基于多肽和溫敏聚合物的光交聯(lián)囊泡的制備及表征
袁 康 周 雪 杜建忠
基于多肽和溫敏聚合物的光交聯(lián)囊泡的制備及表征
袁 康 周 雪 杜建忠*
(1同濟(jì)大學(xué)材料科學(xué)與工程學(xué)院,高分子材料系,上海201804;2同濟(jì)大學(xué)附屬上海市第十人民醫(yī)院,上海200072)
解決聚合物囊泡降解性和穩(wěn)定性的矛盾是一個(gè)重要問(wèn)題。本文通過(guò)可逆加成斷裂鏈轉(zhuǎn)移(RAFT)聚合和開(kāi)環(huán)聚合(ROP)合成了一種聚[(N-異丙基丙烯酰胺-無(wú)規(guī)-7-(2-甲基丙烯酰氧基乙氧基)-4-甲基香豆素)-嵌段-(L-谷氨酸)][P(NIPAM45-stat-CMA5)-b-PGA42]的兩親嵌段共聚物。囊泡膜由溫敏性的聚N-異丙基丙烯酰胺(PNIPAM)和可光交聯(lián)聚7-(2-甲基丙烯酰氧基乙氧基)-4-甲基香豆素(PCMA)組成。由囊泡膜向內(nèi)外舒展的聚谷氨酸(PGA)鏈?zhǔn)鼓遗莘€(wěn)定分散在水中,并且可進(jìn)一步官能化。透射電子顯微鏡(TEM)和動(dòng)態(tài)光散射(DLS)表征證實(shí)了囊泡的形貌和尺寸分布。本研究為制備基于多肽共聚物的可降解溫敏囊泡提供了一個(gè)范例,并有望在納米生物醫(yī)藥領(lǐng)域得到應(yīng)用。
囊泡;多肽;膠囊;可逆加成斷裂鏈轉(zhuǎn)移聚合;N-羧基酸酐(NCA)
1 Introduction
Thermo-responsive polymeric nanoparticles have attracted much attention over the past decades due to their potential applications in drug delivery and tissue engineering,etc1-8.Vesi-cles2,9,10,micelles11-13,and polymer conjugates14-17are typical drug delivery carriers.PNIPAM(poly(N-isopropyl acrylamide))is a typical polymer for preparing thermo-sensitive nanoparticles, which undergoes a sharp transition in water at lower critical solution temperature(LCST)5,18-20.Polypeptides have been introduced into different copolymers to prepare functional nanoparticles due to their biodegradability and biocompatibility21-26.Recently,the controlled polymerization of N-carboxyanhydride (NCA)by ring-opening polymerization afforded a range of functional polypeptides on a large scale and at a low cost17,27.Also, polymer vesicles have attracted much attention due to their promising potential applications in a wide range of fields28,29. Therefore,PNIPAM and polypeptide-based copolymer vesicles may be useful nanocarriers with better properties.More importantly,it is an important challenge to balance the degradability and stability of polymer vesicles.
Herein,we synthesized a new thermo-responsive,polypeptidecontaining and photo-cross-linkable copolymer by RAFT and ROP (Scheme 1).The block copolymer is P(NIPAM45-stat-CMA5)-b-PGA42,which can be self-assembled into vesicles.The hydrophilic PGA block forms the coronas,while the thermo-responsive PNIPAM and photo-cross-linkable PCMA segments are incorporated in the vesicle membrane.
2 Materials and methods
2.1 Materials
Stannous 2-ethylhexanoate(Sn(Oct)2)(96%),N-(tert-butoxycarbonyl)ethylenediamine,trifluoroacetic acid(TFA)(>99.5%), α-pinene(99%),hydrogen bromide(33%(w,mass fraction)in acetic acid),and triphosgene(99%)were purchased fromAladdin. γ-Benzyl-L-glutamate(98%)was obtained from Shanghai Hanhong Chemical Co.,Ltd.Tetrahydrofuran(THF)(≥99.0%), dichloromethane(DCM)(≥99.5%),toluene(≥99.0%),ethyl acetate(≥99.5%),N,N′-dimethylformamide(DMF)(≥99.5%) and other reagents were purchased from Sinopharm Chemical Reagent Co.,Ltd.(SCRC,Shanghai,China)and used without further purification.
2.2 Methods
2.2.1 Synthesis of P(NIPAM45-stat-CMA5)by RAFT
N-isopropylacrylamide(NIPAM;1.15 g,0.0102 mol),7-(2-methacryloyloxyethoxy)-4-methylcoumarin(CMA;0.250 g,0.848 mmol)and 4-cyanovaleric acid dithiobenzoic acid(CPAD;0.0450 g,0.170 mmol)were placed in a round bottom flask,and 1.0 mL of anhydrous N,N-dimethylformamide(DMF)was added.Argon was used to remove oxygen from the solution,then 5.0 mg of azobisisobutyronitrile(AIBN)was added and the flask was placed in a 70°C oil bath for 10 h.After completion of the reaction,the mixture was precipitated in diethyl ether three times to yield the polymer.Yield:~75%.
2.2.2 Synthesis of Bz-Glu NCAmonomer
Bz-Glu NCA monomer was synthesized according to literature27.γ-Benzyl-L-glutamate(5.00 g,21.0 mmol)and α-pinene (14.6 g,105 mmol)were dissolved in 80 mL of anhydrous THF in a three-necked round-bottomed flask.Triphosgene(4.78 g,15.8 mmol)was dissolved in 15.0 mL of anhydrous THF in a constant pressure funnel.Then,triphosgene solution was added dropwise over a period of 1 h at 55°C with the protection of argon.The mixture gradually turned clear in 4 h.The mixture was precipitated by dropping into 500.0 mL of hexane with fast stirring.The crude product was dissolved in dry THF and recrystallized twice by dropping into hexane.The obtained solid was dried in vacuum. Yield:~82%.
2.2.3 Synthesis of P(NIPAM45-stat-CMA5)-NH-Boc
The mixture of P(NIPAM45-stat-CMA5)(0.680 g,0.150 mmol) and dicyclohexylcarbodiimide(DCC,0.0230 g,0.110 mmol)were dissolved in 1.0 mLof DMF in a round bottom flask.After 10 min, 4-dimethylaminopyridine(DMAP,0.0150 g,0.120 mmol)and NBoc-ethylenediamine(0.0320 g,0.200 mmol)were added.The resulting reaction mixture was stirred for 6 h.Then the solution was cooled to room temperature and the resulting solution was dialyzed against deionized water for 2 days and then lyophilized. Yield:~60%.
2.2.4 Synthesis of P(NIPAM45-stat-CMA5)-NH2
P(NIPAM45-stat-CMA5)-NH-Boc(0.500 g)was dissolved in 3.0 mL of anhydrous DCM under argon,and then 3.0 mL of TFAwas added into the solution.The solution was stirred at room temperature for 4 h.Then the solvent was removed under vacuum. The polymer was redissolved in DMF and dialyzed against deionized water for 2 days to remove traces of residual TFA.The white powder was obtained after freeze-drying.Yield:~85%.

Scheme 1 Schematic illustration of self-assembly of P(NIPAM45-stat-CMA5)-b-PGA42copolymer into vesicles and their thermo-responsive behaviour
2.2.5 Synthesis of P(NIPAM45-stat-CMA5)-b-PBLG42
This copolymer was prepared using a typical ROP reaction.A round-bottom flask was charged with P(NIPAM45-stat-CMA5)-NH2(0.330 g,0.0500 mmol)macro-initiator,Bz-Glu NCA(0.400 g) monomer and anhydrous dimethylformamide.The reaction mixture was stirred under vacuum at room temperature for 24 h. The solvent was removed by rotary evaporator.The product was washed with water to remove the spare DMF,filtered and then dried in a vacuum oven.Yield:~72%.
2.2.6 Synthesis of P(NIPAM45-stat-CMA5)-b-PGA42
This diblock copolymer was prepared by hydrolysis of the P(NIPAM45-stat-CMA5)-b-PBLG42diblock copolymer.The diblock polymer P(NIPAM45-stat-CMA5)-b-PBLG42(0.500 g)was dissolved in 33%HBr/CH3COOH solution.After stirring for 4 h,the byproducts and impurities were removed by precipitation in diethyl ether four times.For further purification,the precipitation was dissolved in DMF,transferred into a dialysis tube,and dialyzed against deionized water for 2 days to remove traces of residual impurities.A white powder was obtained after lyophilization.Yield:~68%.
2.2.7 Self-assembling P(NIPAM45-stat-CMA5)-b-PGA42into vesicles
Polymer vesicle was prepared by a solvent switching method. P(NIPAM45-stat-CMA5)-b-PGA42(5.00 mg)diblock polymer was dissolved in 2.5 mL of DMF and 5.0 mL of deionized water was added dropwise to induce the formation of polymer vesicles. Subsequently,DMF was removed by dialysis against deionized water in a dialysis tubing.
2.2.8 Photo-cross-linking of vesicles
The polymer vesicles(400μg·mL-1)were placed under a UV spot curing system(8000 mW·cm-2)at a wavelength of 365 nm to immobilize the vesicle membrane.First,the vesicles solution was exposed to UV light for 10 s,and then measured every 20 s. After 430 s,the vesicles were fully cross-linked.
2.2.9 Nuclear magnetic resonance(NMR)
1H NMR(400 MHz)spectra were recorded by a Varian spectrometer at 25°C using CDCl3or DMSO-d6as a solvent.
2.2.10 Dynamic light scattering(DLS)
The dynamic light scattering measurements of polymer vesicles were carried out by a Nano-ZS 90 Nanosizer(Malvern Instruments Ltd.,Worcestershire,UK)at a fixed scattering angle of 90°. Each measurement was conducted for three runs.
2.2.11 UV-Vis spectroscopy
UV-Vis studies were conducted by using a UV-Vis spectrophotometer(UV-759S,Q/YXL270,Shanghai Precision&Scientific Instrument Co.,Ltd.)with a scan rate of 300 nm·min-1.
2.2.12 Transmission electron microscope(TEM)
TEM images were obtained using a JEOL JEM-2100F electron microscope operating at an acceleration voltage of 200 kV equipped with a Gatan 894 Ultrascan 1k CCD camera.To prepare TEM samples,10 μL of vesicle solution at a concentration of 200 μg·mL-1was dropped onto a carbon-coated copper grid and dried at ambient temperature.The sample was then stained with 1% neutral aqueous phosphotungstic acid solution for 90 s.
3 Results and discussion
3.1 Synthesis of P(NIPAM45-stat-CMA5)-b-PGA42copolymer
This thermo-responsive copolymer was synthesized in five steps(Fig.1):(a)P(NIPAM45-stat-CMA5)was synthesized by one-pot RAFT polymerization.(b)P(NIPAM45-stat-CMA5)with a―COOH end group reacted with N-Boc-ethylenediamine at room temperature to form P(NIPAM45-stat-CMA5)-NH-Boc.(c) P(NIPAM45-stat-CMA5)-NH2was obtained by deprotection of P(NIPAM45-stat-CMA5)-NH-Boc with TFA.(d)P(NIPAM45-stat-CMA5)-b-PBLG42was synthesized by ROP of Bz-Glu-NCAmonomer using P(NIPAM45-stat-CMA5)-NH2as the macroinitiator. (e)The benzyl ester protecting group in the PBLG side chains was removed in the presence of 33%of HBr/CH3COOH to give P(NIPAM45-stat-CMA5)-b-PGA42copolymer.

Fig.1 Synthetic route of P(NIPAM45-stat-CMA5)-b-PGA42copolymer by RAFT polymerization and ROP
The chemical structures of related monomer,macro initiator and intermediate product were confirmed by1H NMR(Figs.S1-S6 in the Supporting Information).Because the polymer contains PGA segments,it will form hydrogen bonds,which cannot be well dissolved in DMF solvent.Therefore,the copolymer is not proper for characterization by GPC.
3.2 Self-assembly of copolymer into vesicles
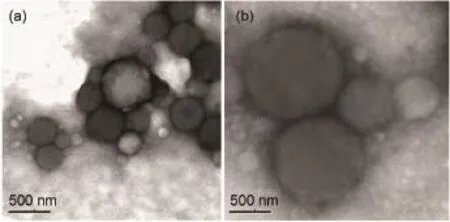
Fig.2 TEM images of polymer vesicles

Fig.3 (a)Cross-linking degrees of polymer vesicles exposed to UV light at different time;(b)DLS study of P(NIPAM45-stat-CMA5)-b-PGA42vesicles at a concentration of 0.4 mg·mL-1upon different temperatures color online
The polymer vesicles were prepared via solvent-switch method28by dissolving P(NIPAM45-stat-CMA5)-b-PGA42copolymer in DMSO,followed by adding water into the solution(DMSO/H2O= 1/2;V/V).DMSO was removed by dialysis against deionized water at 40°C.The hydrophilic peptide(glutamic acid)segments form the coronas of the vesicles,while the thermo-responsive PNIPAM and cross-linkable PCMA segments form the membrane.The morphology and the size of the vesicles were characterized by TEM(Fig.2)and DLS(Fig.3).TEM analysis was conducted to reveal the morphology of the vesicles,as shown in Fig.2 and Fig. S7(Supporting Information).The corresponding hydrodynamic diameter(Dh)of vesicles is 572 nm with a PDI of 0.271(Fig.3(b)). The size of the vesicles by TEM is around 200-300 nm,which is reasonably smaller than that determined by DLS.The zeta potential(ξ)was-34.1 mV because of the carboxyl groups in the PGAcoronas.
3.3 Photo-cross-linking and thermo-responsive behavior of vesicles
PNIPAM segment is hydrophobic at 32°C or above,and hydrophilic below 32°C.Thus the polymer can self-assemble into vesicles at 40°C.The vesicles were diluted to 0.4 mg·mL-1and irradiated with UV light to cross-link the membrane(Fig.3(a)). After cross-linking of CMA,the vesicle structure is fixed.When the temperature is decreased to 20°C,the structure of the crosslinked vesicles doesn′t change but the permeability of the membrane will increase30.So the cross-linked polymer vesicle can be used as drug carriers to load drugs at lower temperature and to deliver them at higher temperature.
The thermo-responsive behavior of the cross-linked polymer vesicles was studied by DLS.The sizes of vesicles before and after cross-linking are 572 and 576 nm at 40°C,respectively.At 20°C, the size of cross-linked vesicles is increased to 627 nm because the PNIPAM chains become hydrophilic.This process is reversible for cross-linked vesicles but not reversible for un-cross-linked vesicles.
4 Conclusions
In summary,we present a new type of thermo-responsive polypeptide-based polymer vesicle.TEM and DLS studies confirmed the formation of polymer vesicles.The membrane of the vesicles consists of thermo-responsive PNIPAM and photo-crosslinkable PCMA.The vesicles can be photo-cross-linked to afford stable vesicles at various temperatures.DLS studies reveal the excellent stability of the vesicle after photo-cross-linking.This multifunctional vesicle may be applied in a range of fields such as delivery of guest molecules in the future.
Supporting Information:1H NMR spectra and TEM images (Fig.S1-Fig.S7).This information is available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1) Du,J.Z.;O′Reilly,R.K.Soft Matter 2009,5,3544. doi:10.1039/b905635a
(2)Fan,L.;Lu,H.;Zou,K.D.;Chen,J.;Du,J.Z.Chem.Commun. 2013,49,11521.doi:10.1039/c3cc45873c
(3)Li,Y.;Lokitz,B.S.;McCormick,C.L.Angew.Chem.Int.Ed. 2006,45,5792.doi:10.1002/anie.200602168
(4) Schmaljohann,D.Adv.Drug Delivery Rev.2006,58,1655. doi:10.1016/j.addr.2006.09.020
(5) Yang,X.L.;Luo,Y.L.;Xu,F.;Chen,Y.S.Pharm.Res.2014, 31,291.doi:10.1007/s11095-013-1160-y
(6) Yu,L.;Fu,W.X.;Li,Z.B.Soft Matter 2015,11,545. doi:10.1039/c4sm02270j
(7) Bajpai,A.K.;Shukla,S.K.;Bhanu,S.;Kankane,S.Prog. Polym.Sci.2008,33,1088.doi:10.1016/j. progpolymsci.2008.07.005
(8) Leal,M.P.;Torti,A.;Riedinger,A.;La Fleur,R.;Petti,D.; Cingolani,R.;Bertacco,R.;Pellegrino,T.ACS Nano 2012,6, 10535.doi:10.1021/nn3028425
(9) Liu,Q.M.;Zhu,H.S.;Qin,J.Y.;Dong,H.Q.;Du,J.Z. Biomacromolecules 2014,15,1586.doi:10.1021/bm500438x
(10) Hao,W.J.;Zhang,J.Q.;Shang,Y.Z.;Xu,S.H.;Liu,H.L.Acta Phys.-Chim.Sin.2016,32,2628.[郝偉舉,張俊琪,尚亞卓,徐首紅,劉洪來(lái).物理化學(xué)學(xué)報(bào),2016,32,2628.]doi:10.3866/ PKU.WHXB201606296
(11) Park,Y.J.;Lee,J.Y.;Chang,Y.S.;Jeong,J.M.;Chung,J.K.; Lee,M.C.;Park,K.B.;Lee,S.J.Biomaterials 2002,23,873. doi:10.1016/s0142-9612(01)00196-x
(12)Gao,C.;Wang,Y.;Zhu,W.P.;Shen,Z.Q.Chin.J.Polym.Sci. 2014,32,1431.doi:10.1007/s10118-014-1528-4
(13) Fang,Y.;Lai,Z.Y.;Pang,P.P.;Jiang,M.Acta Phys.-Chim.Sin. 2011,27,1712.[方 云,賴(lài)中宇,龐萍萍,江 明.物理化學(xué)學(xué)報(bào),2011,27,1712.]doi:10.3866/PKU.WHXB20110703
(14)Menjoge,A.R.;Kannan,R.M.;Tomalia,D.A.Drug Discovery Today 2010,15,171.doi:10.1016/j.drudis.2010.01.009
(15)Yang,Z.C.;Li,Y.C.;Li,F.;Huang,Q.R.;Zhang,G.;Shi,T.F. Chin.J.Polym.Sci.2016,34,280.doi:10.1007/s10118-016-1757-9
(16) Wu,L.;Pang,T.;Guan,Y.B.Chin.J.Polym.Sci.2016,34,523. doi:10.1007/s10118-016-1784-6
(17) Fu,X.H.;Ma,Y.N.;Sun,J.;Li,Z.B.Chin.J.Polym.Sci. 2016,34,1436.doi:10.1007/s10118-016-1861-x
(18) Zheng,X.M.;Jiang,T.;He,F.Acta Polym.Sin.2011,(8),895. doi:10.3724/sp.j.1105.2011.11120
(19) Chung,J.E.;Yokoyama,M.;Aoyagi,T.;Sakurai,Y.;Okano,T. J.Controlled Release 1998,53,119.doi:10.1016/s0168-3659 (97)00244-7
(20)Wang,F.Y.K.;Du,J.Z.Chem.Commun.2015,51,11198. doi:10.1039/c5cc02641e
(21) Wang,M.Z.;Zhou,C.C.;Chen,J.;Xiao,Y.;Du,J.Z. Bioconjugate Chem.2015,26,725.doi:10.1021/acs. bioconjchem.5b00061
(22)Zhou,C.C.;Wang,M.Z.;Zou,K.D.;Chen,J.;Zhu,Y.Q.;Du, J.Z.ACS Macro Lett.2013,2,1021.doi:10.1021/mz400480z
(23) Wang,M.Z.;Du,J.Z.Acta Polym.Sin.2014,No.9,1183. doi:10.11777/j.issn1000-3304.2014.14125
(24)Chen,J.;Wang,F.Y.K.;Liu,Q.M.;Du,J.Z.Chem.Commun. 2014,50,14482.doi:10.1039/c4cc03001j
(25)Wang,M.Z.;Wang,T.;Yuan,K.;Du,J.Z.Chin.J.Polym.Sci. 2016,34,44.doi:10.1007/s10118-016-1725-4
(26) Liu,Q.M.;Song,L.W.;Chen,S.;Gao,J.Y.;Zhao,P.Y.;Du,J. Z.Biomaterials 2017,114,23.doi:10.1016/j. biomaterials.2016.10.027
(27) Liu,Q.M.;Chen,S.;Chen,J.;Du,J.Z.Macromolecules 2015, 48,739.doi:10.1021/ma502255s
(28) Zhu,Y.Q.;Yang,B.;Chen,S.;Du,J.Z.Prog.Polym.Sci.2017, 64,1.doi:10.1016/j.progpolymsci.2015.05.001
(29) Liu,X.X.;Jiang,M.Acta Polym.Sin.2011,No.9,1007. doi:10.3724/sp.j.1105.2011.11169
(30)Wang,X.R.;Liu,G.H.;Hu,J.M.;Zhang,G.Y.;Liu,S.Y. Angew.Chem.Int.Ed.2014,53,3138.doi:10.1002/ anie.201310589
Synthesis and Characterization of Thermo-Responsive Polypeptide-Based Vesicles with Photo-Cross-Linked Membranes
YUAN Kang ZHOU Xue DU Jian-Zhong*
(1Department of Polymeric Materials,School of Materials Science and Engineering,Tongji University,Shanghai 201804,P.R.China;2Shanghai Tenth People′s Hospital,Tongji University School of Medicine,Shanghai 200072,P.R.China)
It is an important challenge to balance the degradability and stability of polymer vesicles.We report a thermo-responsive vesicle based on poly[(N-isopropyl acrylamide-stat-7-(2-methacryloyloxyethoxy)-4-methylcoumarin)-b-(L-glutamic acid)][P(NIPAM45-stat-CMA5)-b-PGA42]diblock copolymer,which was synthesized by reversible addition fragmentation chain transfer(RAFT)polymerization and ring-opening polymerization (ROP).The membrane of the vesicle consists of thermo-responsive PNIPAM and photo-cross-linkable PCMA. The PGA chains in the vesicle coronas can colloidally stabilize the vesicles in water and can be postfunctionalized for further applications.Transmission electron microscopy and dynamic light scattering studies confirmed the formation of vesicles.Overall,we prepared a new functional thermo-responsive vesicle based on polypeptide copolymers that may be used as nanocarriers for the facile loading of a range of molecules in future.
Vesicle;Peptide;Polymersome;Reversible addition fragmentation chain transfer; N-carboxyanhydride(NCA)
O648
10.3866/PKU.WHXB201701162
Received:December 12,2016;Revised:January 16,2017;Published online:January 16,2017.
*Corresponding author.Email:jzdu@tongji.edu.cn;Tel:+86-21-69580239.
The project was supported by the National Natural Science Foundation of China(21374080,21674081,21611130175),Shanghai International Scientific Collaboration Fund,China(15230724500),Shanghai 1000 Talents Plan,and Fundamental Research Funds for the Central Universities, China(0500219211,1500219107).
國(guó)家自然科學(xué)基金(21374080,21674081,21611130175),上海市科委國(guó)際合作項(xiàng)目(15230724500),上海千人計(jì)劃,中央高校基本科研業(yè)務(wù)費(fèi)(0500219211,1500219107)資助項(xiàng)目

