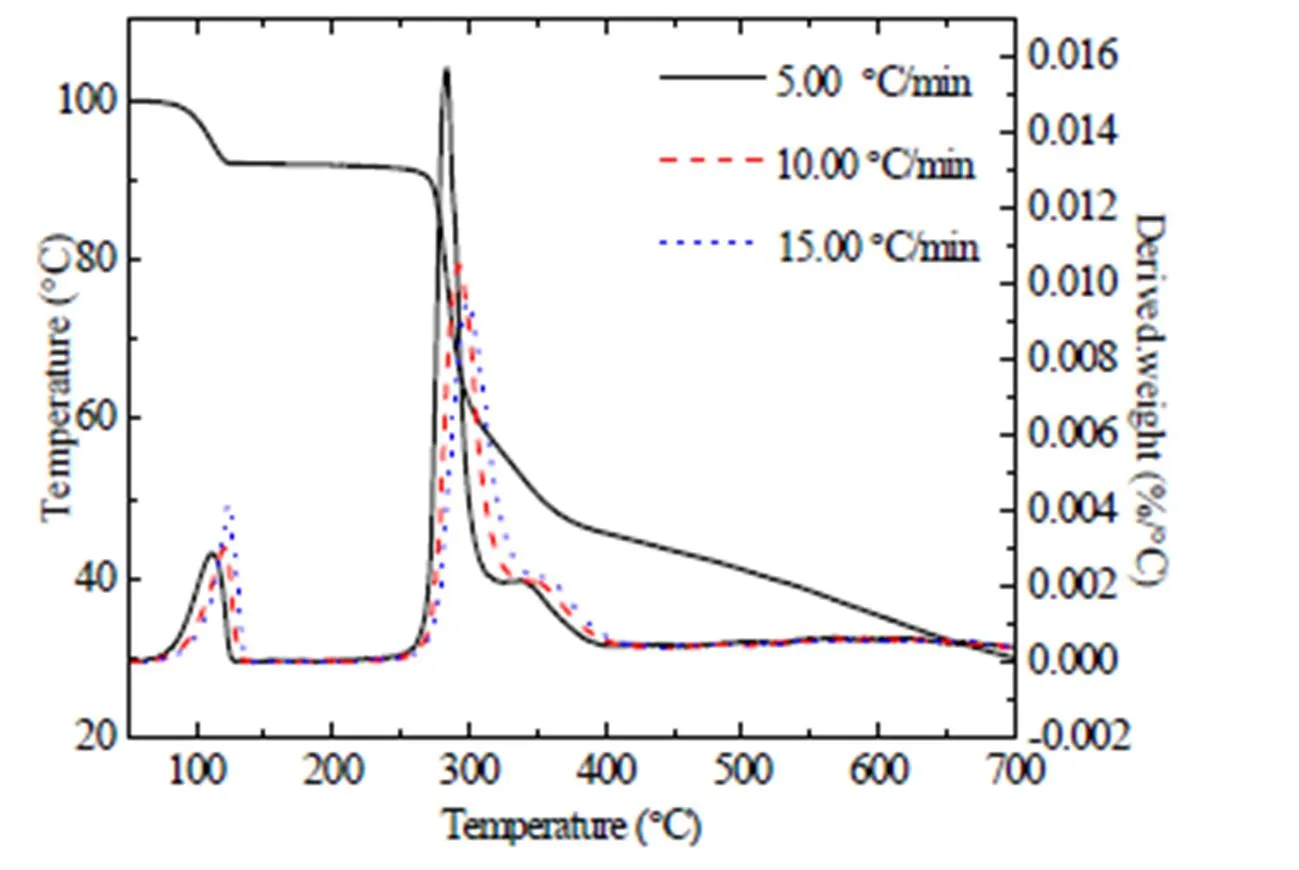
Fig. 5. TG-DTG curves of compound1
The kinetic parameters of decomposition pro- cesses for three compounds are calculated by Kis- singer (3) and Ozawa (4) equations[27]:

wherepis the maximum temperature of endo- thermic peak,the pre-exponential factor,athe apparent activation energy,the gas constant,the heating rate, and() the integral mechanism function.The calculation results are shown in Table 5.
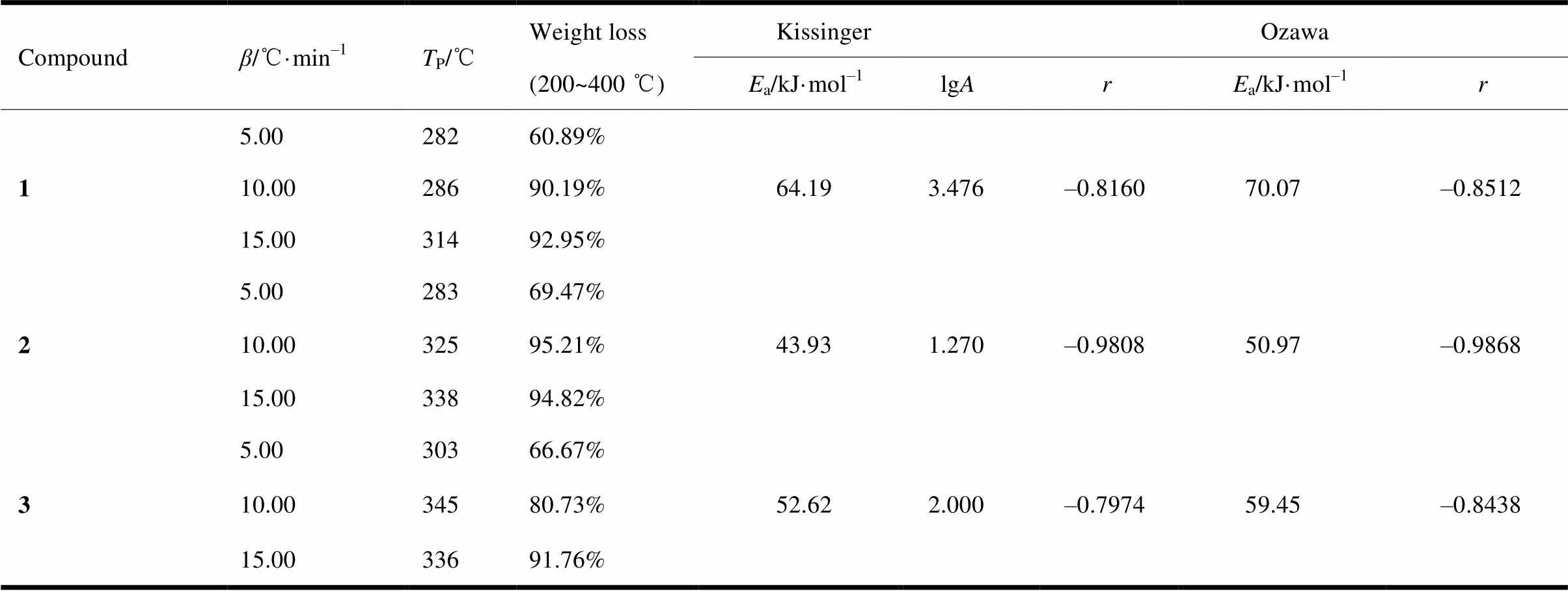
Table 5. Kinetic Parameters of Thermal Decomposition for Three Compounds
3. 3 Interactions of compounds with CT-DNA
3. 3. 1 UV–Vis absorption spectra
UV-Vis absorption spectra are often used to study the interactions of compounds with CT-DNA.Fig.6 shows the absorption spectra of compound1 interaction with CT-DNA. With increasing the concentration of CT-DNA, the absorbance of 1 decreases. The absorption band of 1 exhibits hypochromism at 294 and 365 nm. For 2 and 3, the hypochromisms are observed at 299, 367 nm and 301, 366 nm.It can be inferred that all the com- pounds could bind with CT-DNA through intercala- tion. Generally, hypochromic effect is the spectral feature of intercalative binding mode. Because the* orbital of the intercalated compound could couplewith theorbital of CT-DNA base pairs after the compound inserts into CT-DNA, the coupling* orbital ispartially filled by electrons, which makes the transitionpossibilities decrease[28, 29].
The binding constants (b) of three compounds with CT-DNA are calculated by using the following function equation (5)[30]:

whereDNAis the concentration of CT-DNA,a,bandfthe molar extinction coefficient of compound that is apparent, free and fully bonding with CT– DNA.
The plots ofDNA/(a–f) versusDNAare pre- sented as inset in Fig. 6 and theb-of three com- pounds are determined by the ratio of slope to intercept.b-is shown in Table 6 and follows the order of 2 >1 >3. Thebvalues of three compounds are larger than that of classical DNA intercalator such as ethidium bromide (b-= 3.3 × 105L·mol–1)[31]. Therefore, three compounds have strong binding abilities to the CT-DNA and 2 binds more effectively to CT-DNA than 1 and 3.

Table 6. Parameters of Interaction with CT-DNA Obtained from UV-Vis Spectra for 1~3 with CT-DNA

Fig. 6. UV-Vis spectra of the interaction of 1 with CT-DNA (Inset:the plot ofDNA/(a-f) againstDNA)
3. 3. 2 Fluorescence spectra
The interaction between small molecules and CT-DNA is often studied by fluorescence probe[32]. In this paper, the competitive binding experiment has been carried out using methylene-blue (MB) as a probe. MB has strong intrinsic fluorescence and could insert into the base pair of CT-DNA by hydrophobic interactions and-interactions when the concentration ratio of CT-DNA to MB is more than 6[33]. The interaction of MB with CT-DNA results in a decrease in the fluorescence intensity. After adding compounds into the solution, the compound could displace the MB from CT-DNA causing the fluorescence intensity increase.
Fig. 7 shows the fluorescence spectra of the CT-DNA-MB system of 1. The emission intensities of the CT-DNA-MB system increase as the concentration of the three compounds increase, which indicates that three compounds react with CT-DNA by intercalation effect[20].
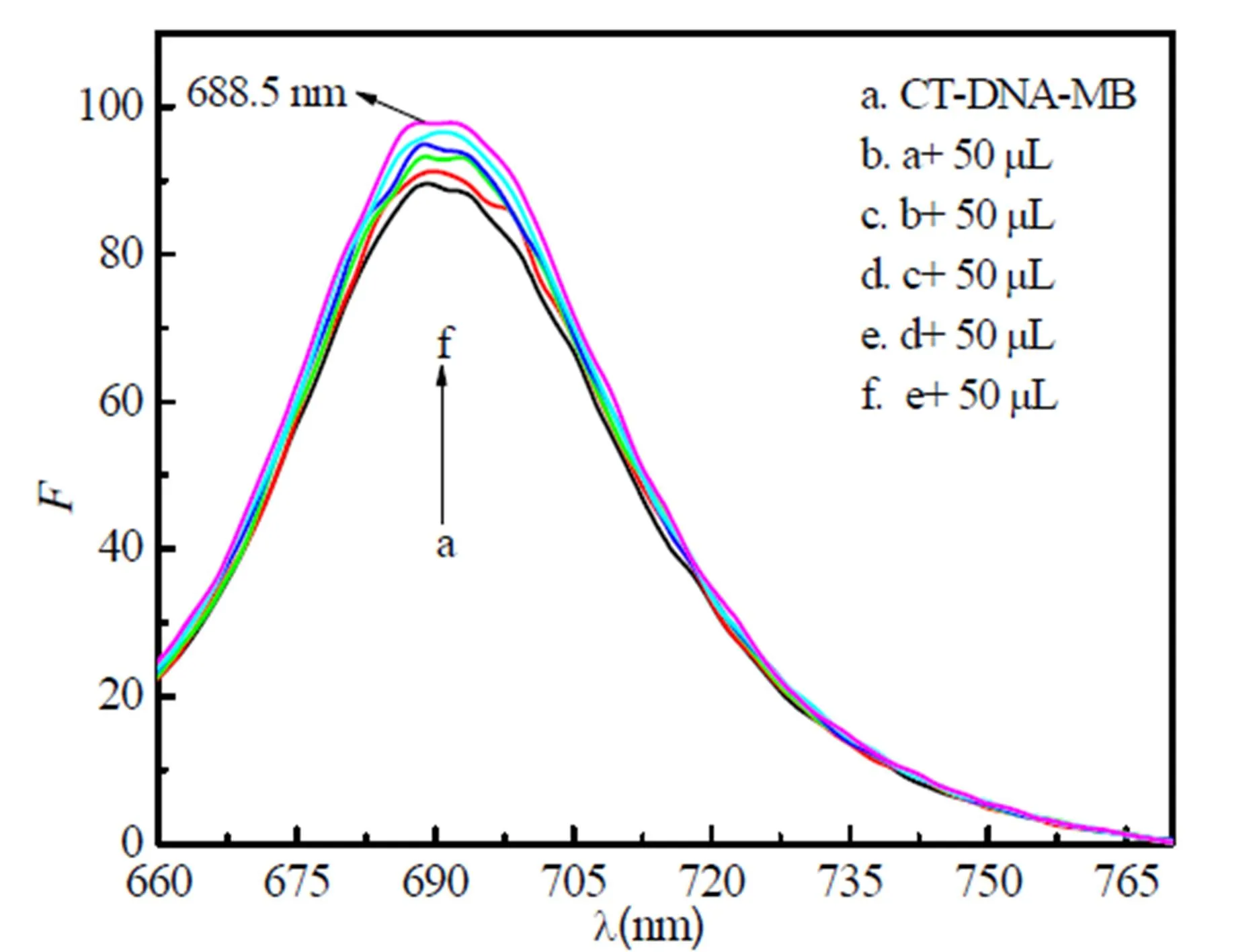
Fig. 7. Fluorescence spectra of the interaction of 1 with CT-DNA-MB
3. 3. 3 Viscosity measurements
Viscositymeasurement is usually considered as one of the most accurate and effective methods to study the interaction between small molecule compounds and DNA[34]. In classical intercalation,the viscosity of CT-DNA solution increases because DNA base pairs were separated to accommodate the bound ligand, which results in DNA helix leng- thens. Instead, partial non-classical intercalation of compounds could bend or kink the DNA helix, resulting in the decrease of DNA length as well as the viscosity. And in groove binding or electrostatic mode, the viscosity of the DNA solution does not change significantly[35, 36].
According to our experiment, as illustrated in Fig. 8, the relative viscosities of CT-DNA increase continuously with the addition of compounds. The results show that three compounds interact with CT-DNA by intercalation and2 exhibits the strongest biological activity, which is consistent with UV-Vis absorption spectra.
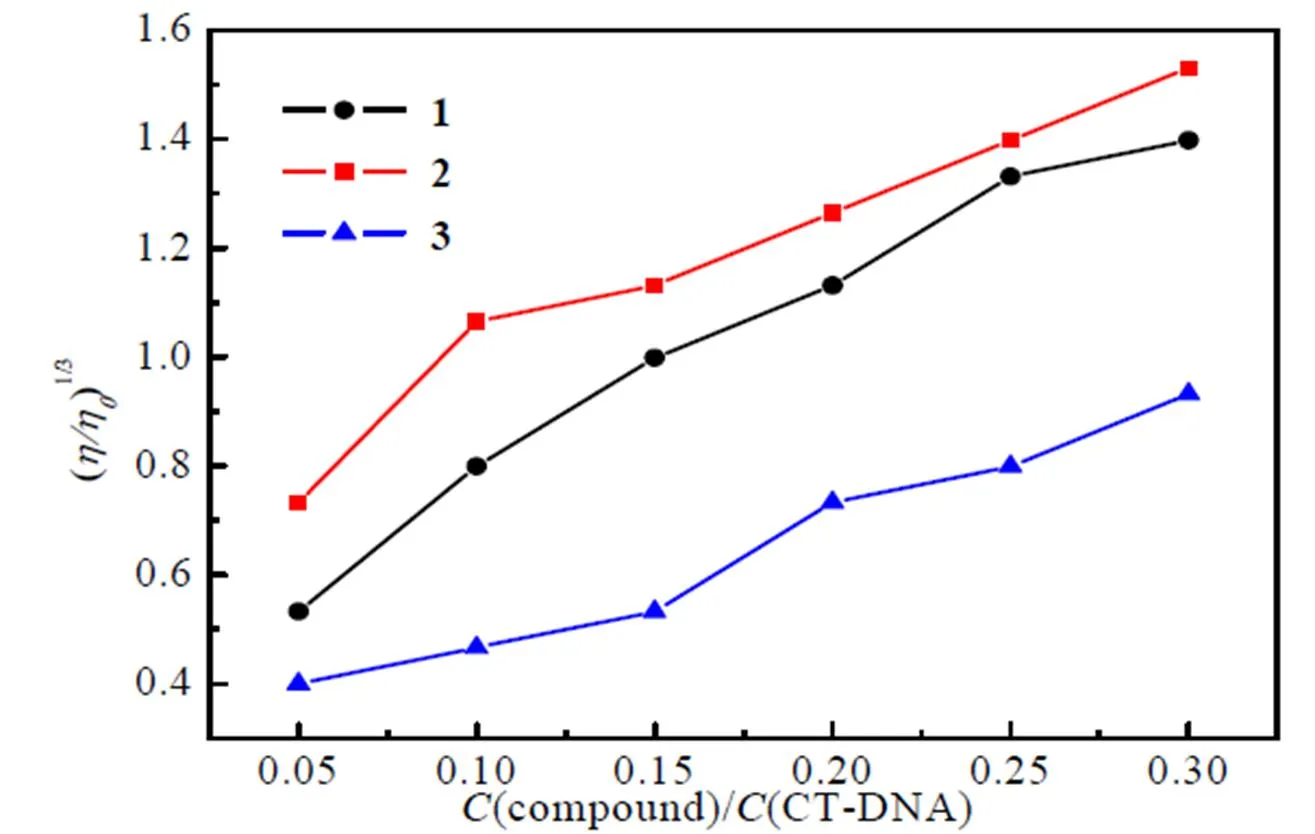
Fig. 8. Plots of (/0)1/3versus(compound)/(CT-DNA)
Fig. 9. Fluorescence spectra of the interaction of 1 with BSA (Inset: Stern-Volmer plot: F/)1~7,VOL= 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6M
3. 4 The interactions of compounds with BSA
Bovine serum proteins (BSA) emit strong endo- genous fluorescence due to the tyrosine, tryptophan and phenylalanine[37]. The effect of 1 on BSA is shown in Fig. 9, which is the same with 2 and 3. Along with the addition of three compounds, the endogenous fluorescence of BSA is quenched regularly, suggesting that 1~3 caninteract with BSA.
Fluorescence quenching is divided into two types: dynamic quenching and static quenching[38]. Assuming three compounds quenched the fluore- scence of BSA through a dynamic process. Fluore- scence quenching is described by Stern-Volmer formular[39]:

where0andare the fluorescence intensities of BSA in the absence and presence of the compounds,qthe quenching rate constant and 2.0 × 1010the highest limit for dynamic quenching[40],0the average life of BSA without the quencher, at approximately 10–8s–1. [] is the concentration of compound andsvthe Stern-Volmer dynamic quenching constant,svandqwere obtained from the plots of/0versus [] (as insets in Fig. 9) and presented in Table 7.

Table 7. Parameters of Interaction with BSA Obtained from Fluorescence Spectra for 1~3 with BSA
As seen from Table 7, all the threeqvalues are more than the maximum dynamic fluorescence quenching rate constant (2.0 × 1010)[40]. Therefore, the assumption of dynamic quenching is not true. The quenching mechanisms of 1~3 with BSA are static quenching process with non fluorescence complex formed between compounds and BSA.
For the static quenching process, the binding constants and the number of binding sites can be calculated from formula 7[41]:
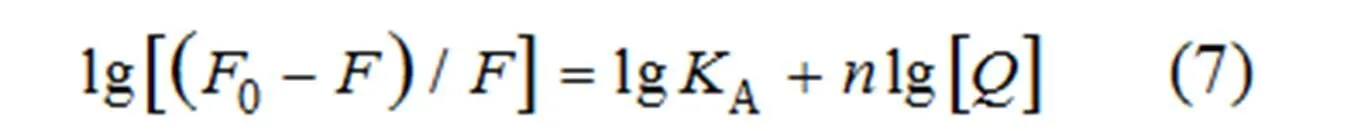
where0andare the fluorescence intensities of BSA in the absence and presence of the compounds,Athe binding constant,the number of binding site and [] the concentration of three compounds.Aandwere obtained from the plots of lg[(0–)/] versus lg[] (Fig. 10) and presented in Table 8.
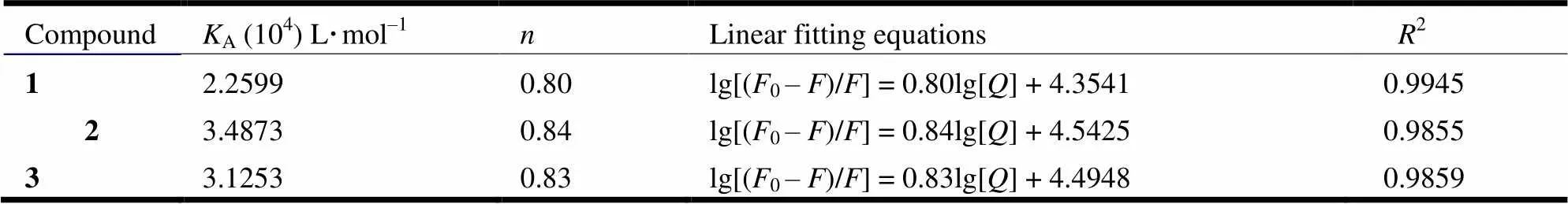
Table 8. Parameters of Interaction with BSA Obtained from Fluorescence Spectra for 1~3 with BSA

Fig. 10. Plots of lg[(F–)/] versus lg[]
The results show that the number of binding sites is less than one, so three kinds of compounds are partially bound to BSA. The binding constants are all in 104~105L·mol–1and decreased in the order of 2>3>1, revealing strong interaction between BSA and the three compounds[42]. Compound 2 exhibits superior binding ability. As shown above, compound 2 possesses better coplanarity than 1 and3,which makes it easily insert into the DNA base pairs or BSA[43].
3. 5 Antimicrobial activity
The antimicrobial activities of three compounds were determined against,and. The results are shown in Table 9, indicating that all the three compounds show an appreciable activity against. Compound 2 with IC50values of 7.75 μmol·L–1exhibited the highest activity among the three compounds. It is also noted that compound 2 is more effective against. Compounds 1~3 show less activity against. Generally, 1 exhibits better activities against bacteria than 2 and 3, which is the same order with the interaction of compounds with DNA and BSA.

Table 9. IC50 of Compounds 1~3 against the Tested Bacteria
3. 6 Cytotoxic activity
Compounds were screened against human lung cancer cells A549 by using MTT assay. As shown in Fig. 11, compound 1 displays better inhibitory activities against the human cancer cell lines with IC50values of 72.16 μmol·L–1in comparison to other tested compounds. The IC50values of 2 and 3 against cells A549 were 428.03 and 424.33 μmol·L–1, respectively. It has been reported in other literatures that the introduction of fluorine atom in phenyl ring could result in a higher anticancer activity. The fluorine atom as substituent can enhance the liposolubility of the compound, which is beneficial for compound molecules to go through the biomembranes[44]. The IC50value of 1 againstA549 iscomparable to that of the standard drug Etoposide (33.13 μmol·L–1)[45].

Fig. 11. Inhibition effects of three compounds on cell A549
4 CONCLUSION
(1) Lu, W. G.; Liu, H. W. Synthesis and crystal structure of a copper(II) complex with 2,4-dihydroxybenzylidene benzoylhydrazone ligand.2005, 24, 1078–1082.
(2) Ni, Z. J.; Xue, S. J.; Wang, J.; Meng, W.Synthesis and anti-leukemia activity of 1-substituted piperidin-4-one arylformylhydrazones.2011, 31, 222–226.
(3) Xiong, Q. Z.; Liu, J. H.; Lin, X. F.; Bao, X. P.Synthesis and bioactivities of novel 1,2,4-triazolo[1,5-a]pyrimidine derivatives containing 1,2,4-triazole-5-thione Schiff base unit.2012, 32, 1951–1957.
(4) Bao, X. P.; Xiong, Q. Z.; Zou, L. B.; Zang, F.; Liu, Y.; Jian, J. Y. Synthesis and fungicidal activities of 2-benzyithio-5-methyl-1,2,4-triazolo [1,5-a]pyrimidine-7-oxoacetohydrazone derivatives.. 2013, 01, 53–57.
(5) Shang, H. J.; Gao, L. Z.; Xie, Y. S.; Yan, Q.; Wu, S. M.; Ni, L. L.; Huang, W. L.; Xie, S. Q.; Hu, G. Q.Synthesis and antitumor activity of N-methyl ciprofloxacin acylhydrazone..2015, 05, 597–601.
(6) Nath, M.; Saini, P. K. Chemistry and applications of organotin(IV) complexes of Schiff bases.. 2011, 40, 7077–7121.
(7) Wang, D. B.; Chen, B. H.; Ma, Y. X.Aroyl hydrazones containing triazole and their divalent nickel complexes.1997, 27, 479–486.
(8) Li, K.; Li, S. J.; Yao, X. J.; Niu, J. J.; Qiu, X. Y. Synthesis, crystal structures and antimicrobial activity of vanadium(V) complexes with similar tridentate hydrazone ligands.2015, 34, 885–893.
(9) Wei, S. P.; Zhang, H.; Xu, X. B.; Yang, Y. Y.Natural existed pyrazine derivants and their application to flavors.. 2000,6, 25–31.
(10) Li, Y. F.; Jin, L. Q.; Liu, Z. Q.; Zheng, Y. G.The research progress on pyrazinamide.. 2010, 04, 307–312.
(11) Zhu, H. L.; Wang, X. L.; Tang, J. F.; Hu, Y.; Yang, Y. S.; Zhang, Y. B.; Zhang, F.103373992A 2013–10–30.
(12) Zhu, H. L.; Wang, X. L.; Zhang, F.; Zhang, Y. B.; Yang, Y. S.; Tang, J. F..103373988A, 2013–10–30.
(13) Xu, Z. P.; Shao, X. S.The unique position of halogen substituents in modern pesticide design.2011, 01, 12–19+38.
(14) Zhang, M.Synthesis and antibacterial activities research of C–N bridged-hydroxy diphenyl compounds.2008.
(15) Wang, Y. Studies on the synthesis, crystal structure biological activity of first-row transition metal complexes with 3,5-dichlorosalicylaldehyde.2009.
(16) Milczarska, B.; Gobis, K.; Foks, H.; Golunski, L.; Sowinski, P.The Synthesis of 3-amino-pyrazine-2-carbohydrazide and 3-amino-N′-methylpyrazine-2-carbohydrazide derivatives.2012, 49, 845–850.
(17) Sheldrick, G. M.. University of G?ttingen, Germany 1996.
(18) Sheldrick, G. M.University of G?ttingen, Germany 1997.
(19) Liu, X. R.; Sun, X. C.; Yang, Z. W.; Zhao, S. S.; Yang, S. L.; Yan, S.2-furancarbaldehyde-4-hydroxy-benzoylhydrazone and its Cu(Ⅱ) complex: crystal structures and binding ability with CT-DNA.2016, 02, 250–258.
(20) Zhang, H. Y. The spectroscopy study on the interaction of the three drugs and BSA/ct–DNA.2014.
(21) Guo, Q.; Li, L. Z.; Dong, J. F.; Liu, H. Y.; Xue, Z. C.; Xu, T.Synthesis, crystal structure and interactions with DNA and BSA of an oxovanadium(IV) complex [VO(-Van-Asn)(Phen)]·1.5CH3OH.2012, 15, 1617–1624.
(22) Sathyadevi, P.; Krishnamoorthy, P.; Bhuvanesh, N. S. P.Organometallic ruthenium(II) complexes: synthesis, structure and influence of substitution at azomethine carbon towards DNA/BSA binding, radical scavenging and cytotoxicity.2012, 55, 420–431.
(23) Zhou, Z. X.; Huang, Q. H.; Zhu, S.; Zhou, L. Establishment of rapid determining method for antibacterial activity by microplate reader.2014, 3, 29–35.
(24) Zhang, Q.; Wang, Q.; Chen, Z. S. In vitro antibacterium and antitumor activities of sesquiterpenes compound from cremanthodium discoideum Msxim.2002, 18, 597–598.
(25) Yai, Z. W.; Ding, Z. C.; Liu, X. R.; Zhao, S. S.; Zhang, R. L.; Yang, S. L.Crystal structures and thermochemical properties of phenyl-acetic acid furan-2-ylmethylene-hydrazide and its Ni(Ⅱ) complex.2015, 08, 1520–1528.
(26) Wei, Z. B.; Wang, J. C.; Jiang, X.; Li, Y. Q.; Chen, G. H.; Xie, Q. F.Experimental and DFT studies of pyridine-4-carboxylic acid (2,4-dihydroxy-phenylethylidene)-hydrazide Schiff base: synthesis, crystal structure, properties and quantum chemistry calculation.2015,09, 1014–1021.
(27) Hu, R. Z.; Gao, S. L.; Zhao, F. Q.. Beijing 2008, 79–120.
(28) Jian, Y.; Li, G.; Yang, P.; Deng, T.; Zhou, X.; Xu, H. Y.Syntheses, spectral properties of novel carbazole derivatives and evaluations of its Ct–DNA interaction.2014, 34, 809–816.
(29) Wei, Q.; Dong, J. F.; Li, W. B.; Zhao, P. R.; Ding, F. F.; Li, L. Z.Syntheses, crystal structures, DNA interactions and SOD activities of two nickel(H) complexes with L-histidine Schiff Base.2016, 05, 789–798.
(30) Yan, H.; Yang, L.; Chang, G. L.; Li, X.; Niu, M. J. DNA interaction and cytotoxic activity of a chiral amino-alcohol Schiff base derived Cu(Ⅱ) complex.2016, 35, 465–471.
(31) Stothkamp, R. E. J. Fluorescence measurements of ethidium binding to DNA.1993,71, 77–79.
(32) Luan, J. M.; Zhang, X. D. Advances of fluorimetric determination of DNA.2007, 43, 241–245.
(33) Hu, Z.Analysis of DNA by the fluorescence probe of methylene blue and its application in the study of the interactions between heavy metals and paraquat with DNA2006.
(34) Kashanian, S.; Dolatabadi, J. E. N.In vitro studies on calf thymus DNA interaction and 2-tert-butyl-4-methylphenol food additive.2010, 230, 821–825.
(35) Kumar, A. K.; Reddy, K. L.; Satyanarayana, S.Study of the interaction between ruthenium(II) complexes and CT-DNA: synthesis, characterisation, photocleavage and antimicrobial activity studies.. 2010, 22, 629–643.
(36) Chen, J. X.; Tian, Y.; Xiang, Q. X.; Zhang, L. Q.; Xiong, J. R.Spectroscopy studies of interactions between DNA and N3O2-donor macrocycle bearing naphathalic sulfonic group.2010, 30, 66–71.
(37) Yan, C. N.; Shang, G. Y. F.; Tong, J. Q.; Liu, Y.; Pang, D. W.; Pan, Z. T.; Qu, S. S.Study on thermodynamics of binding reaction of dipyridamole with bovine serum albumin.2003, 23, 543–546.
(38) Tian, Z. Y.; Su, L. P.; Xie, S. Q.; Zhao, J.; Wang, C. J.Synthesis, biological activity and fluorescence spectroscopy of naphthalimide-polyamine conjugates.2013, 33, 1514–1521.
(39) Akbay, N.; Seferoglu, Z.; G?k, E.Fluorescence interaction and determination of calf thymus DNA with two ethidium derivatives.2009, 19, 1045–1051.
(40) Wang, J.; Liu, L. J.; Liu, B.; Guo, Y.; Zhang, Y. Y.; Xu, R.; Wang, S. X.; Zhang, X. D.Spectroscopic study on interaction of bovine serum albumin with sodium magnesium chlorophyllin and its sonodynamic damage under ultrasonic irradiation.2010, 75, 366–374.
(41) Zhang, G. W.; Zhao, N.; Hu, X.; Tian, J.Interaction of alpinetin with bovine serum albumin: probing of the mechanism and binding site by spectroscopic methods.2010, 76, 410–417.
(42) Chen, Z. F.; Luo, Y. D.; Hua, L. G.; Zhang, J.Reactivities towards DNA and protein and cytotoxic activities of benzimidazole derived mononuclear cobalt(Ⅱ) and nickel(Ⅱ) complexes.2014, 07, 1525–1534.
(43) Yang, L. N.; Yao, L.; Yu, L. L.; Yang, L. Y.; Yu, J. Study on synthesis of nitroderivatives of 2,2?--biimidazoleand their interaction with DNA.2012, 07, 56–59.
(44) Zhong, G. X. The research progress of fluorine-bearing antitumor drugs.2005, 1, 46–48.
(45) Reddy, O. S.; Suryanarayana, C. V.; Sharmila, N.; Ramana, G. V.;Anuradha, V.;Babu, B. H. Synthesis and cytotoxic evaluation for some new dihydropyrimidinone derivatives for anticancer activity.2013, 10, 699–705.
7 August 2017;
10.14102/j.cnki.0254-5861.2011-1799
27 November 2017 (CCDC 1473040 for 1, 1477347 for 2 and 1474331 for 3)
① Project supported by the National Natural Science Foundation of China (Nos. 21073139, 21103135 and 21301139)
②. E-mail: liuxiangrongxk@163.com
- 結構化學的其它文章
- Synthesis, Crystal Structure and Photoluminescent Property of a New Zn(II) Complex Based on 3,4-Bis(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole①
- Transitional Area of Ce4+ to Ce3+ in SmxCayCe1-x-yO2-δ with Various Doping and Oxygen Vacancy Concentrations: A GGA + U Study①
- A New Dinuclear Zinc Polymer Based on 3-Methoxy-2-hydroxybenzaldehyde:Synthesis, Structure, Spectral Characterization and Hirshfeld Surface Analysis①
- Fabrication of WO3/TiO2 Heterostructures for Efficiently Photocatalytic Gaseous Hydrocarbons Degradation: Origin of Photoactivity and Revisit the Role of WO3 Decoration①
- Two Copper Complexes Based on Pyrazole- 3-carboxylic Acid as Heterogeneous Catalysts for Highly Selective Oxidation of Alkylbenzenes①
- Synthesis, Crystal Structure and Cytotoxic Activities of Oxazolidin-2-one Derivatives①

