喹啉酰腙銅鋅配合物的結構及熒光性質
常慧琴 原知則 賴曉晴 吳偉娜*, 王 元*,
(1河南理工大學化學化工學院,焦作454000)
(2焦作市教育局基礎教育教學研究室,焦作454000)
(3中國石油集團鉆井工程技術研究院,北京102206)
喹啉酰腙銅鋅配合物的結構及熒光性質
常慧琴1原知則2賴曉晴3吳偉娜*,1王元*,1
(1河南理工大學化學化工學院,焦作454000)
(2焦作市教育局基礎教育教學研究室,焦作454000)
(3中國石油集團鉆井工程技術研究院,北京102206)
合成了配合物{[Cu(L)(OAc)]·MeOH}n(1)和[Zn(L)(OAc)]n(2)(HL為喹啉-8-甲醛縮異煙肼),并通過單晶X射線衍射、元素分析、紅外光譜表征了結構。單晶衍射結果表明,除了結晶溶劑不同,2個配合物結構類似。每個配合物中,中心金屬離子與來自烯醇化脫質子配體L-的2個O原子和1個N原子,1個單齒配位的醋酸根和相鄰配體4-吡啶N原子配位,形成一維鏈狀結構。但配合物中金屬離子的配位構型分別為扭曲的四方錐和三角雙錐。在360 nm紫外光激發下,甲醇溶液中配合物2在510 nm左右有很強的熒光發射,這是由鋅離子配位熒光增強效應所導致。
喹啉;酰腙;Cu(Ⅱ)配合物;Zn(Ⅱ)配合物;熒光;晶體結構
It is well known that Schiff bases are an important class of ligands in coordination chemistry and have been found extensive application in different fields[1-2].Among them,the Schiff base derivatives of 8-formylquinoline and their metal complexes have been widely investigated due to their high biological and pharmaceutical activities[2-5].However,studies on the fluorescence properties of the metal complexes with such series of ligands are relatively few[1].
Generally,both Cu(Ⅱ)and Zn(Ⅱ)ions are closely related to biochemistry,clinical diagnostics as well as environmental pollution[6].Furthermore,a large amount of Zn(Ⅱ)acylhydrazones have been reported for their fluorescence properties[7].Therefore,in this paper,Cu(Ⅱ)and Zn(Ⅱ)complexes with an acylhydrazone ligand derived from 8-formylquinoline and isonicotinohydrazide have been synthesized and structural determined by single-crystal X-ray diffraction.In addition,the fluorescence properties of three compounds in methanol solution were inves tigated.
1 Experimental
1.1Materials and measurements
Solvents and starting materials for synthesis were purchased commercially and used as received. Elemental analysis was carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1) were determined by the KBr pressed disc method on a Bruker V70 FTIR spectrophotometer.1H NMR spectra of HL was acquired with Bruker AV400 NMR instrument in DMSO-d6solution with TMS as internal standard.The UV spectra were recorded on a Purkinje General TU-1800 spectrophotometer.Fluorescence spectra were determined on a Varian CARY Eclipse spectrophotometer,in the measurements of emission and excitation spectra the pass width is 5 nm.
As shown in Scheme 1,a mixture of 8-formylquinoline(1.57 g,10 mmol)and isonicotinohydrazide(1.37 g,10 mmol)in ethanol(30 mL)were stirred at room temperature for 0.5 h,clear brown solution was formed.After stirring for another 4 h, the pale-yellow solid was gained,then filtered and washed three times with ethanol.Yield:2.26 g(82%). m.p.262~264℃.Elemental analysis Calcd.for HL (C16H12N4O)(%):C,69.55;H,4.38;N,20.28;Found: C,69.38;H,4.27;N,20.44.FTIR(cm-1):ν(O=C) 1 681,ν(C=N,imine)1 605,ν(C=N,quinoline)1 552, ν(C=N,pyridine)1 517.1H NMR(400 MHz,DMSO-d6):δ 12.30(1H,s,NH);9.75(1H,s,CH=N);7.56~7.72(2H),7.85~7.87(1H),8.06~8.09(2H),8.36~8.43 (2H),8.75~8.77(1H),8.95~8.97(2H)for Ar-H.
The complexes 1 and 2 were generated by reaction of HL(5 mmol)with equimolar of Cu(OAc)2and Zn(OAc)2in methanol solution,respectively. Crystals of 1 and 2 suitable for X-ray diffraction analysis were obtained by evaporating the reaction solutions at room temperature.
1:Green blocks.Yield:46%(based on HL). Anal.Calcd.for C19H18N4O4Cu(%):C,53.08;H,4.22; N,13.03.Found(%):C,52.87;H,4.42;N,12.95.IR (KBr,cm-1):ν(N=C-O)1 623,ν(C=N,imine)1 591, ν(C=N,quinoline)1 520,ν(C=N,pyridine)1 502, νas(COO)1 562,νs(COO)1 379.
2:Colorless blocks.Yield:59%(based on HL). Anal.Calcd.for C18H14N4O3Zn(%):C,54.09;H,3.53; N,14.02.Found(%):C,53.88;H,3.67;N,13.89.IR (KBr,cm-1):ν(N=C-O)1 612,ν(C=N,imine)1 589, ν(C=N,quinoline)1 522,ν(C=N,pyridine)1 508, νas(COO)1 568,νs(COO)1 413.
1.3.1X-ray crystallography
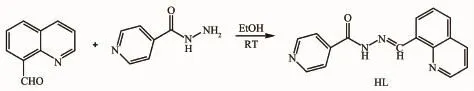
Scheme 1 Reaction scheme for the synthesis of HL
The X-ray diffraction measurement for complexes 1(size:0.22 mm×0.18 mm×0.16 mm)and 2(size:0.20mm×0.18 mm×0.15 mm)was performed on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphite monochromatized Mo Kα radiation(λ= 0.071 073 nm)by using φ-ω scan mode.Semiempirical absorption correction was applied to the intensity data using the SADABS program[8].The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-97 program[9].All non-hydrogen atoms were refined anisotropically.All H atoms were positioned geometrically and refined using a riding model. Details of the crystal parameters,data collection and refinements for both complexes are summarized in Table 1.
在CPIKN中,將協同成員節點pi的點權權重作為其重要程度的衡量指標。然后,定義協同成員節點pi重要程度排序序號為xi,協同成員節點pi的重要程度信息獲取狀態為εi,若協同成員節點pi的重要程度信息已知,則εi=1,反之εi=0。
CCDC:1414665,1;1489193,2.
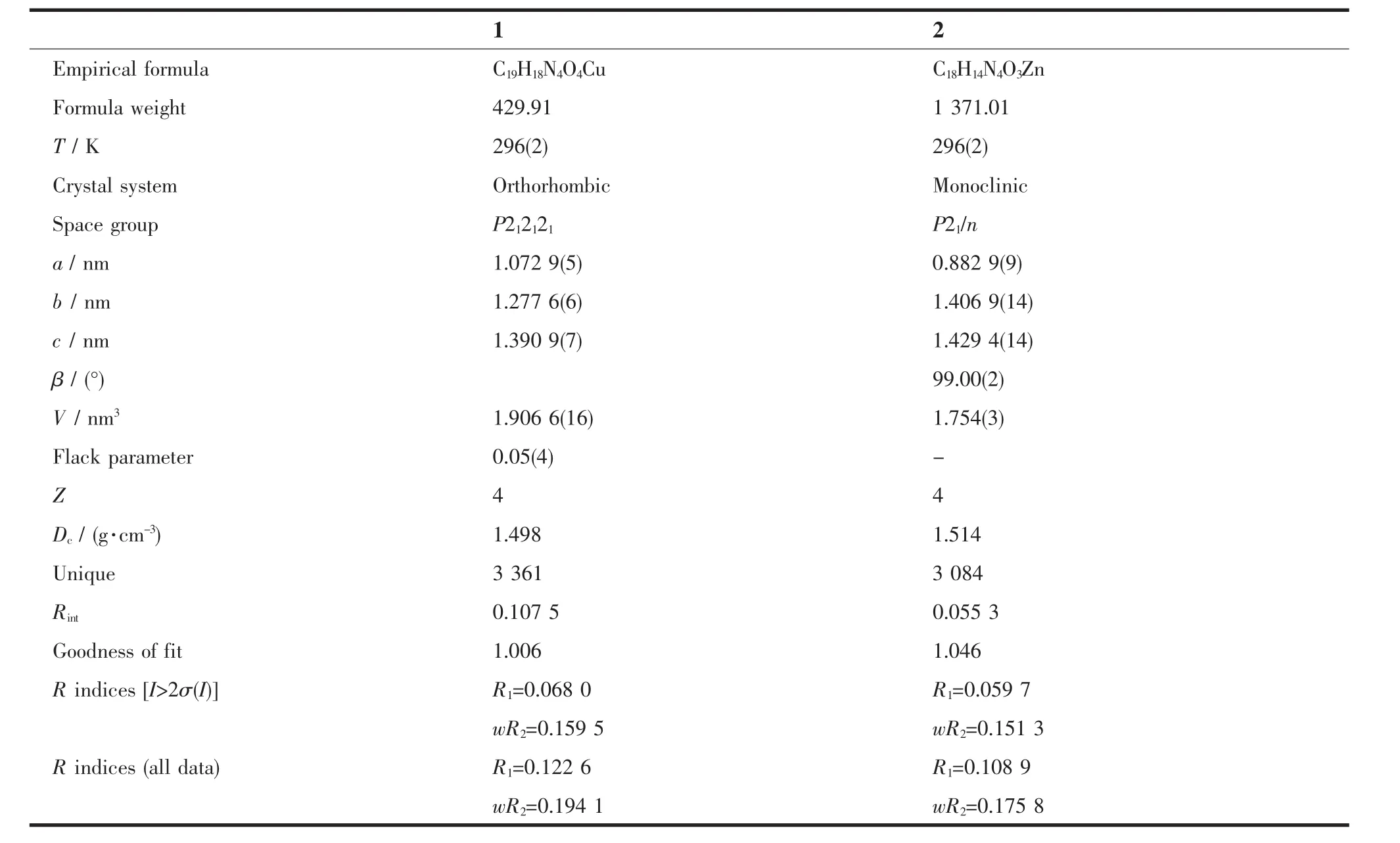
Table1 Crystal data and structure refinement for the complexes 1 and 2
2 Results and discussion
2.1Crystal structures description
Selected bond distances and angles for complexes 1 and 2 are summarized in Table 2.As shown in Fig. 1a and 1b,the structures of both complexes are quite similar except for lattice solvents.Thus the structure of complex 1 is discussed in detail for an example.In complex 1,the acylhydrazone ligand HL is deprotonated,and the distances of the enolized C-O (C11-O1,0.128 1(7)nm)and imine C-N(C11-N3, 0.130 9(7)nm)bands are intermediate between single and double bond,suggesting an extended conjugation in anionic ligand after complexation.The centre Cu(Ⅱ)ion is surrounded by one monodentate acetate anion, one NO2donor set of an enolizated ligand L-and one pyridine nitrogen atom from another adjacent acylhydrazone ligand,thus forming one dimension chain-like framework along c axis(Fig.1c).According to the Addison rule[10],the geometric index τ is 0.267, indicating that the coordination geometry of Cu(Ⅱ)ion is best described as a distorted tetragonal pyramid rather than a trigonal biyramid.The equatorial plane is made up of O1,O2,N1 and O2 atoms,while N4i(Symmetry codes:i2.5-x,2-y,0.5+z)atom occupy the axial position.In addition,the distances of Cu-N/Obonds were in the range of 0.194 5(7)~0.229 1(7)nm, comparable with those in some reported complexes with similar donor set[3].In the crystal of 1,intermolecular O-H…O hydrogen bonds(O4-H4…O3vii, with D…A distance being 0.282 6(15)nm,D-H…A angle being 173.8°,Symmetry codes:vii3-x,0.5+y, 0.5-z)between the lattice methanol and acetate anion are also present.By contrast,the coordination geometry of Zn(Ⅱ)ion in 2 is a distorted trigonal bipyramid with the geometric index τ being 0.715. The structure of complex 2 has similar chain-like framework(Fig.1d)as that of complex 1,but possesses none classical hydrogen bonds as expected.
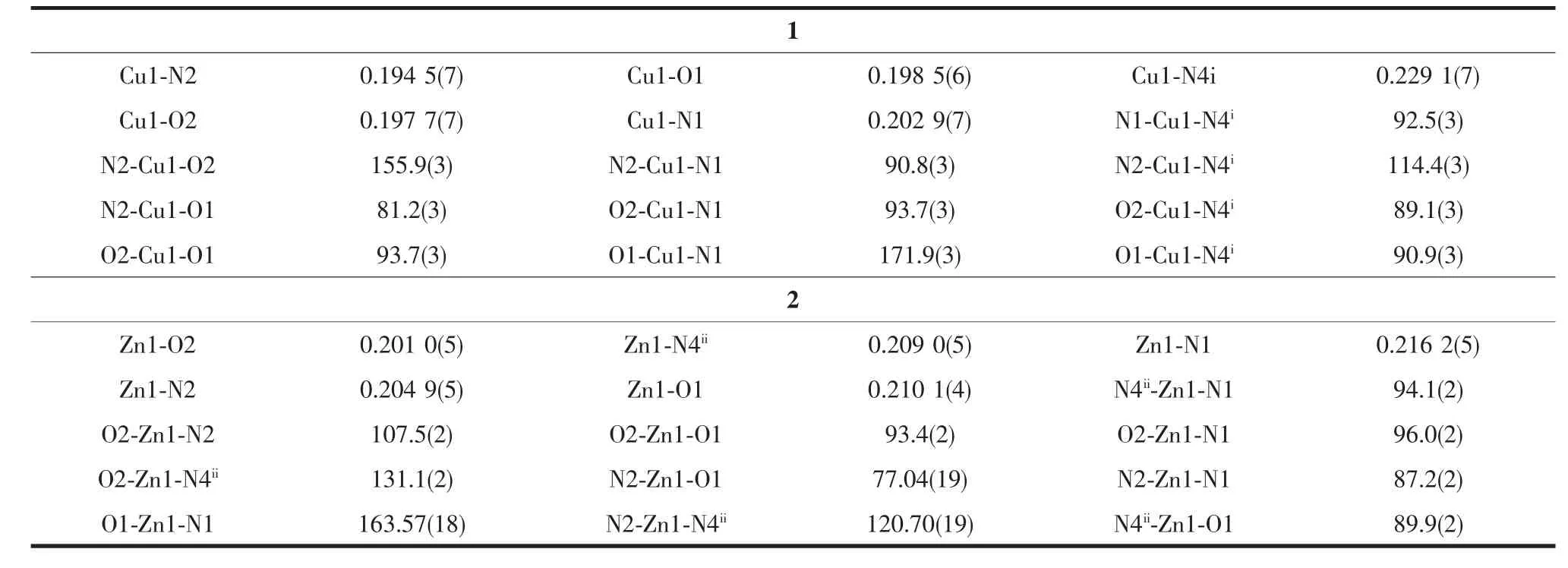
Table2 Selected bond lengths(nm)and angles(°)in the complexes 1 and 2
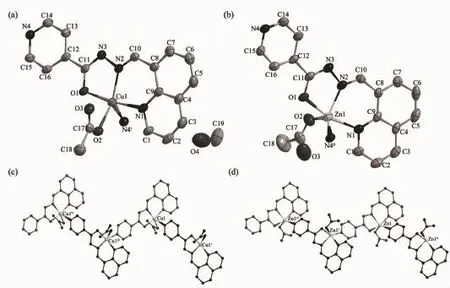
Fig.1 ORTEP drawing of complexes 1(a)and 2(b)with 30%thermal ellipsoids;Chain-like structure along c axis in complexes 1(c)and 2(d)
2.2IR spectra
The FTIR spectral region for both complexes is more or less similar due to the similar coordination modes of the ligands.The ν(C=O)of the free ligand is 1 681 cm-1,it is disappeared in the spectra of complexes.Meanwhile,new(N=C-O)stretchingvibration absorption is observed at 1 623 and 1 612 cm-1in complexes 1 and 2,respectively,revealing that the C=O in O=C-N moiety has enolizated and the oxygen atom coordinates to the metal ion in both complexes[3,7].The ν(C=N)bands of the imine group, quinoline and pyridine ring in the hydrazone ligand shift to lower frequency values in the complexes, indicating that the N atoms of such three units take part in the coordination[7].In addition,the general pattern of the IR spectroscopy for complexes 1 and 2 supports the normal coordination of monodentate acetate group[11].It is in accordance with the crystal structure study.
2.3UV spectra
The UV spectra of the ligand HL,complexes 1 and 2 in methanol solution(concentration:1×10-5mol·L-1) were measured at room temperature(Fig.2).The spectra of L features one main band located around 327(ε= 33 444 L·mol-1·cm-1),which could be assigned to characteristic π-π*transition of hydrazone unit[2]. However,it shifts to 375 nm in the spectra of 1(ε= 29 135 L·mol-1·cm-1)and 2(ε=21 787 L·mol-1·cm-1), probably due to the extended conjugation effect of the anionic ligand in the complexes.
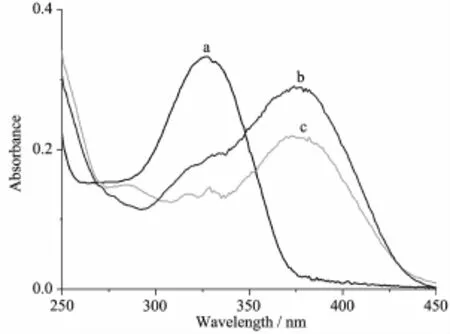
Fig.2 UV spectra of the ligand HL(a),1(b),and 2(c)in the methanol solution at room temperature
2.4Fluorescence spectra
The fluorescence spectra of the ligand HL, complexes 1 and 2 have been studied in methanol solution(concentration:1×10-5mol·L-1)at room temperature.As shown in Fig.3,the emission spectra of the ligand HL and complex 1 exhibit almost none emission peak in the visible range when excited at 340 nm.By contrast,complex 2 shows a remarkable broad peak at about 510 nm(λex=360 nm),primarily due to the CHEF(chelation enhancement of the fluorescence emission)effect of the Zn(Ⅱ)ion[7].
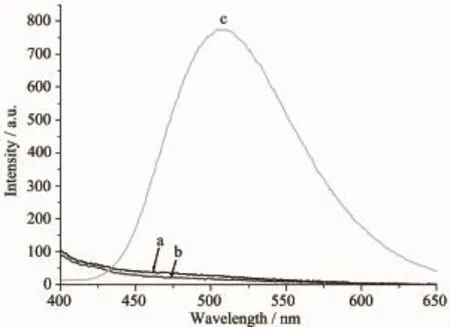
Fig.3 Fluorescence emission spectra of the ligand HL (a),1(b)and 2(c)in the methanol solution at room temperature
References:
[1]LI Xiao-Jing(李曉靜),WU Wei-Na(吳偉娜),XU Zhou-Qing (徐周慶),et al.Chinese J.Inorg.Chem.(無機化學學報), 2015,31:2265-2271
[2]MAO Pan-Dong(毛盼東),YAN Ling-Ling(閆玲玲),WANG Wen-Jing(王文靜),et al.Chinese J.Inorg.Chem.(無機化學學報),2016,32:555-560
[3]Bourosh P N,Revenko M D,Stratulat E F,et al.Russ.J. Inorg.Chem.,2009,54:530-538
[4]Bourosh P N,Revenko M D,Stratulat E F,et al.Russ.J. Inorg.Chem.,2014,59:545-557
[5]Revenko M D,Bourosh P N,Stratulat E F,et al.Russ.J. Inorg.Chem.,2010,55:1387-1397
[6]Chen X,Pradhan T,Wang F,et al.Chem.Rev.,2012,112: 1910-1956
[7]LI Xiao-Jing(李曉靜),CAI Hong-Xin(蔡紅新),WU Wei-Na (吳偉娜),et al.Chinese J.Inorg.Chem.(無機化學學報), 2015,31:1661-1666
[8]Sheldrick G M.SADABS,University of G?ttingen,Germany, 1996.
[9]Sheldrick G M.SHEL X-97,Program for the Solution and the Refinement of Crystal Structures,University of G?ttingen, Germany,1997.
[10]Addison A W,Rao T N.J.Chem.Soc.,Dalton Trans.,1984, 1349-1356
[11]Yang Z Y,Wang Y,Wang Y.Bioorg.Med.Chem.Lett., 2007,17:2096-2101
Cu(Ⅱ)and Zn(Ⅱ)Complexes with an Acylhydrazone Ligand Bearing Quinoline Unit:Crystal Structures and Fluorescence Property
CHANG Hui-Qin1YUAN Zhi-Ze2LAI Xiao-Qing3WU Wei-Na*,1WANG Yuan*,1
(1College of Chemistry and Chemical Engineering,Henan Polytechnic University,Jiaozuo Henan 454000,China)
(2Basic education and Research Office,Jiaozuo Education Bureau,Jiaozuo,Henan 454000,China)
(3CNPC Drilling Research Institute,Beijing 102206,China)
Two complexes,{[Cu(L)(OAc)]·MeOH}n(1)and[Zn(L)(OAc)]n(2)(HL is the acylhydrazone ligand derived from 8-formylquinoline and isonicotinohydrazide)have been synthesized and characterized by single-crystal X-ray diffraction,elemental analysis and IR spectroscopy.X-ray diffraction analysis results show that the structures of both complexes are quite similar.The metal ion in each complex is five-coordinated,involving one monodentate acetate anion,NO2donor set of an enolizated ligand L-and one pyridine nitrogen atom from another adjacent acylhydrazone ligand,thus forming one dimension chain-like framework.The coordination geometry of the centre ion is a distorted tetragonal pyramid in 1,whilst a trigonal bipyramid in 2.When excited at 360 nm,complex 2 exhibits strong emission at about 510 nm in methanol solution,primarily due to the CHEF effect of the Zn(Ⅱ)ion. CCDC:1414665,1;1489193,2.
quinoline;hydrazone;Cu(Ⅱ)complex;Zn(Ⅱ)complex;fluorescence;crystal structure
O614.121;O614.24+1
A
1001-4861(2016)11-2058-05
10.11862/CJIC.2016.240
2016-07-01。收修改稿日期:2016-09-12。
國家自然科學基金(No.21001040,21404033,21401046)、河南省科技廳基礎與前沿項目(No.162300410011)和河南省教育廳自然科學基金(No.12B150011,14B150029)資助。
*通信聯系人。E-mail:wuwn08@hpu.edu.cn;wangyuan08@hpu.edu.cn

