在離子液體介質(zhì)中催化合成β羰基膦酸酯
向建南 朱永鋼 楊世平

摘 要:報(bào)道了一種由炔基膦酸酯制備β羰基膦酸酯的簡(jiǎn)便、高效新合成方法. 該方法以離子液體為反應(yīng)介質(zhì),質(zhì)子酸為催化劑催化炔基膦酸酯水合制備β羰基膦酸酯.優(yōu)化實(shí)驗(yàn)條件為:反應(yīng)溫度為60 ℃,離子液體用量3.5 mmol,濃硫酸用量3.5 mmol, 0.5 mmol炔基膦酸酯與1 mmol水反應(yīng)20 h,β羰基膦酸酯的產(chǎn)率達(dá)到98%.該合成方法具有無(wú)金屬催化劑、β羰基膦酸酯產(chǎn)率高以及多種基團(tuán)適用性等特點(diǎn).本文合成的化合物2p未見(jiàn)文獻(xiàn)報(bào)道.
關(guān)鍵詞:合成;水合反應(yīng);β羰基膦酸酯;離子液體
中圖分類(lèi)號(hào):O622.4 文獻(xiàn)標(biāo)識(shí)碼:A
Abstract:A simple and efficient synthesis of βketophosphonates was described. The hydrolysis reactions of alkynylphosphonates in the presence of sulphuric acid (3.5 mmol) as a catalyst in the recyclable ionic liquid N·(HSO4)2 afforded the desired βketophosphonates in excellent yields(98%). This method has the advantages of metalfree and tolerance of multifunctional groups. Synthesized compound 2p has not been reported.
Key words:synthesis; hydration reaction; βketophosphonates; ionic liquid
有機(jī)磷化合物在有機(jī)合成、醫(yī)藥研究方面一直有著重要的應(yīng)用,β羰基膦酸酯是一類(lèi)十分重要的有機(jī)磷化合物,具有廣泛的生物活性[1-2],可以作為其他很多有機(jī)合成反應(yīng)的原料[3-4].β羰基膦酸酯是通過(guò)霍納爾沃茲沃思埃蒙斯反應(yīng)[5]制備α,β不飽和羰基化合物的一種不可缺少的底物之一. 同時(shí)β羰基膦酸酯也是制備手性β氨基[6]和手性β羥基[7]膦酸化合物的前體.
由于β羰基膦酸酯在化學(xué)領(lǐng)域研究中的重要作用,多個(gè)實(shí)驗(yàn)室報(bào)道了其制備β羰基膦酸酯的方法 [8-12]. 但這些制備β羰基膦酸酯的方法存在著一些缺點(diǎn):嚴(yán)苛的反應(yīng)條件,復(fù)雜的實(shí)驗(yàn)操作步驟,需要使用昂貴的且對(duì)環(huán)境有害的金屬催化劑. 其中使用過(guò)渡金屬催化炔基磷酸酯水合成β羰基膦酸酯的方法操作相對(duì)簡(jiǎn)單[13-14],且水合反應(yīng)的原子利用率高[15]. 隨著不斷的改進(jìn),此類(lèi)水合反應(yīng)所用金屬催化劑從最初的汞鹽[8]到此后的鈀鹽[9]、金化合物[10]及銀鹽[11],但此類(lèi)方法所使用的金屬催化劑將不可避免地對(duì)環(huán)境造成一定污染.
基于此前實(shí)驗(yàn)室對(duì)合成β羰基膦酸酯所做的工作[10-12],我們發(fā)現(xiàn)了一種綠色無(wú)污染的方法來(lái)制備β羰基膦酸酯. 離子液體[16]是一種不易損耗且可循環(huán)使用的低熔點(diǎn)鹽,很多有機(jī)反應(yīng)在離子液體介質(zhì)中都能很好地進(jìn)行[17]. Wong實(shí)驗(yàn)組報(bào)道了一種新型的離子液體催化介質(zhì),此種介質(zhì)包含有少量的硫酸,能夠在溫和的條件下催化炔烴水合轉(zhuǎn)化成酮類(lèi)化合物[18].本文探討了一種在溫和無(wú)金屬催化劑參與的條件下高效地制備β羰基膦酸酯的方法.
1 實(shí)驗(yàn)部分
1.1 試劑與儀器
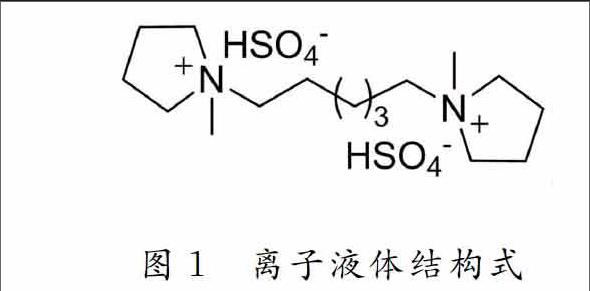
乙酸乙酯和正己烷及其他部分藥品和試劑均購(gòu)自九鼎化學(xué)試劑公司,藥品和試劑均為分析純,離子液體(N ·(HSO4)2) (結(jié)構(gòu)式見(jiàn)圖1)參照Wong實(shí)驗(yàn)組的方法[18]制備,薄層和柱層析用硅膠均為青島海洋化工廠(chǎng)產(chǎn)品;1H NMR,31P NMR和13C NMR(內(nèi)標(biāo)為T(mén)MS, 溶劑為CDCl3)用Brucker ARX 400 FT型核磁共振儀測(cè)定;IR用FD5DX型紅外儀測(cè)定;MS用GCTTOF型高分辨質(zhì)譜儀測(cè)定.
1.2 目標(biāo)產(chǎn)物的合成
在圓底燒瓶中依次加入離子液體N·(HSO4)2 (3.5 mmol, 1 565 mg),炔基膦酸酯(0.5 mmol)和水(1.0 mmol, 18 mg). 攪拌5 min后,向混合液中緩慢滴加濃硫酸(3.5 mmol, 98% H2SO4, 350 mg),然后將反應(yīng)體系溫度逐步提升到60 ℃,持續(xù)攪拌20 h,用薄層色譜法監(jiān)測(cè)反應(yīng)進(jìn)程. 反應(yīng)結(jié)束后,向反應(yīng)混合物中加入6 mL水,并用二氯甲烷萃取所得的混合物,水層保留用作循環(huán)使用. 有機(jī)層用15 mL水洗滌一次,15 mL飽和食鹽水洗滌2次,分離出的水相用15 mL二氯甲烷萃取3次,合并有機(jī)相用無(wú)水硫酸鈉干燥,過(guò)濾,減壓蒸餾去除溶劑,得到殘留黏稠液體,經(jīng)過(guò)柱層析法分離提純得到目標(biāo)產(chǎn)物2a-2p.
為證明離子液體的良好活性和可回收性,將含有濃硫酸的離子液體體系循環(huán)5次作為反應(yīng)催化體系,每次循環(huán)過(guò)程中,將反應(yīng)產(chǎn)物萃取出,分離提純,計(jì)算反應(yīng)產(chǎn)率. 剩余部分加入新的反應(yīng)底物炔基膦酸酯和水,繼續(xù)循環(huán). 循環(huán)反應(yīng)的產(chǎn)物平均產(chǎn)率達(dá)到94%,這個(gè)結(jié)果證明了離子液體的體系能夠循環(huán)使用.
(2氧代2苯乙基)膦酸二乙酯(2a)[10]:黃色油狀物,產(chǎn)率96% (123 mg),1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 7.2 Hz, 2H), 7.57~7.53 (m, 1H), 7.46~ 7.42 (m, 2H), 4.14~4.06 (m, 4H), 3.60 (d, J = 22.8 Hz, 2H), 1.24 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 191.8 (d, J = 6.5 Hz), 136.3, 133.5, 128.9, 128.4, 62.5 (d, J = 6.6 Hz), 38.3 (d, J = 129.1 Hz), 16.1 (d, J = 6.6 Hz); 31P NMR (162 MHz, CDCl3) δ 20.0.
[2氧代2(4甲基苯乙基)]膦酸二乙酯(2b) [11]:黃色油狀物,產(chǎn)率97% (131 mg),1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 8.4 Hz, 2H), 7.24 (d, J = 8.0 Hz, 2H), 4.12~4.05 (m, 4H), 3.56 (d, J = 22.8 Hz, 2H), 2.36 (s, 3H) , 1.23 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 191.3 (d, J =6.5 Hz), 144.5, 134.0, 129.1, 129.0, 62.5 (d, J = 6.6 Hz), 38.1 (d, J = 129.0 Hz), 21.5, 16.0 (d, J = 6.6 Hz); 31P NMR (162 MHz, CDCl3) δ 20.3.
[2氧代2(4甲氧基苯乙基)]膦酸二乙酯(2c) [11]:黃色油狀物,產(chǎn)率98% (140 mg),1H NMR (400 MHz, CDCl3) δ 7.95 (d,J = 8.4 Hz, 2H), 6.90 (d,J = 8.4 Hz, 2H), 4.13~4.06 (m, 4H), 3.83 (s, 3H), 3.55 (d, J = 22.8 Hz, 2H), 1.24 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 190.1 (d, J = 6.5 Hz), 163.9, 131.3, 129.4, 113.6, 62.5 (d, J = 5.8 Hz), 55.4, 38.0 (d, J = 129.0 Hz), 16.1 (d, J = 5.9 Hz); 31P NMR (162 MHz, CDCl3 ) δ 20.6.
[2氧代2(4乙酰氧基苯乙基)]膦酸二乙酯(2d) [12]:黃色油狀物,產(chǎn)率90% (140 mg),1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 8.8 Hz, 2H), 7.20 (d, J = 8.8 Hz, 2H), 4.15~4.08 (m, 4H), 3.60 (d, J = 22.8 Hz, 2H), 2.30 (s, 3H), 1.26 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 190.6 (d, J = 6.6 Hz), 168.6, 154.7, 133.9, 130.6, 121.7, 62.6 (d, J = 6.6 Hz), 38.4 (d, J = 129.1 Hz), 21.0, 16.1 (d,J = 6.6 Hz); 31P NMR (162 MHz, CDCl3 ) δ 19.9.
[2氧代2(4氟苯乙基)]膦酸二乙酯(2e) [12]:黃色油狀物,產(chǎn)率87% (118 mg),1H NMR (400 MHz, CDCl3) δ 8.05~8.02 (m, 2H), 7.15~7.10 (m, 2H), 4.15~4.08 (m, 4H), 3.58 (d, J = 22.8 Hz, 2H), 1.26 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 190.2 (d, J = 6.5 Hz), 166.0 (d, J = 254.4 Hz), 132.8, 131.4 (d, J = 9.5 Hz), 115.6 (d, J = 21.9 Hz), 62.6 (d,J = 6.5 Hz), 38.5 (d, J = 129.1 Hz), 16.1 (d, J = 6.5 Hz); 31P NMR (162 MHz, CDCl3 ) δ 19.7.
[2氧代2(4乙酰基苯乙基)]膦酸二乙酯(2f) [12]:黃色油狀物,產(chǎn)率86% (128 mg),1H NMR (400 MHz, CDCl3) δ 8.08 (d, J = 8.0 Hz, 2H), 8.02 (d, J = 8.0 Hz, 2H), 4.16~4.09 (m, 4H), 3.64 (d, J = 22.8 Hz, 2H), 2.63 (s, 3H), 1.27 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 197.3, 191.4 (d, J = 6.6 Hz), 140.4, 139.5, 129.2, 128.3, 62.7 (d, J = 6.5 Hz), 38.8 (d, J = 128.4 Hz), 26.8, 16.1 (d, J = 5.8 Hz); 31P NMR (162 MHz, CDCl3 ) δ 19.2.
[2氧代2(4三氟甲基苯乙基)]膦酸二乙酯(2g) [11]:黃色油狀物,產(chǎn)率81% (131 mg),1H NMR (400 MHz, CDCl3) δ 8.12 (d, J = 8.4 Hz, 2H), 7.74 (d, J = 8.4 Hz, 2H), 4.17~4.10 (m, 4H), 3.64 (d, J = 23.2 Hz, 2H), 1.28 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 191.0 (d, J = 6.5 Hz), 139.0, 134.7 (dd, J = 32.8 Hz, 32.6 Hz), 129.3, 125.5 (q, J =3.7 Hz), 122.0, 62.7 (d, J = 6.5 Hz), 38.7 (d, J = 128.3 Hz), 16.0 (d, J = 6.5 Hz); 31P NMR (162 MHz, CDCl3 ) δ 19.0.
[2氧代2(3甲基苯乙基)]膦酸二乙酯(2h) [10]:黃色油狀物,產(chǎn)率90% (120 mg),1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 7.6 Hz, 2H), 7.36~7.29 (m, 2H), 4.12~4.05 (m, 4H), 3.57 (d, J = 22.8 Hz, 2H), 2.36 (s, 3H), 1.23 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 191.9 (d, J = 6.6 Hz), 138.2, 136.4, 134.3, 129.2, 128.3, 126.1, 62.4 (d, J = 6.6 Hz), 38.2 (d, J = 129.1 Hz), 21.1, 16.0 (d, J = 5.8 Hz); 31P NMR (162 MHz, CDCl3 ) δ 20.1.
[2氧代2(2甲基苯乙基)]膦酸二乙酯(2i) [11]:黃色油狀物,產(chǎn)率75% (100 mg),1H NMR (400 MHz, CDCl3) δ 7.67 (d, J = 7.6 Hz, 1H), 7.34~7.30 (m, 1H), 7.23~7.17 (m, 2H), 4.08~4.00 (m, 4H), 3.52 (d, J = 22.8 Hz, 2H), 2.44 (s, 3H), 1.19 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 195.0 (d, J = 6.6 Hz), 138.8, 137.1 (d, J = 2.2 Hz), 131.9, 131.8, 129.5, 125.6, 62.4 (d, J = 6.6 Hz), 40.9 (d, J = 128.3 Hz), 21.2, 16.1 (d, J =5.8 Hz); 31P NMR (162 MHz, CDCl3 ) δ 20.2.
(2氧代2萘乙基)膦酸二乙酯(2j) [12]:黃色油狀物,產(chǎn)率83% (126 mg),1H NMR (400 MHz, CDCl3) δ 8.53 (s, 1H), 8.03 (d, J = 8.8 Hz, 1H), 7.96 (d,J = 8.0 Hz, 1H), 7.88~7.83 (m, 2H), 7.60~7.51 (m, 2H), 4.16~4.09 (m, 4H), 3.74 (d, J = 22.8 Hz, 2H), 1.25 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 191.7 (d, J = 6.6 Hz), 135.6, 133.7, 132.2, 131.3, 129.6, 128.7, 128.3, 127.6, 126.8, 124.0, 62.6 (d, J = 6.6 Hz), 38.4 (d, J = 129.1 Hz), 16.1 (d, J = 6.5 Hz); 31P NMR (162 MHz, CDCl3 ) δ 20.2.
[2氧代2(2乙基噻吩基)]膦酸二乙酯(2k) [12]:黃色油狀物,產(chǎn)率84% (109 mg),1H NMR (400 MHz, CDCl3) δ 7.79 (d, J = 3.6 Hz, 1H), 7.66 (d, J = 4.8 Hz, 1H), 7.12~7.10 (m, 1H), 4.14~4.07 (m, 4H), 3.51 (d, J = 22.8 Hz, 2H), 1.25 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 184.1 (d, J = 6.6 Hz), 143.7, 135.0, 134.1, 128.2, 62.6 (d, J = 6.6 Hz), 39.2 (d, J = 129.1 Hz), 16.1 (d, J = 6.5 Hz); 31P NMR (162 MHz, CDCl3 ) δ 19.4.
[2氧代2(3乙基噻吩基)]膦酸二乙酯(2l) [10]:黃色油狀物,產(chǎn)率87% (113 mg),1H NMR (400 MHz, CDCl3) δ 8.16~8.15 (m, 1H), 7.50 (d, J = 5.2 Hz, 1H), 7.25~7.24 (m, 1H), 4.10~4.03 (m, 4H), 3.46 (d, J = 22.8 Hz, 2H), 1.22 (t, J = 7.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 185.5 (d, J = 6.6 Hz), 141.7, 134.2, 127.1, 126.3, 62.5 (d, J = 6.5 Hz), 39.8 (d,J = 128.4 Hz), 16.0 (d,J = 6.6 Hz); 31P NMR (162 MHz, CDCl3 ) δ 19.9.
2氧代癸基膦酸二乙酯(2m) [10]:無(wú)色油狀物,產(chǎn)率30% (43 mg),1H NMR (400 MHz, CDCl3) δ 4.17~4.09 (m, 4 H), 3.05 (d, J = 22.8 Hz, 2 H), 2.60 (t,J = 7.2 Hz, 2 H), 1.56 (t, J = 7.2 Hz, 2 H), 1.34~1.25 (m, 16 H), 0.86 (t,J = 7.2 Hz, 3 H); 13C NMR (100 MHz, CDCl3) δ 202.2 (d, J = 5.8 Hz), 62.5 (d, J = 5.9 Hz), 44.0, 42.2 (d, J = 126.9 Hz), 31.7, 29.2, 29.0, 28.9, 23.4, 22.5, 16.2 (d, J = 5.8 Hz), 14.0; 31P NMR (162 MHz, CDCl3 ) δ 20.1.
(2氧代2新戊酰氧基丙基)膦酸二乙酯(2n) [11]:無(wú)色油狀物,產(chǎn)率35% (52 mg),1H NMR (400 MHz, CDCl3) δ 4.77 (s, 2 H), 4.18~4.10 (m, 4 H), 3.09 (d, J = 22.8 Hz, 2 H), 1.32 (t, J = 7.2 Hz, 6 H), 1.24 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 195.2 (d, J = 5.8 Hz), 177.6, 68.0, 62.8 (d, J = 5.9 Hz), 39.0 (d, J = 128.3 Hz), 38.6, 27.0, 16.2 (d, J = 6.6 Hz); 31P NMR (162 MHz, CDCl3 ) δ 18.4.
(2氧代2苯乙基)膦酸二異丙酯(2o) [12]:黃色油狀物,產(chǎn)率96% (136 mg),1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 7.6 Hz, 2H), 7.53~7.50 (m, 1H), 7.42~7.39 (m, 2H), 4.71~4.62 (m, 2H), 3.54 (d,J = 22.8 Hz, 2H), 1.21 (q, J = 4.0 Hz, 12H); 13C NMR (100 MHz, CDCl3) δ 191.9 (d, J = 6.5 Hz), 136.4, 133.3, 128.9, 128.3, 71.3 (d, J = 7.3 Hz), 39.4 (d,J =129.8 Hz), 23.7 (d, J = 4.3 Hz), 23.5 (d, J =5.1 Hz); 31P NMR (162 MHz, CDCl3 ) δ 17.7.
(2氧代2苯乙基)膦酸二異丁酯(2p):黃色油狀物,產(chǎn)率94% (146 mg),1H NMR (400 MHz, CDCl3) δ 7.94 (d, J = 7.2 Hz, 2H), 7.53~7.50 (m, 1H), 7.42~7.38 (m, 2H), 3.78~3.74 (m, 4H), 3.58 (d, J = 23.2 Hz, 2H), 1.86~1.76 (m, 2H), 0.80 (d, J = 6.8 Hz, 12H); 13C NMR (100 MHz, CDCl3) δ 191.8 (d, J = 6.6 Hz), 136.5, 133.5, 128.9, 128.5, 72.3 (d, J = 7.3 Hz), 38.0 (d, J = 129.1 Hz), 29.0 (d, J = 6.6 Hz), 18.5 (d, J = 1.5 Hz); 31P NMR (162 MHz, CDCl3 ) δ 19.7; IR (neat): 2 958, 1 685, 1 594, 1 465, 1 273, 1 026, 1 004, 905 cm-1; HRMS (ESI): m/z [M + H]+ calcd for C16H26O4P: 313.156 9, found: 313.156 4.
2 結(jié)果與討論
2.1 反應(yīng)條件的優(yōu)化
為高產(chǎn)率地合成目標(biāo)產(chǎn)物,我們進(jìn)行了一系列的實(shí)驗(yàn),尋找最佳反應(yīng)條件. 2苯乙炔基膦酸二乙酯(1a)作為模型底物來(lái)進(jìn)行實(shí)驗(yàn),得到目標(biāo)產(chǎn)物(2a)的分離產(chǎn)率為44% (Table 1, Entry 1). 當(dāng)加入的濃硫酸的量由3摩爾逐漸提高到7摩爾時(shí)目標(biāo)產(chǎn)物(2a)的產(chǎn)率逐漸提高到了83% (Entries 2~6). 不向反應(yīng)體系中加入濃硫酸時(shí),反應(yīng)不能正常進(jìn)行,沒(méi)有目標(biāo)產(chǎn)物生成,回收物為初始投入原料(Entry 7), 因而濃硫酸加入的量對(duì)反應(yīng)的產(chǎn)率有很大影響. 溫度的變化也會(huì)影響反應(yīng)的進(jìn)程,對(duì)反應(yīng)溫度進(jìn)行了篩查,將反應(yīng)溫度由25 ℃升高至60 ℃,產(chǎn)率由83%升高到91% (Entries 5, 8, 9). 但繼續(xù)將溫度提高到80 ℃,產(chǎn)率沒(méi)有繼續(xù)升高(Entry 10).從表1中的條目8~10的結(jié)果可以看出,最佳反應(yīng)溫度為 60 ℃. 同時(shí)篩選了反應(yīng)時(shí)間 (Entries 11~14),反應(yīng)時(shí)間縮短到8 h時(shí),產(chǎn)率下降到86%(Entry 11). 當(dāng)反應(yīng)時(shí)間逐漸延長(zhǎng)時(shí),反應(yīng)產(chǎn)率在逐漸升高,反應(yīng)時(shí)間超過(guò)20 h,反應(yīng)產(chǎn)率趨于平穩(wěn)(Entries 12~14),因而最佳反應(yīng)時(shí)間為20 h(Entry14).
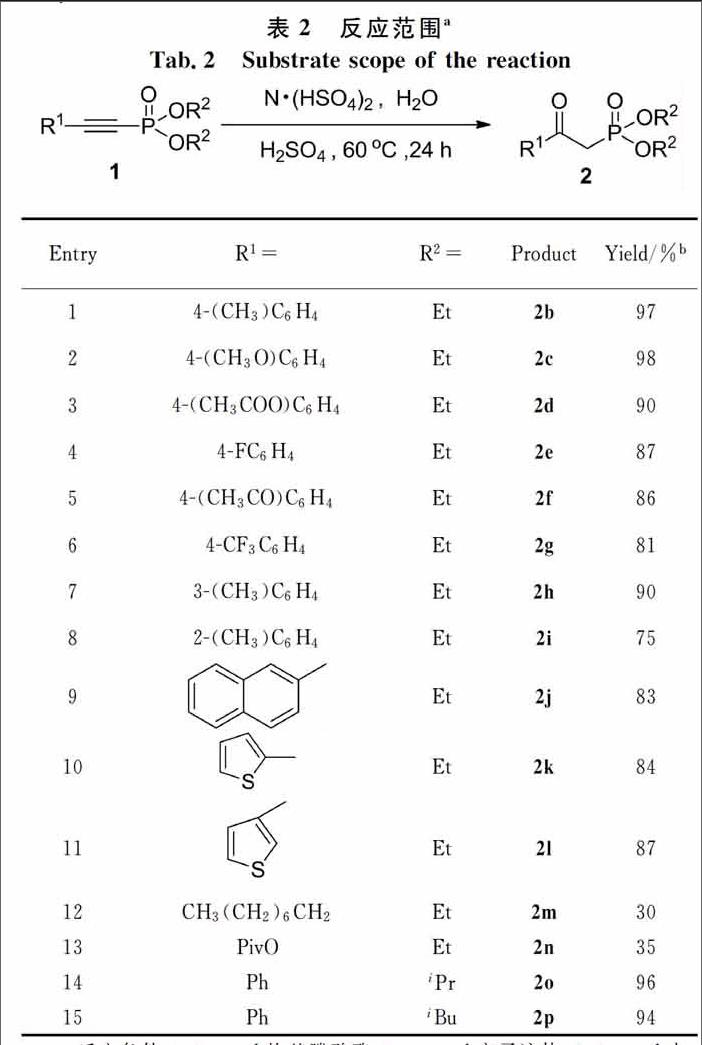
在最優(yōu)的反應(yīng)條件下,我們對(duì)各種芳香族和脂肪族的炔基膦酸酯進(jìn)行探究,來(lái)考察底物上取代基團(tuán)對(duì)于反應(yīng)的影響. 這些反應(yīng)的結(jié)果都統(tǒng)計(jì)在表2中.
供電子基團(tuán)取代的β羰基膦酸酯都能容易地合成且產(chǎn)率高(Tab.2, Entries 1~3),同時(shí)吸電子基團(tuán)取代的β羰基膦酸酯也能夠在較高的產(chǎn)率下制備(Entries 4~6),但其產(chǎn)率略低于供電子基團(tuán)取代. 然而由于位阻效應(yīng)間位取代和鄰位取代的β羰基膦酸酯的產(chǎn)率都比對(duì)位取代的β羰基膦酸酯的產(chǎn)率低(Entries 7, 8). 萘環(huán)取代的β羰基膦酸酯也能順利地制備(Entry 9),十分重要的、具有生物活性的噻吩取代的β羰基膦酸酯也同樣能夠合成(Entries 10, 11). 結(jié)果表明,盡管β羰基膦酸酯的產(chǎn)率會(huì)受到取代基的電子效應(yīng)和位阻效應(yīng)的影響,但很多芳香取代的β羰基膦酸酯都能容易地合成(Entries 1~11). 脂肪族取代的炔基膦酸酯也同樣能進(jìn)行此反應(yīng),但反應(yīng)的產(chǎn)率偏低(Entries 12, 13).其他的膦酸酯底物也能順利地進(jìn)行水合反應(yīng)得到相應(yīng)的β羰基膦酸酯(Entries 14, 15).
為了探討大量反應(yīng)的可行性,將模型反應(yīng)底物的量增大到20 mmol,同樣也得到了92%的高產(chǎn)率,結(jié)果與預(yù)想的一致,能夠用離子液體體系制備大量的β羰基膦酸酯產(chǎn)物.
2.2 機(jī)理探究
此反應(yīng)的機(jī)理一般認(rèn)為是通過(guò)碳碳三鍵的限速質(zhì)子化,然后水快速地連接上來(lái),形成烯醇結(jié)構(gòu),通過(guò)烯醇結(jié)構(gòu)的轉(zhuǎn)換最終得到羰基化合物[19-20]. 我們的實(shí)驗(yàn)結(jié)果及得到的相應(yīng)產(chǎn)物也印證了這一反應(yīng)機(jī)制.
3 結(jié) 論
我們成功地發(fā)展了一種利用酸性離子液體在溫和的條件下合成β羰基膦酸酯的綠色、實(shí)用的方法.該方法對(duì)于反應(yīng)底物的取代基團(tuán)具有很好的兼容性,在體系中無(wú)論是缺電子還是富電子的芳香族取代炔基磷酸酯都能得到非常高的產(chǎn)率. 更值得一提的是,這種方法避免了使用昂貴且復(fù)雜的金屬催化劑,是一種環(huán)境友好的合成方法.
參考文獻(xiàn)
[1] BALG C, BLAIS S P, BERNIER S, et al. Synthesis of betaketophosphonate analogs of glutamyl and glutaminyl adenylate, and selective inhibition of the corresponding bacterial aminoacyltRNA synthetases[J]. Bioorganic & Medicinal Chemistry, 2007,15(1):295-304.
[2] PERUMAL S K, ADEDIRAN S A, PRATT R F. Betaketophosphonates as betalactamase inhibitors: Intramolecular cooperativity between the hydrophobic subsites of a class D betalactamase[J]. Bioorganic & Medicinal Chemistry, 2008,16(14):6987-6994.
[3] DEBROUWER W, HEUGEBAERT T S, VAN HECKE K, et al. Synthetic entry into 1Phosphono3azabicyclo[3.1.0]hexanes[J]. The Journal of Organic Chemistry, 2013,78(17):8232-8241.
[4] ESSID I, TOUIL S. βKetophosphonates as substrates in the Biginelli multicomponent reaction: an efficient and straightforward synthesis of phosphorylated dihydropyrimidinones[J]. ARKIVOC, 2013,4:98-106.
[5] WADSWORTH W S, EMMONS W D. The utility of phosphonate carbanions in olefin synthesis[J]. Journal of the American Chemical Society, 1961,83(7):1733-1738.
[6] RYGLOWSKI A, KAFARSKI P. Preparation of 1aminoalkylphosphonic acids and 2aminoalkylphosphonic acids by reductive amination of oxoalkylphosphonates[J]. Tetrahedron, 1996,52(32):10685-10692.
[7] KITAMURA M, TOKUNAGA M, NOYORI R. Asymmetric hydrogenation of .beta.keto phosphonates: a practical way to fosfomycin[J]. Journal of the American Chemical Society, 1995, 117(10): 2931-2932.
[8] POSS A J, BELTER R K. Diethyl 3iodopropynylphosphonate: an alkylative .beta.keto phosphonate equivalent[J]. The Journal of Organic Chemistry, 1987,52(21):4810-4812.
[9] LI X, HU G, LUO P, et al. Palladium(II)catalyzed hydration of alkynylphosphonates to βKetophosphonates[J]. Advanced Synthesis & Catalysis, 2012,354(13):2427-2432.
[10]XIE L, YUAN R, WANG R, et al. Gold(I)catalyzed hydration of alkynylphosphonates: efficient access to βketophosphonates[J]. European Journal of Organic Chemistry, 2014,2014(13):2668-2671.
[11]XIANG J, YI N, WANG R,et al. Synthesis of βketophosphonates via AgNO3catalyzed hydration of alkynylphosphonates: a rateenhancement effect of methanol[J]. Tetrahedron, 2015,71(4):694-699.
[12]YI N, WANG R, ZOU H,et al. Copper/ironcatalyzed aerobic oxyphosphorylation of terminal alkynes leading to βketophosphonates[J]. The Journal of Organic Chemistry, 2015, 80(10):5023-5029.
[13]BAIDOSSI W, LAHAV M, BLUM J. Hydration of alkynes by a PtCl4 CO catalyst[J]. The Journal of Organic Chemistry, 1997, 62(3): 669- 672.
[14]SUZUKI T, TOKUNAGA M, WAKATSUKI Y. Ruthenium complexcatalyzed antiMarkovnikov hydration of terminal alkynes[J]. Organic Letters, 2001,3(5):735-737.
[15]HINTERMANN L, LABONNE A. Catalytic hydration of alkynes and its application in synthesis[J]. Synthesis, 2007(8):1121-1150.
[16]STEINRCK H P, WASSERSCHEID P. Ionic liquids in catalysis[J]. Catalysis Letters, 2014,145(1):380-397.
[17]PLECHKOVA N V, SEDDON K R. Applications of ionic liquids in the chemical industry[J]. Chemical Society Reviews, 2008,37(1):123-150.
[18]WONG W L, HO K P, LEEL Y S,et al. Sulfuric acidcatalyzed conversion of alkynes to ketones in an ionic liquid medium under mild reaction conditions[J]. ACS Catalysis, 2011,1(2):116-119.
[19]MAMEDA N, PERAKA S, MARRI M R, et al. Solventfree hydration of alkynes over Hβ zeolite[J]. Applied Catalysis A: General, 2015,505:213-216.
[20]NOYCE D S, SCHIAVELLI M D. Acidcatalyzed hydration of phenylacetylene. evidence for the vinyl cation intermediate[J]. Journal of the American Chemical Society, 1968,90(4):1020-1022.

