2-苯亞胺官能化吲哚基銪胺基配合物與芳基取代甲脒的反應性
馮志君崔巧玉韋蕓周雙六吳運軍王少印
(1皖南醫學院醫用基礎化學教研室,蕪湖241002)
(2功能分子固體教育部重點實驗室,安徽師范大學化學與材料科學學院分子材料實驗室,蕪湖241000)
2-苯亞胺官能化吲哚基銪胺基配合物與芳基取代甲脒的反應性
馮志君*,1,2崔巧玉1韋蕓2周雙六2吳運軍1王少印1
(1皖南醫學院醫用基礎化學教研室,蕪湖241002)
(2功能分子固體教育部重點實驗室,安徽師范大學化學與材料科學學院分子材料實驗室,蕪湖241000)
2-(苯亞胺基次甲基)吲哚銪胺基配合物[η1∶η1-2-(C6H5NH=CH)C8H5N]2Eu[N(SiMe3)2](1)與二芳基取代甲脒(2,6-R2C6H3N= CHNH(C6H3R2-2,6)(R=iPr(2),Me(3))經過配體交換反應,分別得到了含吲哚基脒基銪配合物[η1∶η1-2-(C6H5NH=CH)C8H5N]Eu[(η3-2,6-iPr2C6H3)N=CHN(C6Hr2-2,6)][N(SiMe3)2](4)和含脒基的稀土銪配合物[(η3-2,6-Me2C6H3)N=CHN(C6H3Me2-2,6)]2Eu[N(SiMe3)2] (5)。結果表明,脒基的位阻顯著影響了吲哚基稀土金屬胺基配合物與二芳基取代甲脒的配體交換反應。配合物4和5通過IR、元素分析和X射線單晶衍射分析進行了表征。
稀土金屬;銪;吲哚基配體;胺基配合物
Duringthecourseoflanthanidechemistry studies,novel reactivity of rare-earth metal complexes has been one of the most important research fields[1-3], because such kinds of reactions are usually the basis for lots of important catalytic procedures,organic transformationsandproposedpathwayforthe formation of lanthanide compounds.To date,much effort has been devoted to study the reactivity of rare-earth metal complexes[4-15],which involves a number of small molecular substrates including H2[4],CO[5],CO2[6-9], alkane[10],alkene[8,11],alkyne[11],amine[9],carbodiimide[12], silane[4,13],phosphine[14],nitrile[7-8],isocyanate[8-9],amidine[13],carbonylcompound[7,11],heterocyclic compound[11]and so on[15].Among them,amidines,used as ligands or substrates for reactivity studies[16-18],have drawn much attention due to being easily prepared and tuned sterically and electronically.Amidines have played an important role in the investigation on the influence of spatial hindrance and electronic effect on the synthesis, bonding modes,structures and performances of rareearthmetalcomplexes.Thus,reactivitystudies between indolyl rare-earth metal amido complexes with formamidines are still the interesting work.
Recently,we have reported the reactivity of 2-(2, 6-diisopropylphenylaminomethine)indolyl europiumamido complex with formamidines having different spatial hindrance afforded novel complexes incorporating indolyl ligands bonded with metal in novel μ-η3∶η1∶η1and μ-η2∶η1∶η1bonding manners[19],respectively. In addition,we have also reported the reactivities of rare-earth metal monoalkyl complexes incorporating bidentate indolyl ligands with PhSiH3or amidine,and have obtained the first example of trivalent rare-earth metalcomplexeshavinganamido-functionalized indolyl ligand bonded to a metal in the μ-η6∶η1∶η1fashions[20].
Continuing our investigation on the amino,iminofunctionalized indolyl ancillary ligand system,we have synthesized europiumamido complex having 2-(phenyliminomethine)indoly ligands[21].Encouraged by above-mentioned results,we further investigated the reactivity of the indolyl europiumamide with formamidines,as shown in Scheme 1.According to our experimental results,when treating of europiumcomplexcontainingimino-functionalizedindolyl groups and N(SiMe3)2group as ancillary ligands with formamidines,the attack occurs primarily onto the Eu-N bonds between indolyl ligand and central metal. Moreover,the results also made it clear that the reactivity of europiumamido complex having the functionalized indolyl ligands and the structures of the resultants are significantly dependent on the steric hindrance of formamidines.Herein,we will report these results and structural studies of new complexes.
1 Experimental
1.1 Materials and methods

Scheme 1
All manipulations of air-and moisture-sensitive materials were performed using Schlenk techniques or in a glovebox under an atmosphere of purified argon. Tolueneandhexanewererefluxedandfreshly distilled over sodium benzophenone ketyl under argonpriortouse.[2-(C6H5NH=CH)C8H5N]2Eu[N(SiMe3)2] (1)[21]and 2,6-R2C6H3N=CHNH(C6H3R2-2,6)(R=iPr(2), Me(3))[22]were prepared according to the literature methods.Elemental analyses data were obtained on a Perkin-Elmer 2400SeriesⅡelemental analyzer and analyses were carried out in the microanalytical laboratory of Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences.IR spectra were run on a Shimadzu FTIR-8400s spectrometer(KBr pellet). Melting points were determined in sealed capillaries and are uncorrected.
1.2 Syntheses of complexes
1.2.1 Synthesis of[η1∶η1-2-(C6H5NH=CH)C8H5N]Eu
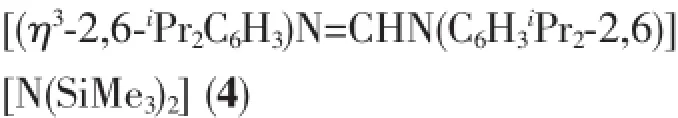
A Schleck flask was charged with complex 1(0.90g,1.20mmol),2,6-iPr2C6H3N=CHNH(C6H3iPr2-2,6)(2)(0.43g,1.20mmol)and toluene(30mL).The reaction mixture was stirred at 70℃for 16h.The red-brown solution was evaporated to dryness and extracted with n-hexane(14mL).Orange-red crystals that were suitable for X-ray diffraction were obtained upon crystallization from hexane at 0℃after a few days(0.59g,55%).m.p.187℃.Anal.Calcd.for C46H65N5Si2Eu(%):C,61.65;H,7.31;N,7.81.Found (%):C,61.16;H,7.63;N,8.22.IR(KBr pellet,cm-1): 2959(w),2865(w),1660(s),1614(s),1589(s),1483(m),1445(m),1423(m),1298(w),1194(w),1065(w),957(w),908(w),872(w),800(m),767(m),753(m),737(m),695(s).
1.2.2 Synthesis of[(η3-2,6-Me2C6H3)N=CHN(C6H3Me2-2,6)]2Eu[N(SiMe3)2](5)
The synthesis of complex 5was same to that of complex 4except that 2,6-Me2C6H3N=CHNH(C6H3Me2-2,6)(3)(0.60g,2.40mmol)was used instead of compound 2.Yield:0.55g,56%.m.p.205℃.Anal. Calcd.for C40H58N5Si2Eu(%):C,58.80;H,7.16;N,8.57. Found(%):C,58.38;H,7.42;N,8.68.IR(KBr pellet, cm-1):2961(w),2854(w),1661(s),1613(s),1589(s),1483(m),1444(m),1425(m),1298(w),1195(w), 1066(w),955(w),906(w),870(w),799(m),765(m), 750(m),737(m),696(s).
1.3 X-ray crystallographic analyses
Single crystals of complexes 4and 5suitable for X-ray diffraction studies were sealed in thin-walled glasscapillariesunderargon.Diffractionwas performed on a Bruker SMART CCD area detector diffractometer using graphite-monochromated Mo Kα radiation(λ=0.071073nm).An empirical absorption correction was applied using the SADABS program[23]. All structures were solved by direct methods,completed by subsequent difference Fourier syntheses,refined anisotropically for all nonhydrogen atoms by full-matrix least-squares calculations on F2using the SHELXTL program package[24].All hydrogen atoms were refined using a riding model.A summary of the crystallographic data and selected experimental information are listed in Table 1,while the selected bond lengths and angles are summarized in Table 2.
CCDC:1496184,4;1496185,5.
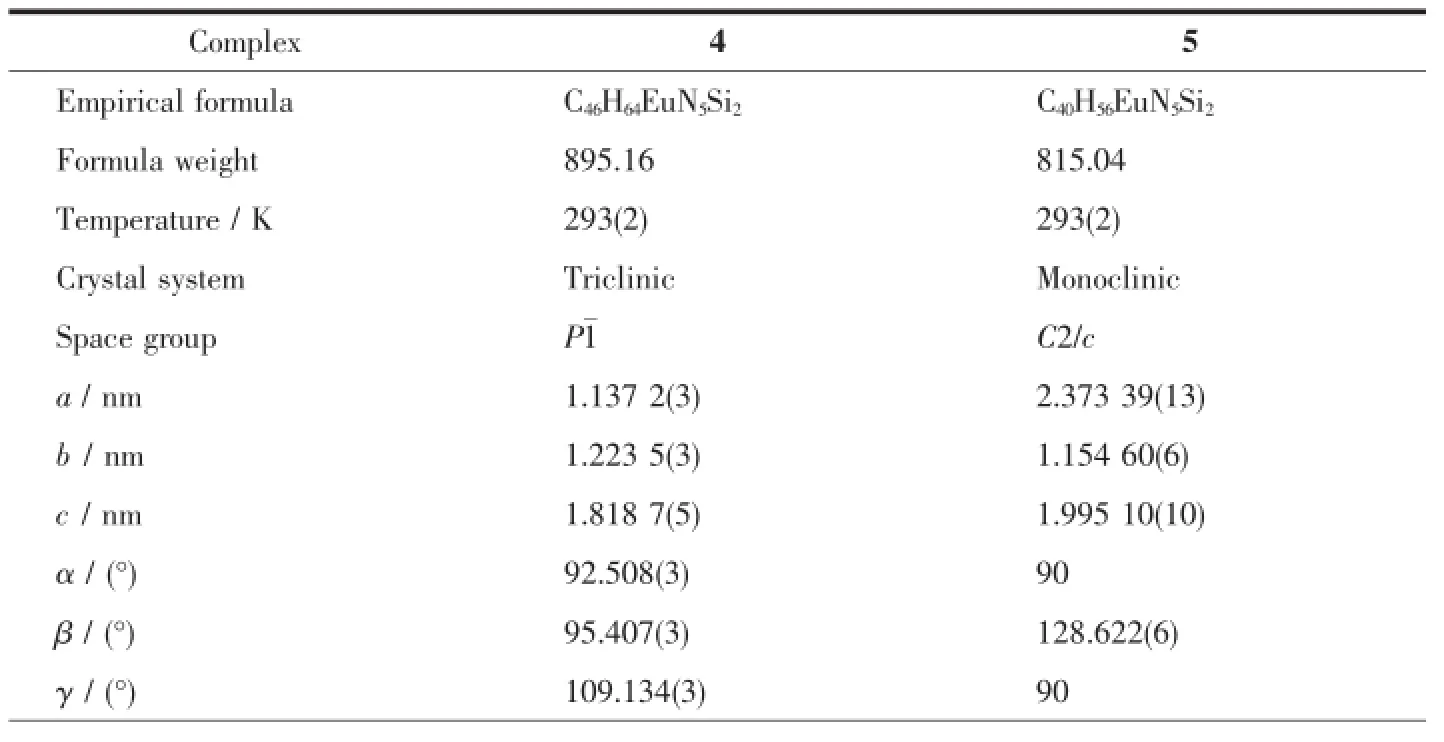
Table 1Crystallographic data and structure refinement for complexes 4and 5
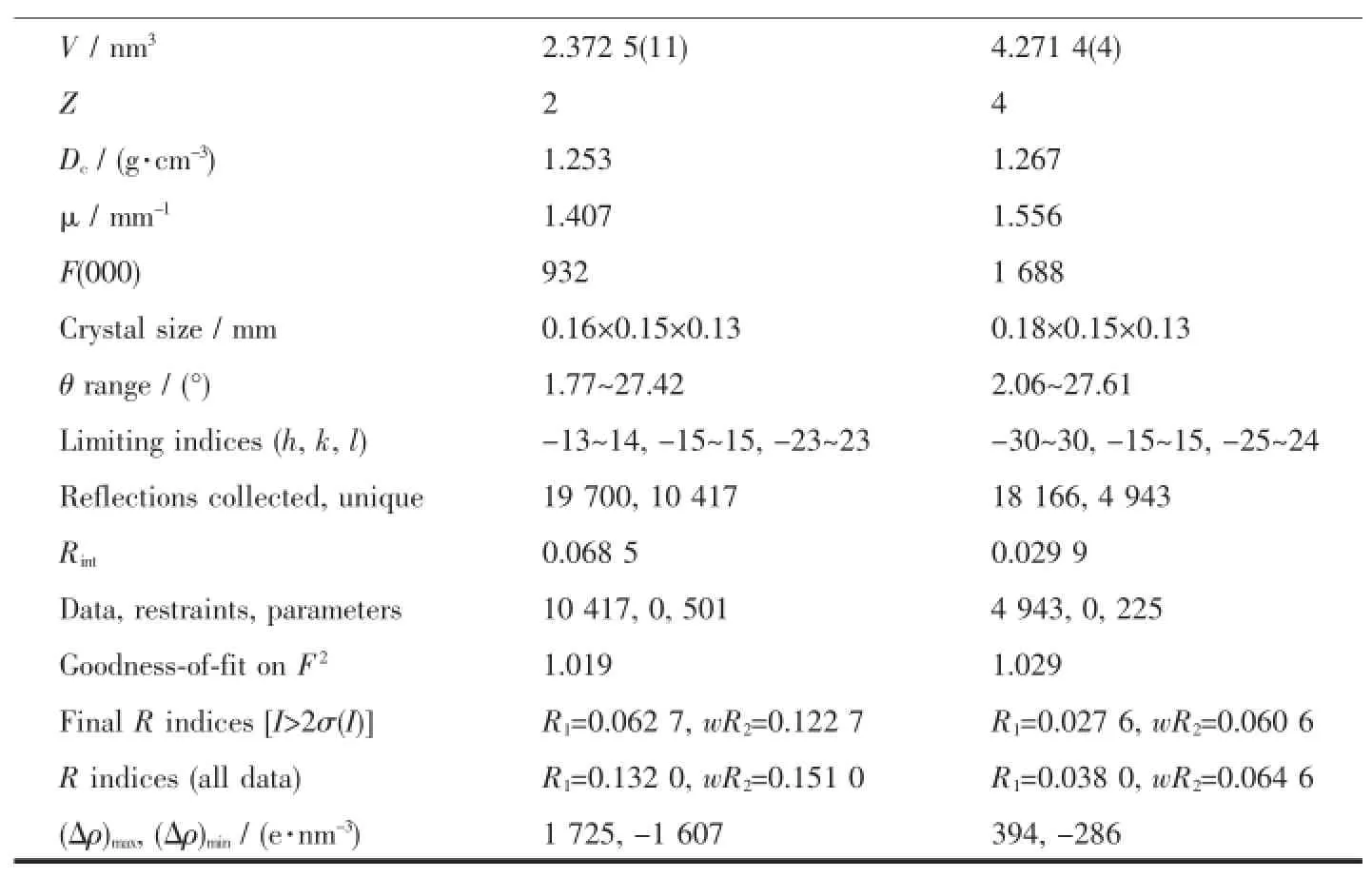
Continued Table 1
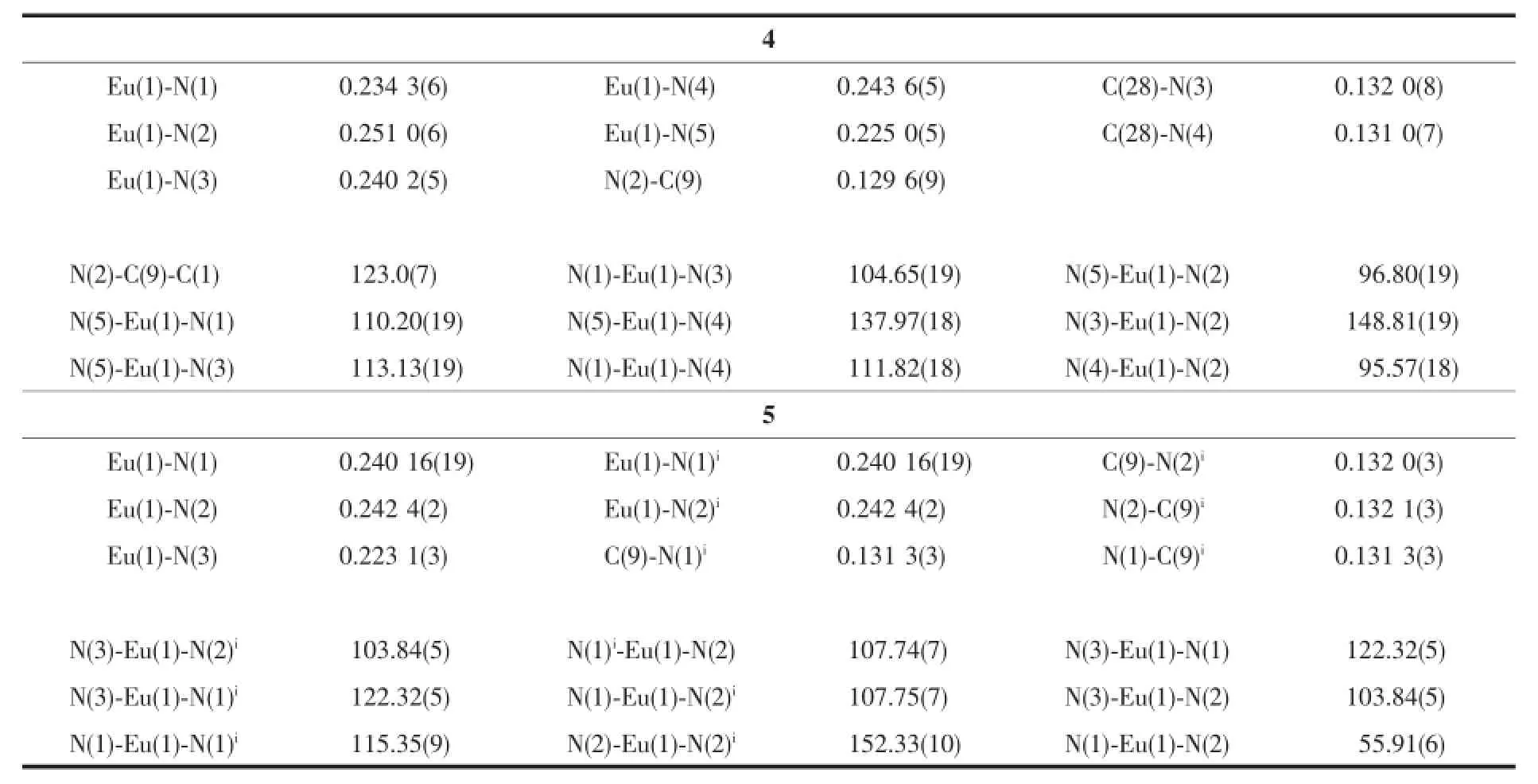
Table 2Selected bond lengths(nm)and angles(°)for complexes 4and 5
2 Results and discussion
As shown in Scheme 1,reaction of europium complex 1with the sterically bulky formamidine[(2, 6-iPr2C6H3)NCHNH(C6H3iPr2-2,6)](2)gave europium complex formulated as[η1∶η1-2-(C6H5NH=CH)C8H5N] Eu[(η3-2,6-iPr2C6H3)N=CHN(C6H3iPr2-2,6)][N(SiMe3)2] (4),containing an indolyl ligand and an formamidinato ligand via ligand exchange process.Treatment of europium complex 1with the less bulky formamidine 2,6-Me2C6H3N=CHNH(C6H3Me2-2,6)(3)afforded europium complex formulated as[(η3-2,6-Me2C6H3)N=CHN (C6H3Me2-2,6)]2Eu[N(SiMe3)2](5),only incorporating formamidinato ligands and N(SiMe3)2.Complexes 4and 5are extremely sensitive to air and moisture.They were characterized by spectroscopic and elemental analyses,and their structures were further elucidated by X-ray diffraction study.The structures of 4and 5are individually displayed in Fig.1and 2,while selected bond lengths and angles are given in Table 2.
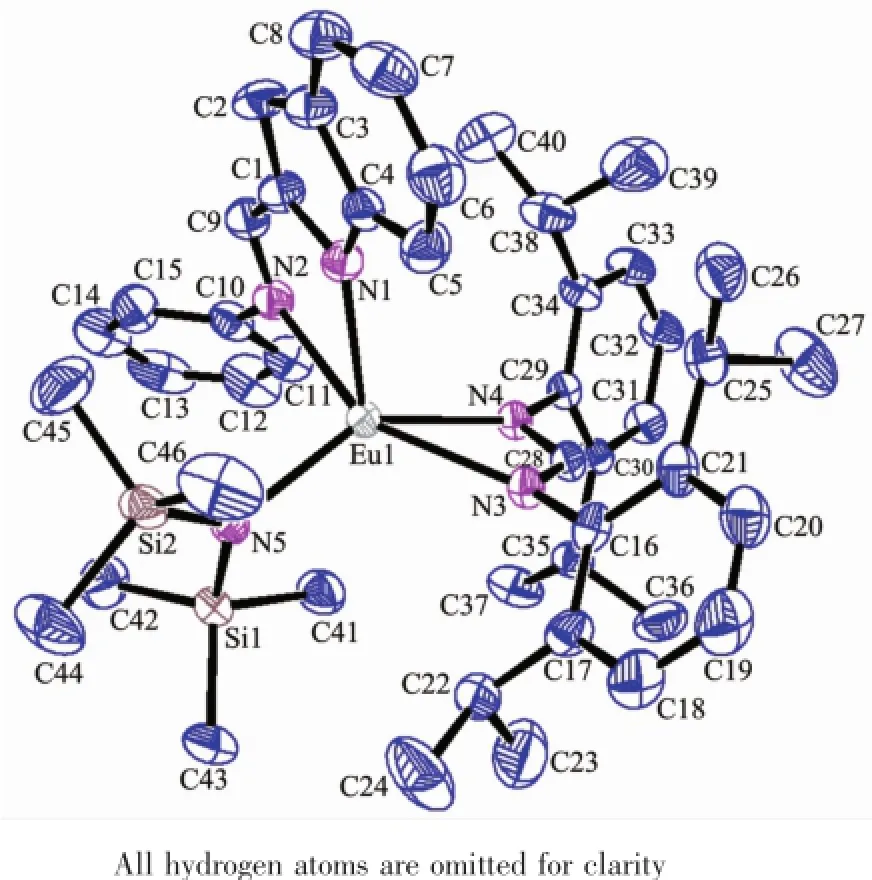
Fig.1Molecular structure of 4with 30%thermal ellipsoids
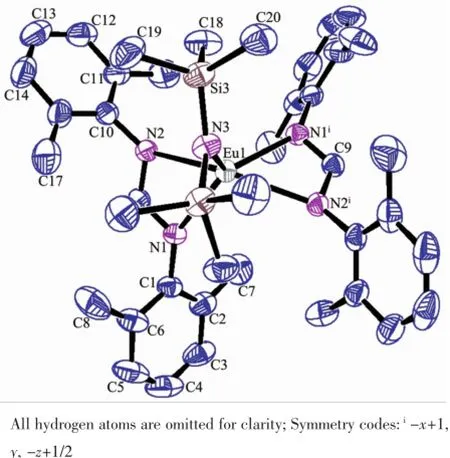
Fig.2Molecular structure of 5with 30%thermal ellipsoids
Complex 4crystallizes in a triclinic crystal system with space group P1.X-ray analyses revealed that 4is five-coordinated mononuclear complex.As shown in Fig.1,the central Eu3+is coordinated by a bidentate indolyl ligand in η1∶η1hapticities,an formamidinato ligand in an η3fashion and N(SiMe3)2ligand adopting a distorted trigonal bipyramid configuration similar to that of complex 1[21].The C(28)-N(3)length of 0.1320(8) nm and C(28)-N(4)of 0.1310(7)nm has become average and close to the value between carbon-carbon length of single bond and that of double bond,showing the characteristic of delocalized bond and indicating that the formamidinato ligand is bonded to the Eu3+with an η3manner.Among the Eu-N lengths in complex 4,the Eu(1)-N(2)length of 0.2510(6)nm of the appendant arm is significantly longer than the others (Eu(1)-N(1)0.2343(6)nm,Eu(1)-N(3)0.2402(5)nm, Eu(1)-N(4)0.2436(5)nm,Eu(1)-N(5)0.2250(5)nm), and close to the corresponding value of 0.2525(3)nm in complex 1and that of 0.2565(2)nm in complex [2-(tBuN=CH)C8H5N]Eu[N(SiMe3)2]2[21],indicating coordination nature of the N2atom.The bond angles of N(5)-Eu(1)-N(1),N(5)-Eu(1)-N(3),N(1)-Eu(1)-N(3), N(1)-Eu(1)-N(4)and N(5)-Eu(1)-N(2)are respectively 110.20(19)°,113.13(19)°,104.65(19)°,111.82(18)° and 96.80(19)°.
Complex 5,crystallizing in a monoclinic crystal systemwithspacegroupC2/c,isalsofivecoordinated.Differently,the coordination environment of the central metal is completed by two formamidino groups and one-N(SiMe3)2ligand as a centrosymmetric tetragonal pyramid arrangement,in which N3atom of -N(SiMe3)2group occupies the vertex,as shown in Fig. 2.Given that the C(9)-N(1)i,N(1)-C(9)ilengths of 0.1313(3)nm,C(9)-N(2)iof 0.1320(3)nm and N(2)-C(9)iof 0.1321(3)nm,the bonding mode of formamidino ligand bonded to Eu3+is described as an η3fashion. The Eu(1)-N(1)ilength of 0.24016(19)nm or Eu(1)-N(2)ilength of 0.2424(2)nm is in accordance with the corresponding Eu(1)-N(1)length of 0.24016(19) nm or Eu(1)-N(2)length of 0.2424(2)nm,indicating a centrosymmetric geometry.Moreover,the centrosymmetric structure is also deduced by the bond angles of N(3)-Eu(1)-N(2)iof 103.84(5)°,N(3)-Eu(1)-N(1)iof 122.32(5)°,N(3)-Eu(1)-N(1)of 122.32(5)°and N(3)-Eu(1)-N(2)of 103.84(5)°.Thus,the result suggested that imino-functionalized indolyl ligands in complex 1are completely replaced by the less bulky formamidino groups,while the geometry of the resultant complex 5is obviously different from that of complex 1.
3 Conclusions
In summary,we have studied the reactivity ofeuropiumamido complex having 2-(phenyliminomethine)indoly ligands[2-(C6H5NH=CH)C8H5N]2Eu[N (SiMe3)2](1)with formamidines.Reaction of 1with the sterically bulky formamidine[(2,6-iPr2C6H3)NCHNH (C6r2-2,6)]or the less bulky formamidine 2,6-Me2C6H3N=CHNH(C6H3Me2-2,6)gave complexes[η1∶η1-2-(C6H5NH=CH)C8H5N]Eu[(η3-2,6-iPr2C6H3)N=CHN (C6H3iPr2-2,6)][N(SiMe3)2](4)and[(η3-2,6-Me2C6H3)N= CHN(C6H3Me2-2,6)]2Eu[N(SiMe3)2](5),respectively.The results showed that the steric hindrance of organic molecular substrates have significantly effects on the reactivityofrare-earthmetalamidocomplexes containing imino-functionalized indolyl ligands and thestructuresoftheresultantnewlanthanide complexes.Further study on the reactivity of the rareearthmetalcompoundswiththerelatedindolyl ligands is in progress in our laboratory and will be reported soon.
[1]Bochkarev M N.Chem.Rev.,2002,102:2089-2117
[2]Edelmann F T,Freckmann D M M,Schumann H.Chem. Rev.,2002,102:1851-1896
[3]Li T S,Kaercher S,Roesky P W.Chem.Soc.Rev.,2014,43: 42-57
[4]Fegler W,Venugopal A,Kramer M,et al.Angew.Chem.Int. Ed.,2015,54:1724-1736
[5]Shima T,Hou Z.J.Am.Chem.Soc.,2006,128:8124-8125
[6]LeBlanc F A,Piers W E,Parvez M.Angew.Chem.Int.Ed., 2014,53:789-792
[7]Xu L,Wang Y C,Xi Z F,et al.Chem.Eur.J.,2015,21:6686-6689
[8]Chu J X,Lu E L,Chen Y F,et al.Angew.Chem.Int.Ed., 2011,50:7677-7680
[9]Hong J Q,Zhang L X,Zhou X G,et al.Organometallics, 2013,32:7312-7322
[10]Cole M L,Deacon G B,Junk P C,et al.Organometallics, 2013,32:1370-1378
[11]Arnold P L,McMullon M W,Rieb J,et al.Angew.Chem. Int.Ed.,2015,54:82-100
[12]Xu L,Wei J N,Xi Z F,et al.Chem.Eur.J.,2015,21:15860-15866
[13]Chu J X,Lu E L,Chen Y F,et al.Organometallics,2013, 32:1137-1140
[14]Wang K,Luo G,Zhang L X,et al.Angew.Chem.Int.Ed., 2014,53:1053-1056
[15]Rong W,He D,Cui D M,et al.Chem.Commun.,2015,51: 5063-5065
[16]Edelmann F T.Chem.Soc.Rev.,2009,38:2253-2268
[17]Yao S,Chan H S,Lee H K,et al.Inorg.Chem.,2009,48: 9936-9946
[18]Cole M L,Deacon G B,Junk P C,et al.Chem.Eur.J., 2013,19:1410-1420
[19]Feng Z J,Zhu X C,Wang S W,et al.Inorg.Chem.,2013, 52:9549-9556
[20]Zhang G C,Wang S W,Zhou S L,et al.Organometallics, 2015,34:4251-4261
[21]Feng Z J,Wei Y,Wang S W,et al.Dalton Trans.,2015,44: 20502-20513
[22]Hirano K,Urban S,Wang C,et al.Org.Lett.,2009:1019-1022
[23]Sheldrick G M.SADABS,A Program for Empirical Absorption Correction of Area Detector Data,University of G?ttingen, Germany,1996.
[24]Sheldrick G M.SHELXTL 5.10for Windows NT,Structure Determination Software Programs,Bruker Analytical X-ray Systems,Inc.,Madison,WI,1997.
Reactivity of 2-Phenylimino-Functionalized Indolyl Europium Amide with Diaryl-Substitued Formamidines
FENG Zhi-Jun*,1,2CUI Qiao-Yu1WEI Yun2ZHOU Shuang-Liu2WU Yun-Jun1WANG Shao-Yin1
(1Department of Chemistry,Wannan Medical College,Wuhu,Anhui 241002,China)
(2Key Laboratory of Functionalized Molecular Solids,Ministry of Education,Anhui Laboratory of Molecule-Based Materials,College of Chemistry and Materials Science,Anhui Normal University,Wuhu,Anhui 241000,China)
Reactions of structurally well-defined europiumamido complex having 2-(phenyliminomethine) indolyl ligands[η1∶η1-2-(C6H5NH=CH)C8H5N]2Eu[N(SiMe3)2](1)with formamidines(2,6-R2C6H3N=CHNH(C6H3R2-2,6)(R=iPr(2),Me(3))afforded the formamidinato indolyl-ligated europium complex[η1∶η1-2-(C6H5NH=CH)C8H5N] Eu[(η3-2,6-iPr2C6H3)N=CHN(C6H3iPr2-2,6)][N(SiMe3)2](4)and formamidinato complex[(η3-2,6-Me2C6H3)N=CHN (C6H3Me2-2,6)]2Eu[N(SiMe3)2](5),respectively.Results showed that the steric hindrance of formamidines has a great influence on the reactivity of the imino-functionalized indolyl rare-earth metal amido complex with diarylsubstitued formamidines.The complexes 4and 5were characterized by IR spectra,elemental analyses and X-ray crystallographic diffraction study.CCDC:1496184,4;1496185,5.
rare-earth metal;europium;indolyl ligand;amido complex
O614.33+8
A
1001-4861(2017)01-0169-06
10.11862/CJIC.2017.006
2016-08-14。收修改稿日期:2016-11-02。
皖南醫學院博士科研啟動基金(No.rcqd201613)和國家級大學生創新訓練項目(No.201510368021)資助。*
。E-mail:fengzhijun73@163.com

