一對手性銪(Ⅲ)配合物的合成和鐵電性質
劉 建 張小朋 李承輝
一對手性銪(Ⅲ)配合物的合成和鐵電性質
劉 建*,1,2張小朋3李承輝2
(1南京林業大學化學工程學院,江蘇省生物質綠色燃料與化學品重點實驗室,南京 210037)
(2南京大學化學化工學院,配位化學國家重點實驗室,人工微結構科學與技術協同創新中心,南京 210023)
(3海南師范大學化學與化工學院,海南省水環境污染治理與資源化重點實驗室,海口 571158)
合成并表征了一對蒎烯修飾的稀土銪(Ⅲ)配合物 Eu(TTA)3L1a(1a)和 Eu(TTA)3L1b(1b)(TTA=2-噻吩甲酰三氟丙酮,L1a=(+)-4,5-雙蒎烯-2,2′-聯吡啶,L1b=(-)-4,5-雙蒎烯-2,2′-聯吡啶)。 化合物的手性特征通過 X 射線單晶結構和圓二色光譜進行了表征。配合物1a結晶于極性空間群P1,中心Eu(Ⅲ)離子呈現出嚴重扭曲的四方反棱柱體的配位環境。鑒于非中心對稱結構的特性,配合物1a也表現出明顯的鐵電性質。
配位化學;手性銪(Ⅲ)配合物;晶體結構;鐵電性質
Recently,multifunctionalmaterialsbased on lanthanide complexes have received much attention due to their potential applications in optoelectronics[1-3].The introduction of chiral environments in lanthanide complexes would lead to a diversification of configuration and crystallize in a polar space group.Thus,interesting properties,such as ferroelectricity,piezoelectricity,triboluminescence,circular polarized luminescence (CPL)and second harmonic generation(SHG)would be expected[4-7].
Ferroelectric materials possessing a spontaneous electric polarization that can be reversed by the application of an external electric field,are of great interest in the field of optics and electronics[8].Compared to the typicalinorganic ABO3-type ferroelectrics,molecular-based ferroelectric materials show some advantages:largerspontaneouspolarization,facile chemical vapor deposition (CVD)and convenient structural modification.Furthermore,owing to the high coordination numbers and the interaction between a central metal ion and its ligand field (LF),the structures of lanthanide complexes could be easily distorted and spontaneous dipole moments can be induced,possibly resulting in interesting multi-ferroic properties.
In previous studies,we have studied multifunctional properties (luminescence,ferroelectricity,magnetism and so on)of chiral lanthanide complexes incorporated pinene functionalized with aromatic amine derivativesaswellassolvent-induced or temperature-induced switching of configurations and properties of these complexes[9-12].A novel Eu(Ⅲ)complex Eu(TTA)3L1a(1a)(TTA=thenoyltrifluoroacetonate,L1a=(+)-4,5-bis(pinene)-2,2′-bipyridine)with a bispinene-containing bipyridyl ligand was used as high efficient luminescent down-shifting material and lead to a significant improvement of the spectral response of c-Si PV module in the UV region[13].
As an extension of these work,the crystal structure and ferroelectric property of 1a has been studied in this paper.The compound 1a crystallizes in anoncentrosymmetricspacegroup (P1),anda distorted square-antiprism configuration exists around the central Eu(Ⅲ) ion,satisfying the prerequisites of ferroelectrics.As expected,complex 1a shows a distinct electric hysteresis loop,making it as potential multifunctional molecular materials.The enantiomer Eu(TTA)3L1b(1b)(L1b=(-)-4,5-bis(pinene)-2,2′-bipyridine)was also prepared,and chiral environments are confirmed by a mirror symmetry of circular dichroism(CD)spectra of 1a and 1b.
1 Experimental
1.1 Chemicals and synthesis
All reagents were purchased from commercial suppliers and used as received.Compounds 1a and 1b were prepared according to our published methods as follows[13]:L1aor L1b(344 mg,1 mmol)was added to a stirred solution of Eu(TTA)3·2H2O (851 mg,1 mmol)in ethanol (40 mL).The resulting mixture was stirred at room temperature for 24 h.The precipitate was collected by filtration to yield the desired product.Crystals of compound 1a suitable for X-ray crystallographic analysis were obtained by slow evaporation over a period of 3 weeks of their CH2Cl2/CH3CH2OH solution.
1.2 Chemical and physical characterizations
Single-crystalX-ray diffraction measurements were carried out on a Bruker SMART APEX CCD based on diffractometer operating at room temperature.Intensities were collected with graphite monochromatized Mo Kα radiation (λ=0.071 073 nm)operating at 50 kV and 30 mA,using ω-2θ scan mode.The data reduction was made with the Bruker SAINT package[14]. Absorption corrections were performed using the SADABS program[15].The structures were solved by direct methods and refined on F2by fullmatrix least-squares using SHELXL-97 with anisotropic displacement parameters for all non-hydrogen atoms in all two structures[16].Hydrogen atoms bonded to the carbon atomswereplaced in calculated positions and refined as riding mode,with dC-H=0.093 nm (methane)or 0.096 nm (methyl)and Uiso(H)=1.2Ueq(Cmethane)or Uiso(H)=1.5Ueq(Cmethyl).All computations were carried out using the SHELXTL-97 program package[16-17].The absorption spectra of complexes 1a and 1b in CH2Cl2solution were recorded on a Shimazu UV-3100 spectrometer.CD spectra were recorded by a Jasco J-810 spectropolarimeter.The Electric hysteresis loops were recorded by a Ferroelectric Tester Precision PremierⅡby using powder samples in pellets at room temperature.CCDC:818185,1a.
2 Results and discussion
2.1 Crystal structure
The crystal data and structure refinements for compound 1a are provided in Table 1.Similar to those europium (Ⅲ) complexes reported previously[4,12],compound 1a is a mononuclear neutral europium(Ⅲ)compound and crystalizes in triclinic system,space group P1.As shown in Fig.1,each molecule contains three β-diketonate anions,one (+)-4,5-bis(pinene)-2,2′-bipyridine,and one eight-coordinated Eu(Ⅲ) ion.Each of the three diketonate anions provides two donor O atoms to coordinate to the Eu(Ⅲ)ion.The other two coordination sites of the Eu(Ⅲ)ion are occupied by two N atomsofthe chiralbipyridine derivative to complete the eight-coordinate configuration.
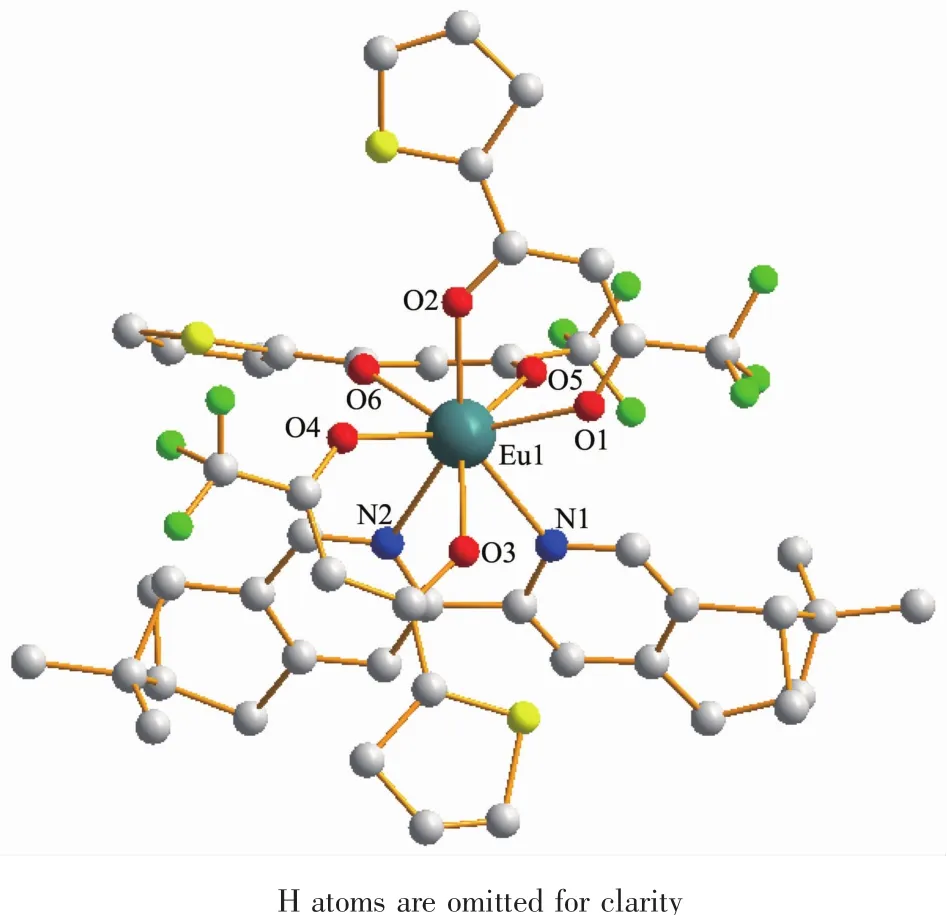
Fig.1 Molecular structure of 1a
Selected bond lengths and angles are summarized in Table 2.The Eu-O bond lengths range from 0.231 8 to 0.238 5 nm,while the two Eu-N bond lengths are 0.258 7(16)and 0.255 6(15)nm,respectively.Eight bonds with different lengths give rise to a strongly distorted square-antiprism environment around the Eu(Ⅲ) ion (Fig.2).The O1-O2-O3-O4 (bottom plane)and O5-O6-N1-N2 atoms (top plane)comprise the two square-basic planesofthe antiprism with mean deviations of 0.002 78 and 0.013 97 nm from each plane,and their dihedral angle is 3.442°.For a regularsquareantiprism,twosquareplanesare parallel to each other and one square rotates 45°from the other.However,in 1a,the top plane rotates only 41.4°from the bottom plane.
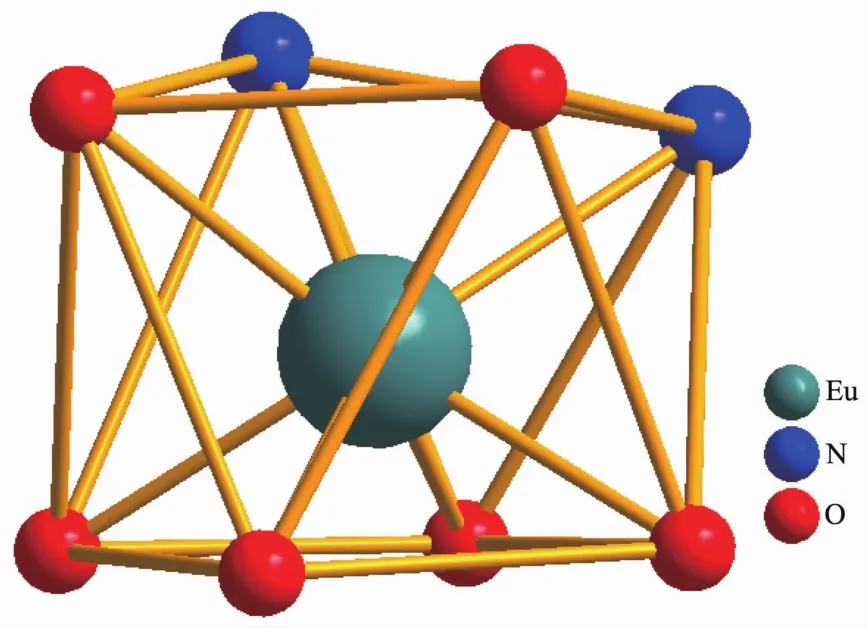
Fig.2 Coordination environments of 1a
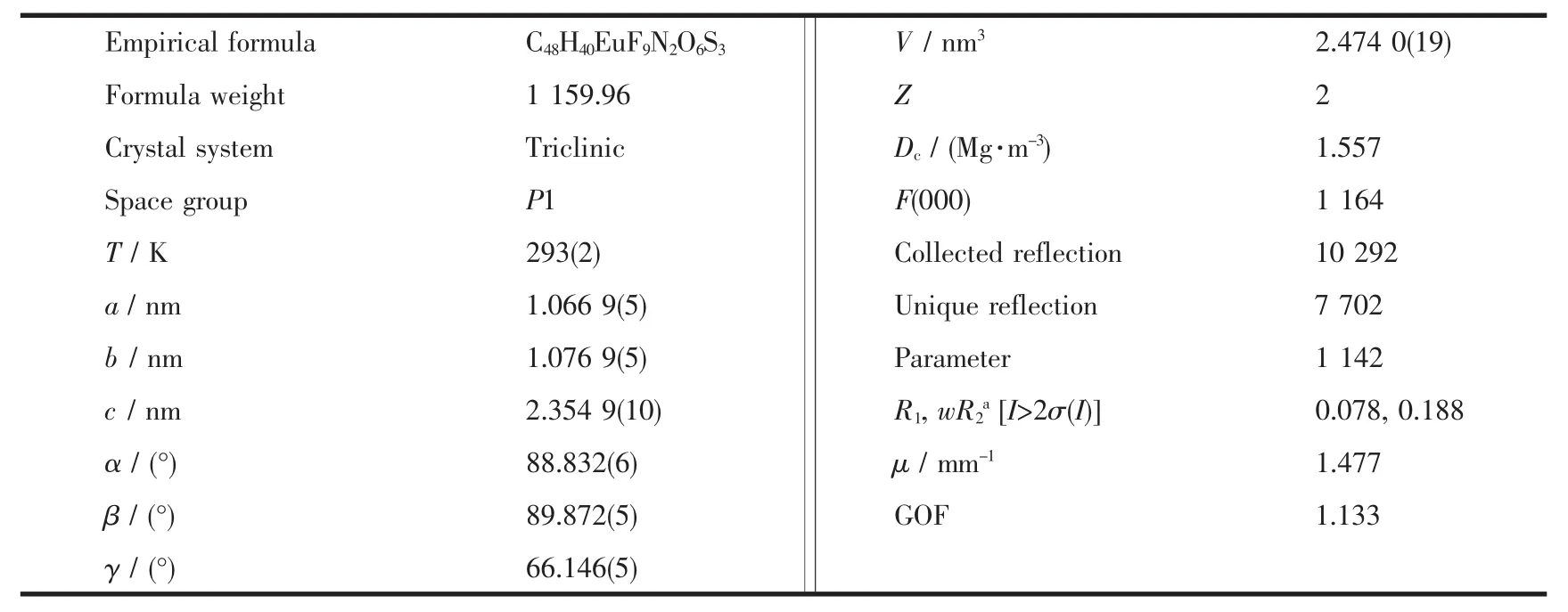
Table 1 Crystal data and structure refinements for complex 1a
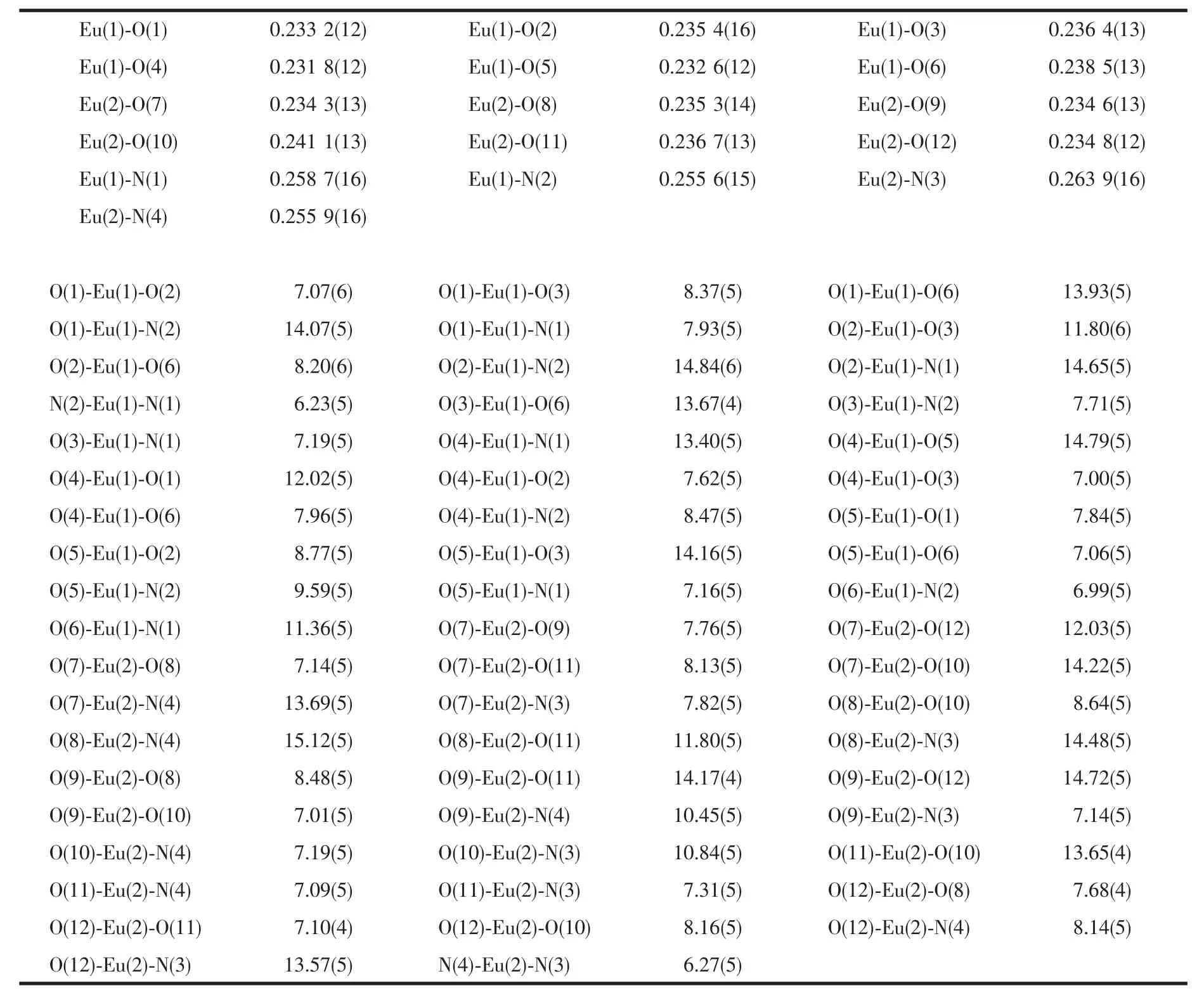
Table 2 Selected bond lengths(nm)and angles(°)for 1a
2.2 Circular dichroism (CD)spectra
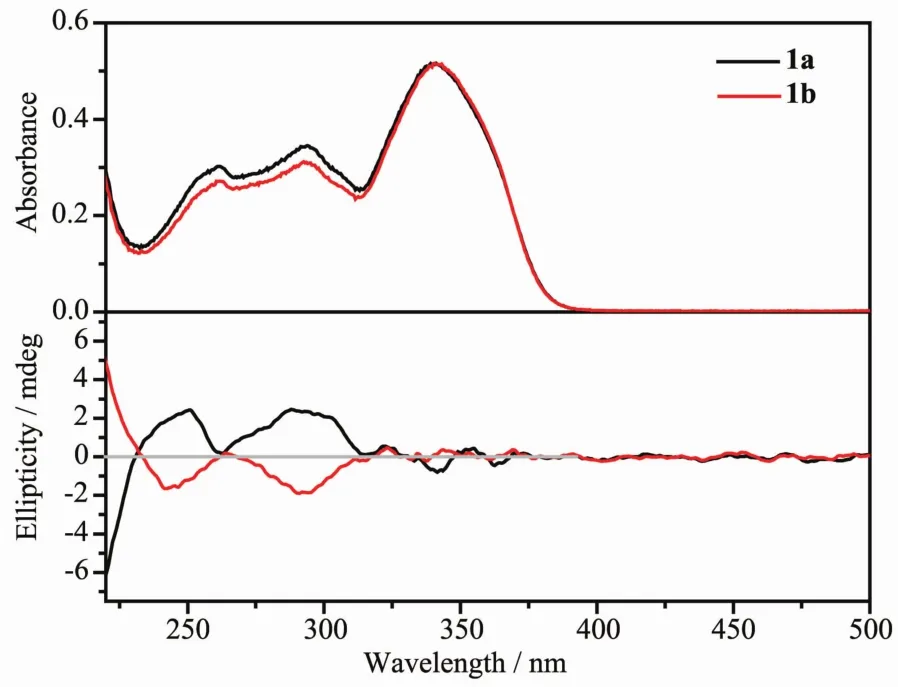
Fig.3 UV-Vis and CD spectra of 1a and 1b in 2×10-5 mol·L-1dichloromethane solution
The chirality ofcomplexes 1a and 1b is confirmed by measuring circulardichroism (CD)spectra (Fig.3).Two distinct Cotton effect at 245 and 292 nm can be observed,and the spectra of 1a and 1b are almostmirror-symmetric.These features indicate that 1a and 1b are chiral and enantiomeric to each other.
2.3 Ferroelectric properties
Since complexes 1a crystalizes in a polar space group (P1),the ferroelectric properties of 1a were investigated at298 K with compressed powder samples as shown in Fig.4. The ferroelectric measurements reveal that complex 1a indeed displays an obvious ferroelectric behavior and almost reaches thesaturated polarization statuswith a remnant polarization (Pr)of ca.0.27 μC·cm-2and Ecof ca.7.2 kV·cm-1by applying an electric field of 15 kV·cm-1at 4 Hz.The saturation spontaneous polarization (Ps)is about 0.43 μC·cm-2,which exceeds the value of typical ferroelectrics NaKC4H4O6·4H2O (Rosal salt,Ps=0.25 μC·cm-2).
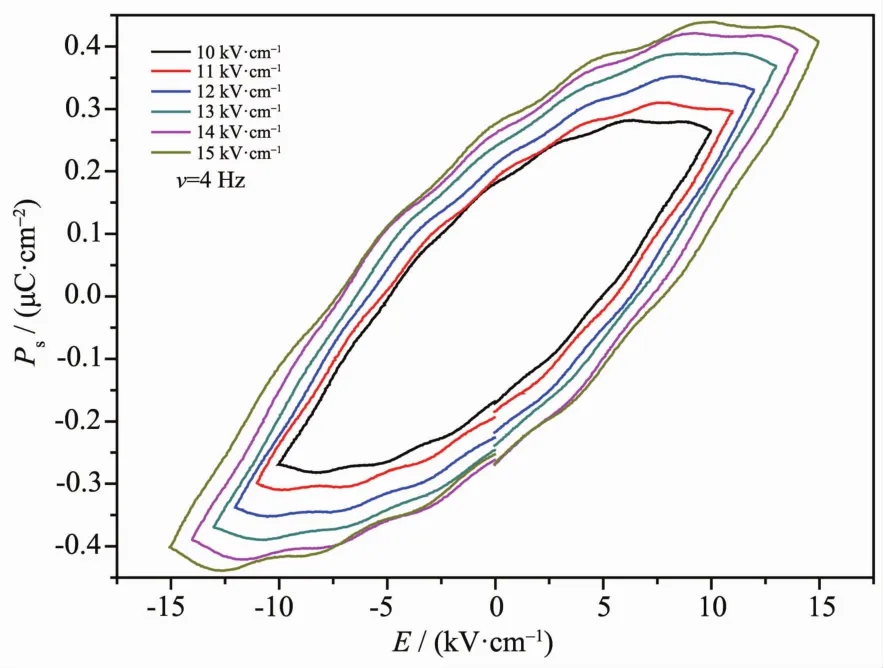
Fig.4 Ferroelectric hysteresis loop for complex 1a at 298 K
3 Conclusions
In summary,a couple of pinene-containing chiral europium(Ⅲ)complexes 1a and 1b were prepared.The chirality of complexes 1a and 1b is confirmed by measuring circular dichroism (CD)spectra.Complexes 1a crystalizes in P1 space group of triclinic system.The coordination polyhedron of the Eu(Ⅲ)ion in 1a can be described as a siginificantly distorted squareantiprism.The complex 1a exhibited distinct ferroelectric behaviors with a remnant polarization (Pr)of ca.0.27 μC·cm-2and Ecof ca.7.2 kV·cm-1by applying an electric field of 15 kV·cm-1at 4 Hz.
Acknowledgements:This work was supported by the Jiangsu Province Science Foundation for Youths (Grant No.BK20150569),the National Natural Science Foundation of China (Grant No.201502088),the project of Scientific and Technological Support Program in Jiangsu province (Grant No.BE2014147-2),and the Doctoral Fund of the Ministry of Education ofChina for financial support (GrantNo.20120091130002).
[1]Walton J W,Bourdolle A,Butler S J,et al.Chem.Commun.,2013,49(16):1600-1602
[2]Kotova O,Kitchen J A,Lincheneau C,et al.Chem.Eur.J.,2013,19(48):16181-16186
[3]Carr R,Di Bari L,Lo Piano S,et al.Dalton Trans.,2012,41(42):13154-13158
[4]Li D P,Li C H,Wang J,et al.Eur.J.Inorg.Chem.,2009(32):4844-4849
[5]Bozoklu G,Gateau C,Imbert D,et al.J.Am.Chem.Soc.,2012,134(20):8372-8375
[6]Harada T,Tsumatori H,Nishiyama K,et al.Inorg.Chem.,2012,51(12):6476-6485
[7]Li X L,Chen C L,Xiao H P,et al.Dalton Trans.,2013,42(43):15317-15325
[8]Hang T,Zhang W,Ye H Y,et al.Chem.Soc.Rev.,2011,40(7):3577-3598
[9]Li D P,Wang T W,Li C H,et al.Chem.Commun.,2010,46(17):2929-2931
[10]Li D P,Zhang X P,Wang T W,et al.Chem.Commun.,2011,47(24):6867-6869
[11]Liu J,Zhang X P,W T,et al.Inorg.Chem.,2012,51(16):8649-8651
[12]Li X L,Chen K,Liu Y,et al.Angew.Chem.Int.Ed.,2007,46(36):6820-6823
[13]Liu J,Wang K,Zheng W,et al.Prog.Photovoltaics Res.Appl.,2013,21(4):668-675
[14]SAINT-Plus,Ver.6.02,Bruker Analytical X-ray System,Madison,WI,1999.
[15]Sheldrick G M.SADABS,an Empirical Absorption Correction Program,Bruker Analytical X-ray Systems,Madison,WI,1996.
[16]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of G?ttingen,Germany,1997.
[17]Sheldrick G M.Acta Crystallogr.Sect.A:Found.Crystallogr.,2008,64:112-122
Syntheses and Ferroelectric Properties of a Couple of Chiral Europium(Ⅲ)Complexes
LIU Jian*,1,2ZHANG Xiao-Peng3LI Cheng-Hui2
(1Jiangsu Key Lab of Biomass-based Green Fuels and Chemicals,College of Chemical Engineering,Nanjing Forestry University,Nanjing 210037,China)
(2Collaborative Innovation Center of Advanced Microstructures,State Key laboratory of Coordination Chemistry,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210023,China)
(3Key Laboratory of Water Pollution Treatment&Resource Reuse of Hainan Province,College of Chemistry and Chemical Engineering,Hainan Normal University,Haikou 571158,China)
A couple of pinene-containing europium(Ⅲ) complexes,Eu(TTA)3L1a(1a)and Eu(TTA)3L1b(1b)(TTA=thenoyltrifluoroacetonate,L1a=(+)-4,5-bis(pinene)-2,2′-bipyridine,L1b=(-)-4,5-bis(pinene)-2,2′-bipyridine),were prepared and characterized.The chiral characterization was confirmed by single crystal X-ray diffraction and distinct electronic circular dichroism (CD)signals.Complex 1a crystallizes in the polar space group P1,and a strongly distorted square-antiprism environment around the Eu(Ⅲ)ion is exhibited.Due to the non-centrosymmetric structure,distinct ferroelectric behavior of complex 1a was observed as expected.CCDC:818185,1a.
coordination chemistry;chiral europium(Ⅲ)complexes;crystal structure;ferroelectric properties
O614.33+8
A
1001-4861(2017)11-2060-05
10.11862/CJIC.2017.256
2017-09-20。收修改稿日期:2017-10-16。
江蘇省自然科學青年基金(No.BK20150569)、國家自然科學青年基金(No.201502088)、江蘇省科技支撐計劃(No.BE2014147-2)、國家教育部博士點專項基金(No.20120091130002)資助項目。
*通信聯系人。E-mail:liu.jian@nju.edu.cn

