二芳基二硫醚與硝基芳烴的反應
李術艷,孫莉娜,沈淑君,程天行,程雙華,陳久喜
?
二芳基二硫醚與硝基芳烴的反應
李術艷1,2,孫莉娜1,2,沈淑君1,2,程天行3,程雙華3,陳久喜3
(1漳州職業技術學院食品與生物工程系,福建漳州 363000;2農產品深加工及安全福建省高校應用技術工程中心,福建 漳州 363000;3溫州大學化學與材料工程學院,浙江溫州 325035)
以二芳基二硫醚(ArSSAr)和硝基芳烴(ArNO2)為原料,在廉價易得的甲醛次硫酸氫鈉(Rongalite?)和碳酸鉀(K2CO3)共同促進下,以二甲亞砜(DMSO)為溶劑,50℃下合成了一系列非對稱二芳基硫醚衍生物,產物結構經1H NMR和13C NMR確證。該方法具有反應條件溫和、原料易得和操作簡單等優點。
二芳基二硫醚;硝基芳烴;甲醛次硫酸氫鈉;二芳基硫醚;反應機理
引 言
二芳基硫醚是一類非常重要的含硫化合物,被廣泛應用于藥物設計與開發[1-4]。許多含有芳基硫醚官能團的化合物具有多種生物活性。因此,二芳基硫醚衍生物的合成備受關注。早在1984年,Lindley等[5-6]報道了在六甲基磷酰胺等極性溶劑中高溫條件下(>200℃)進行芳基鹵化物與硫醇銅鹽或硫酚(醇)的反應。近年來,過渡金屬催化的鹵代芳烴與硫酚的偶聯反應制備硫醚的策略成為研究熱點[7-11]。最近,Bahekara等[12]報道了硝基芳烴與硫酚的偶聯反應,實現了C—S的形成反應以構建二芳基硫醚衍生物(圖1)。
雖然近年來芳基硫醚的合成取得重要進展[13-30],但這些方法仍存在一些缺點,如使用過量的具有惡臭味液態狀的硫醇或硫酚,較長的反應時間,計量甚至過量的不穩定的催化劑,苛刻的反應條件等。因此,開發一種簡便的合成方法制備芳基硫醚仍然具有重要意義。
甲醛次硫酸氫鈉(Rongalite?),也稱雕白粉,是一種非常廉價的還原性試劑,被廣泛應用于化工醫藥中間體的合成[31]。本課題組已報道了雕白粉促進二硫醚的活化,與環氧化合物的開環反應[32]、,-不飽和羰基化合物的Michael反應[33]。而甲醛次硫酸氫鈉促進的二硫醚與硝基芳烴的反應至今沒有報道。本文使用甲醛次硫酸氫鈉為促進劑活化二芳基二硫醚(替代具有惡臭味的硫酚),進而與硝基芳烴通過加成-消除反應制備非對稱芳基硫醚衍生物。
1 實驗部分
1.1 實驗藥品
對硝基苯甲醛(分析純)、對二硝基苯(分析純)、對硝基苯乙酮(分析純)、4,4′-二甲基二苯基二硫醚(分析純)、4,4′-二羥基二苯基二硫醚(分析純)、二苯基二硫醚(分析純)均購于百靈威科技有限公司;二甲亞砜(分析純)、碳酸鉀(分析純)、碳酸鈉(分析純)、碳酸氫鈉(分析純)、碳酸銫(分析純)、,-二甲基甲酰胺(分析純)均購于上海阿拉丁生化科技股份有限公司;環己烷(分析純)、甲苯(分析純)、甲醇(分析純)、三乙胺(分析純)、二水合氟化鉀(分析純)均購于天津市科密歐化學試劑有限公司;薄層層析硅膠板(2.5 cm×8 cm) 購于煙臺江友硅膠開發有限公司。
1.2 反應及分析測試儀器
AVANCE-300型或ADVANCE-500型核磁共振儀,TMS為內標,CDCl3為溶劑,Bruker公司;MicrOTOF-QII高分辨質譜,Bruker公司;WRS-1B數字熔點儀,上海精密科學儀器有限公司;ZF-20D型暗箱式紫外分析儀,河南愛博特科技發展有限公司;DSB-2100型旋轉蒸發儀,上海愛朗儀器有限公司;智能恒溫磁力攪拌器(ZNCL-QS 130-60),河南愛博特科技發展有限公司;TLE104E/02型電子天平,梅特勒-托利多儀器(上海)有限公司。
1.3 非對稱二芳基硫醚化合物的合成
在裝有磁力攪拌器的干燥的Schlenk管中加入硝基芳烴(0.4 mmol)、二芳基二硫醚(0.3 mmol)、甲醛次硫酸氫鈉(0.6 mmol)、K2CO3(0.45 mmol)和DMSO(2 ml),常溫攪拌溶解后,升溫至50℃充分攪拌3 h。整個實驗過程用高效薄層色譜板(TLC)進行監測,反應完畢后,反應液用20ml水洗,然后再用乙醚30 ml分3次萃取,合并有機相,無水硫酸鎂干燥,過濾后濃縮,所得的粗產物用0.037~0.048 mm硅膠柱層析分離純化制得非對稱二芳基硫醚。
當使用對硝基苯甲醛作為反應底物時,反應在氮氣氛圍中進行。
2 實驗結果與討論
2.1 反應條件篩選
選用如圖2所示的對硝基苯乙酮(1a)和二苯基二硫醚(2a)作為模板反應,考察不同條件(如溶劑、堿、配比等)對反應結果的影響。
2.1.1 溶劑的篩選 首先考察了溶劑對反應的影響。如表1所示,溶劑對反應的影響較大,當選用環己烷、甲苯或甲醇作為溶劑時,只得到非常少量的目標產物4-(苯硫基)苯甲醛(3a)。然而,當反應在,-二甲基甲酰胺(DMF)中進行時,3a的收率提高至52%。當選用二甲亞砜(DMSO)為溶劑,3a的收率進一步提高至77%。因此,在接下來的反應條件篩選選取DMSO作為溶劑。
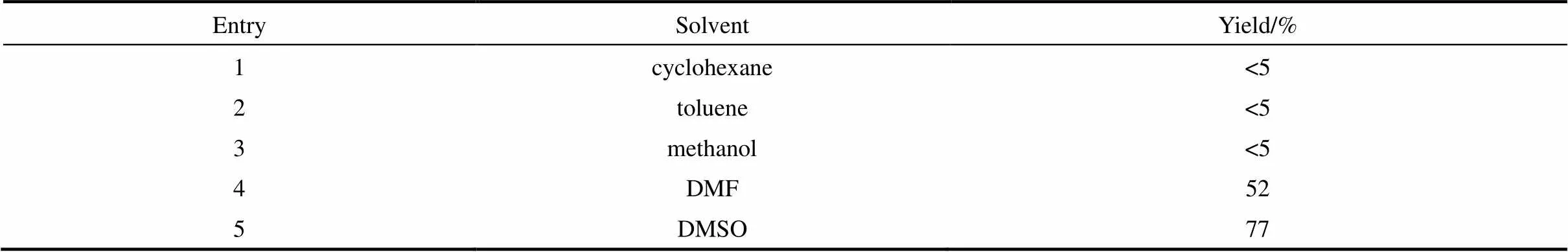
表1 溶劑篩選
Note: Reaction conditions: 1a (0.4 mmol ), 2a (0.3 mmol),K2CO3(0.45 mmol) and Rongalite?(0.6 mmol), solvent (2 ml), 3 h, 50℃.
2.1.2 堿的篩選 如表2所示,堿對該反應也有一定的影響。在所有被考察的堿性化合物中,當選用KF·2H2O作為堿時,反應只得到痕量目標產物3a。
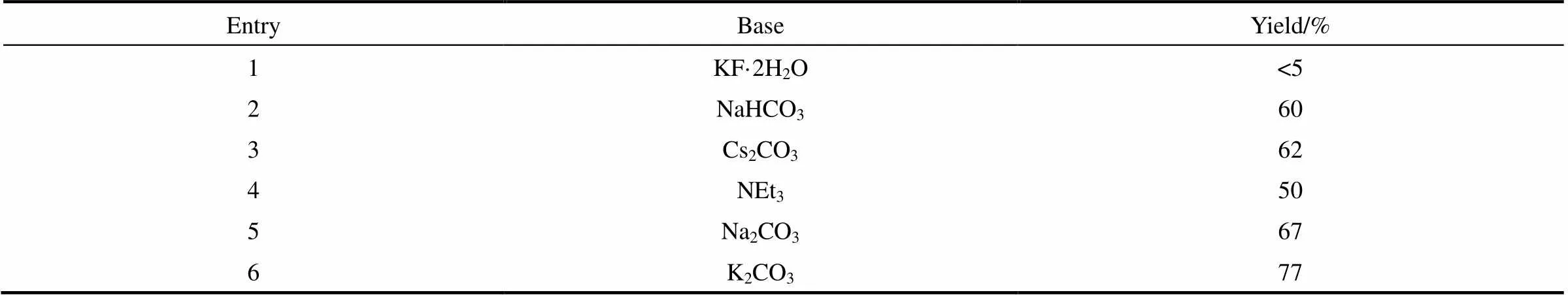
表2 堿篩選
Note: Reaction conditions: 1a (0.4 mmol ), 2a (0.3 mmol),base (0.45 mmol) and Rongalite?(0.6 mmol), DMSO (2 ml), 3 h, 50℃.
當使用其他的無機堿(如NaHCO3、Cs2CO3和Na2CO3)或有機堿(如三乙胺,NEt3),3a的收率分別為60%、62%、67%和50%。以K2CO3作為堿時的收率可達77%。
2.1.3 堿及甲醛次硫酸氫鈉的用量對反應的影響
以DMSO為反應溶劑,K2CO3為堿性條件,考察了堿和甲醛次硫酸氫鈉(Rongalite?)的用量對反應的影響。由表3可見,堿和甲醛次硫酸氫鈉對該反應至關重要,不加兩者中任意一種,該反應無法進行。首先篩選K2CO3的用量,當反應體系中加入0.15 mmol的K2CO3時,產物3a的收率為30%;當K2CO3的量增至0.3 mmol,產物3a的收率提高至65%;當K2CO3的量為0.45 mmol時,產物3a的收率最高(77%);繼續K2CO3的量增至0.6 mmol時,產率并沒有明顯的提高。另一方面,通過篩選Rongalite?的用量發現當反應體系中加入0.6 mmol的Rongalite?時,產物3a的收率為77%。
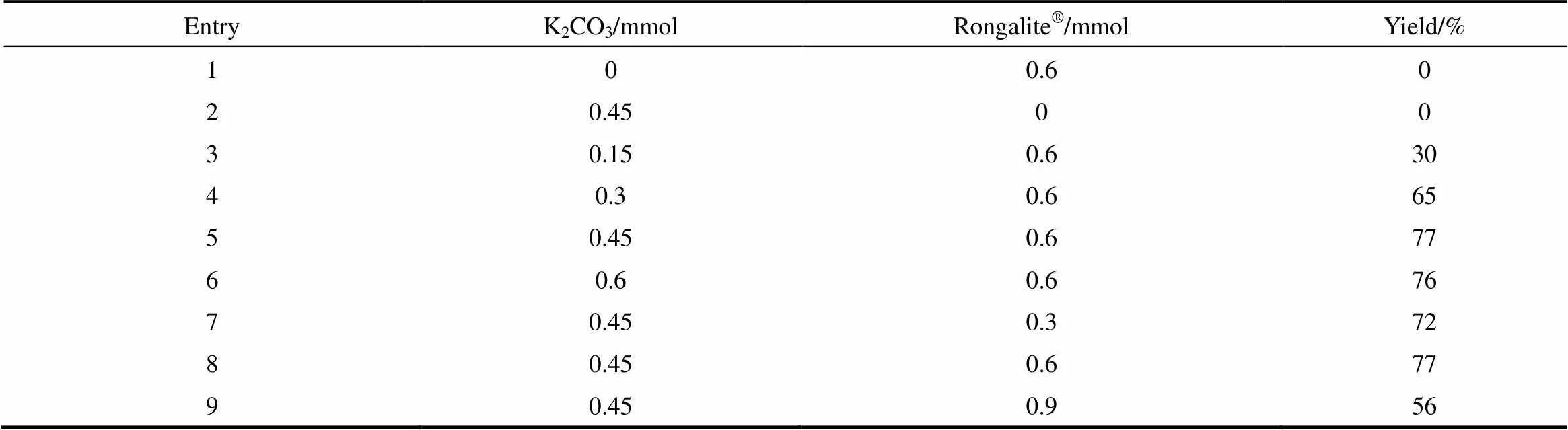
表3 堿及促進劑的用量對反應的影響
Note: Reaction conditions: 1a (0.4 mmol ), 2a (0.3 mmol),DMSO (2 ml), 3 h, 50℃.
2.2 底物范圍
通過以上的篩選,獲得較佳的反應條件如下:對硝基苯乙酮(0.4 mmol)、二苯基二硫醚(0.3 mmol)、Rongalite?(0.6 mmol),K2CO3(0.45 mmol),DMSO (2 ml),50℃,3 h。為進一步研究該反應條件的普適性,探討了含其他取代基團硝基芳烴與二芳基二硫醚的反應情況。
如表4所示,對硝基苯乙酮(1a)與二對甲苯基二硫醚(2b)、二對羥基苯基二硫醚(2c)的反應都能平穩地進行,相應的目標產物3b和3c收率分別為61% 和72%。有趣的是,當使用1,4-二硝基苯(1b)為底物時,反應也能順利進行;如序號4~6所示,選擇性地合成了目標產物3d~3f,收率分別是67%、61%和66%。值得一提的是,當使用對硝基苯甲醛(1c)為底物時,采用在N2保護條件下、降低反應溫度(25℃)的策略避免對硝基苯甲醛的甲酰基(CHO)被氧化。如序號7~9所示,1c與二苯基二硫醚(2a)、二對甲苯基二硫醚(2b)和二對羥基苯基二硫醚(2c)的反應,所得的目標產物3g~3i的收率分別為79%、70%和73%。當然,該方法也存在底物使用范圍窄的局限性。例如,當使用富電子的硝基芳烴作為底物時,該反應無法進行。
表4 底物范圍
Table 4 Substrates scope
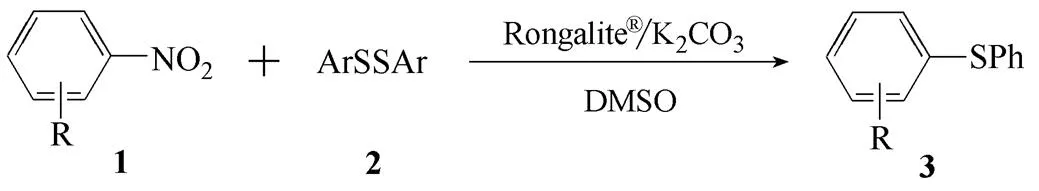

EntryR (1)Ar (2)ProductYield /% 1COCH3 (1a)Ph (2a)3a77① 21ap-MeC6H4 (2b)3b61① 31ap-OHC6H4(2c)3c72① 4NO2 (1b)2a3d67① 51b2b3e61① 61b2c3f66① 7CHO (1c)2a3g79② 81c2b3h70② 91c2c3i73②
① Reaction conditions: 1 (0.4 mmol ), 2 (0.3 mmol), K2CO3(0.45 mmol) and Rongalite?(0.6 mmol), DMSO (2 ml), 3 h, 50℃. ②At 25℃ under N2atmosphere.
2.3 產物結構表征
1-(4-苯硫基)苯乙酮(3a): 熔點: 62~63℃;1H NMR (CDCl3, 300 MHz): 2.55 (s, 3H), 7.83~7.19 (m, 9H);13C NMR (CDCl3, 125 MHz):26.5,127.5, 128.8, 128.9, 129.7, 132.1, 133.9, 134.5, 144.9, 197.1。HRMS (/): [M+H]+calcd. for C14H13OS: 229.0682; found: 229.0677。
1-(對甲苯硫基)苯乙酮(3b): 熔點: 92~93℃;1H NMR (CDCl3, 300 MHz): 2.39 (s, 3H), 2.53 (s, 3H), 7.13~7.23 (m, 4H), 7.39~7.80 (m, 4H);13C NMR (CDCl3, 125 MHz):21.1, 26.3, 126.5, 127.8, 128.7, 130.4, 134.0, 134.3, 139.2, 145.8, 196.9。HRMS (/): [M+H]+calcd. for C15H15OS: 243.0838; found: 243.0835。
1-(對羥基苯硫基)苯乙酮(3c): 油狀物;1H NMR (CDCl3, 300 MHz):2.55 (s, 3H), 5.20 (s, 1H), 6.90~7.81 (m, 8H);13C NMR (CDCl3, 125 MHz):25.8, 117.0, 120.8, 125.8, 130.1, 133.1, 137.2, 149.1, 157.2, 191.4。HRMS (/): [M+H]+calcd. for C14H13O2S: 245.0631; found: 245.0638。
4-(對硝基苯基)苯基硫醚(3d): 熔點: 53~54℃;1H NMR (CDCl3, 300 MHz): 7.16~8.08 (m, 9H);13C NMR (CDCl3, 125 MHz):124.1, 126.7, 129.7, 130.1, 130.5, 134.8, 145.4, 148.5。HRMS (/): [M+H]+calcd. for C12H10NO2S: 232.0427; found: 232.0423。
4-(對硝基苯基)(對甲基苯基)硫醚(3e): 熔點: 83~84℃;1H NMR (CDCl3, 300 MHz): 2.42 (s, 3H), 7.12~8.07 (m, 8H);13C NMR (CDCl3, 125 MHz):21.3, 124.0, 126.1, 126.5, 130.9, 135.1, 140.2, 142.2, 149.3。HRMS (/): [M+H]+calcd. for C13H12NO2S: 246.0583; found: 246.0588。
4-(對硝基苯硫基)苯酚(3f): 油狀物;1H NMR (CDCl3, 300 MHz): 5.21 (s, 1H), 6.933~7.10 (m, 4H), 7.43~8.06 (m, 4H);13C NMR (CDCl3, 125 MHz):117.2, 120.2, 124.0, 125.6, 137.4, 145.0, 150.1, 157.6。HRMS (/): [M+H]+calcd. for C12H10NO3S: 248.0376; found: 248.0379。
4-(苯硫基)苯甲醛(3g): 熔點: 53~54℃;1H NMR (CDCl3, 300 MHz): 7.23~7.45 (m, 5H), 7.52~7.74 (m, 4H), 9.91 (s, 1H);13C NMR (CDCl3, 125 MHz):127.2, 129.2, 129.8, 130.2, 131.3, 133.7, 134.4, 147.3, 191.3。HRMS (/): [M+H]+calcd. for C13H11OS: 215.0525; found: 215.0529。
4-(對甲基苯硫基)苯甲醛(3h): 熔點: 54~55℃;1H NMR (CDCl3, 300 MHz): 2.41 (s, 3H), 7.17~7.26 (m, 4H), 7.42~7.71 (m, 4H), 9.89 (s, 1H);13C NMR (CDCl3, 125 MHz):21.3, 126.5, 127.2, 130.0, 130.6, 133.4, 134.8, 139.7, 148.1, 191.1。HRMS (/): [M+H]+calcd. for C14H13OS: 229.0682; found: 229.0689。
4-(對羥基苯硫基)苯甲醛(3i): 油狀物;1H NMR (CDCl3, 300 MHz): 5.20 (s, 1H), 6.93~7.15 (m, 4H), 7.42~7.71 (m, 4H), 9.88 (s, 1H);13C NMR (CDCl3, 125 MHz):116.9, 125.8, 128.8, 133.8, 137.1, 146.9, 157.0, 196.0。HRMS (/): [M+H]+calcd. for C13H11O2S: 231.0474; found: 231.0479。
3 結 論
本文研究了以廉價的甲醛次硫酸氫鈉為反應促進劑,利用低毒無臭而且易于操作的二芳基二硫醚替代硫酚與硝基芳烴的反應,在溫和的反應條件下實現了非對稱二芳基硫醚的制備。所得產物采用1H NMR和13C NMR進行確證。該方法具有反應條件溫和、原料易得和操作簡單等優點,為非對稱二芳基硫醚衍生物的制備提供了新策略。
References
[1] LIU L P, STELMACH J E, NATARAJAN S R,Sar of 3,4-dihydropyrido[3, 2-]pyrimidone p38 inhibi- tors[J]. Bioorg. Med. Chem. Lett.,2003, 13: 3979-3982.
[2] LIU G, LINK J T, PEI Z,Discovery of novel-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intracellular adhesion molecule-1 interaction (1): Identification of an additional binding pocket based on an anilino diaryl sulfide lead[J]. J. Med. Chem.,2000, 43: 4025-4040.
[3] LIU G, HUTH J R, OLEJNICZAK E T,Novel-arylthio cinnamides as antagonists of leukocyte function-associated antigen-1/intracellular adhesion molecule-1 interaction (2): Mechanism of inhibition and structure-based improvement of pharmaceutical properties[J]. J. Med. Chem.,2001, 44: 1202-1210.
[4] DE MARTINO G, EDLER M C, LA REGINA G,New arylthioindoles: potent inhibitors of tubulin polymerization (2): Structure-activity relationships and molecular modeling studies[J]. J. Med. Chem., 2006, 49: 947-954.
[5] LINDLEY J. Copper assisted nucleophilic-substitution of aryl halogen[J].Tetrahedron,1984, 40: 1433.
[6] YAMAMOTO T, SEKINE Y. Condensation of thiophenols with aryl halides using metallic copper as a reactant. Intermediation of cuprous thiophenolates[J].Can. J. Chem.,1984, 62: 1544-1547.
[7] TERUYUKI K, TAKE-AKI M. Metal-catalyzed carbon-sulfur bond formation[J]. Chem. Rev.,2000, 100: 3205-3220.
[8] KAO H L, LEE C F. Efficient copper-catalyzed S-vinylation of thiols with vinyl halides[J]. Org. Lett.,2011, 13: 5204-5207.
[9] GUILARTE V, FERNáNDEZ-RODRíGUEZ M A, GARCíA-GARCíA P,.A practical, one-pot synthesis of highly substituted thiophenes and benzo[]thiophenes from bromoenynes and-alkynylbromobenzenes[J]. Org. Lett.,2011, 13: 5100-5013.
[10] XU H J, ZHAO Y Q, FENG T,Chan-Lam-type S-arylation of thiols with boronic acids at room temperature[J]. J. Org. Chem.,2012, 77: 2878-2884.
[11] CHEN C K, CHEN Y W, LIN C H,Synthesis of CuO on mesoporous silica and its applications for coupling reactions of thiols with aryl iodides[J]. Chem. Commun.,2010, 46: 282-284.
[12] BAHEKARA S S, SARKATE A P, WADHAI V M,CuI catalyzed C—S bond formation by using nitroarenes[J]. Catal. Commun.,2013, 41: 123-125.
[13] REZAEI N, MOVASSAGH B. Polystyrene resin-supported CuI-cryptand 22 complex: a highly efficient and reusable catalyst for the formation of aryl-sulfur bonds in aqueous media[J]. Tetrahedron Lett.,2016, 57: 1625-1628.
[14] LAI C S, KAO H L, WANG Y J,A general rhodium-catalyzed cross-coupling reaction of thiols with aryl iodides[J]. Tetrahedron Lett.,2012, 53: 4365-4367.
[15] ZHANG P F, YUAN J Y, LI H R,Mesoporous nitrogen-doped carbon for copper-mediated Ullmann-type C-O/-N/-S cross-coupling reactions[J]. RSC Adv.,2013, 3: 1890-1895.
[16] SINGH G, KUMAR A, MALIK S,TBAHS catalyzed coupling reactions of aryl iodides and aryl bromides with thiols under solvent free conditions[J]. Hetero. Lett.,2013, 3: 183-190.
[17] GUZMáN-PERCáSTEGUI E, HERNáNDEZ D J, CASTILLO I. Calix[8]arene nanoreactor for Cu(I)-catalyzed C—S coupling[J]. Chem. Commun.,2016, 52: 3111-3114.
[18] PANDA N, JENA A K, MOHAPATRA S. Heterogeneous magnetic catalyst for S-arylation reactions[J]. Appl. Catal. A—Gen., 2012, 433/434: 258-264.
[19] LAN M T, WU W Y, HUANG S H,. Reusable and efficient CoCl2·6H2O/cationic 2,2'-bipyridyl system-catalyzed S-arylation of aryl halides with thiols in water under air[J]. RSC Adv., 2011, 1: 1751-1755.
[20] MOVASSAGH B, TAKALLOU A, MOBARAKI A. Magnetic nanoparticle-supported Pd(Ⅱ)-cryptand 22 complex: an efficient and reusable heterogeneous precatalyst in the Suzuki-Miyaura coupling and the formation of aryl-sulfur bonds[J].J. Mol. Catal. A: Chem.,2015, 401: 55-65.
[21] FRINDY S, EL KADIB A, LAHCINI M,Copper nanoparticles stabilized in a porous chitosan aerogel as a heterogeneous catalyst for C—S crosscoupling[J]. ChemCatChem,2015, 7: 3307- 3315.
[22] FU W Q, LIU T T, FANG Z X,High activity and stability in the cross-coupling of aryl halides with disulfides over Cu-doped hierarchically porous zeolite ZSM-5[J]. Chem. Commun.,2015, 51: 5890-5893.
[23] ROSTAMI A, ROSTAMI A, GHADERI A. Copper-catalyzed thioetherification reactions of alkyl halides, triphenyltin chloride, and arylboronic acids with nitroarenes in the presence of sulfur sources[J]. J. Org. Chem., 2015, 80: 8694-8704.
[24] MITSUDOME T, TAKAHASHI Y, MIZUGAKI T,Hydrogenation of sulfoxides to sulfides under mild conditions using ruthenium nanoparticle catalysts[J]. Angew. Chem., Int. Ed.,2014, 53: 8348-8351.
[25] SEBASTIAN KRACKL S, COMPANY A, ENTHALER S. Low-valent molybdenum-based dual pre-catalysts for highly efficient catalytic epoxidation of alkenes and deoxygenation of sulfoxides[J]. ChemCatChem,2011, 3: 1186-1192.
[26] JANG Y, KIM K T, JEON H B. Deoxygenation of sulfoxides to sulfides with thionyl chloride and triphenylphosphine: competition with the pummerer reaction[J].J. Org. Chem.,2013, 78: 6328-6331.
[27] ENTHALER S. A straightforward zinc-catalysed reduction of sulfoxides to sulfides, Enthaler, Stephan[J]. Catal. Sci. Technol., 2011, 1: 104-110.
[28] ABBASI M, MOHAMMADIZADEH M R, MORADI Z. Efficient reduction of sulfoxides with NaHSO3catalyzed by I2[J]. Tetrahedron Lett.,2015, 56: 6610-6613.
[29] YOO B W, YU B R, YOON C M. A facile and efficient procedure for the deoxygenation of sulfoxides to sulfides with the HfCl4/Zn system[J].J. Sulfur Chem.,2015, 36: 358-363.
[30] AMIRI K, AMIN ROSTAMI A, SAMADI S,Cu-ZSM5 as reusable catalyst for the one-pot, odorless and ligand-free C—S bond formation[J]. Catal. Comm.,2016, 86: 108-112.
[31] CHEN J X. Rongalite[J]. Synlett, 2012, 23: 157-158.
[32] GUO W X, LV G S, CHEN J X,Rongalite?and base-promoted cleavage of disulfides and subsequent Michael addition to a,b-unsaturated ketones/esters: an odorless synthesis of α,β-sulfido carbonyl compounds[J]. Tetrahedron,2010, 66: 2297-2300.
[33] GUO W X,CHEN J X, WU H Y,.Rongalite?promoted highly regioselective synthesis of β-hydroxy sulfides by ring-opening of epoxides with disulfides[J]. Tetrahedron,2009, 65: 5240-5243.
Reaction of diaryl disulfides with nitroarenes
LI Shuyan1,2, SUN Lina1,2, SHEN Shujun1,2, CHENG Tianxing3, CHENG Shuanghua3, CHEN Jiuxi3
(1Department of Food and Biology Engineering, Zhangzhou Institute of Technology, Zhangzhou 363000, Fujian, China;2The Applied Technical Engineering Center of Further Processing and Safety of Agriculture Products, Higher Education Institution in Fujian Province, Zhangzhou 363000, Fujian, China;3College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, Zhejiang, China)
The cheap and readily available Rongalite?/K2CO3promoted the reaction of diaryl disulfides with nitroarenes in DMSO at 50 ℃, providing a convenient route to the synthesis of unsymmetrica diaryl sulfide. The structures of all products were characterized by1H NMR and13C NMR. This protocol had some distinct advantages of mild conditions, readily available starting materials and simple work-up.
diaryl sulfide; nitroarenes; sodium formaldehyde sulfoxylate; diaryl sulfide; reaction mechanism
10.11949/j.issn.0438-1157.20161755
O 6
A
0438—1157(2017)06—2394—05
程天行。
李術艷(1981—),女,碩士研究生,講師。
浙江省自然科學基金項目(LY16B020012);福建省教育廳科技計劃項目(JA13388)。
2016-12-15收到初稿,2017-03-15收到修改稿。
2016-12-15.
CHENG Tianxing, ctx@wzu.edu.cn
supported by the Natural Science Foundation of Zhejiang Province (LY16B020012) and the Educational Foundation of Fujian Province (JA13388).

