多不飽和脂肪酸對神經(jīng)細胞保護作用的研究進展
劉志國,王華林,王麗梅,劉烈炬
(武漢輕工大學(xué)生物與制藥工程學(xué)院,湖北 武漢 430023)
多不飽和脂肪酸對神經(jīng)細胞保護作用的研究進展
劉志國,王華林,王麗梅,劉烈炬*
(武漢輕工大學(xué)生物與制藥工程學(xué)院,湖北 武漢 430023)
本文從細胞生物學(xué)的角度綜述多不飽和脂肪酸(polyunsaturated fatty acids,PUFAs)改善大腦功能的作用機理,包括PUFAs促進神經(jīng)細胞生成(neurogenesis)、維持神經(jīng)細胞形態(tài)和功能、促進神經(jīng)突生長、防止神經(jīng)細胞變性(neurodegeneration)、抑制神經(jīng)細胞凋亡(apoptosis),以及調(diào)節(jié)神經(jīng)細胞膜流動性和可塑性(plasticity)、端粒(telomere)活性等作用機制,為PUFAs的營養(yǎng)干預(yù)研究應(yīng)用,特別是應(yīng)用于保護大腦正常功能,防止生理性衰老和疾病性腦功能障礙(如阿爾茲海默氏癥和帕金森癥)所致的腦功能障礙提供參考。
多不飽和脂肪酸;神經(jīng)變性;神經(jīng)細胞;神經(jīng)發(fā)生;大腦功能
神經(jīng)細胞(也稱神經(jīng)元)是腦組織的主要功能細胞,其正常的生長分化及功能健全是維持大腦功能的基礎(chǔ)。多不飽和脂肪酸(polyunsaturated fatty acids,PUFAs)是一類在腦組織中含量豐富的脂肪酸,對維護神經(jīng)細胞的正常結(jié)構(gòu)和功能發(fā)揮著至關(guān)重要的作用[1-4],尤其n-3 PUFAs具有促進神經(jīng)細胞增殖、維持神經(jīng)細胞正常形態(tài)及促進神經(jīng)突觸生長、防止神經(jīng)細胞變性與凋亡等功能作用,對促進胎兒和嬰幼兒大腦的正常生長發(fā)育,防止老年期大腦功能的衰退具有重要作用[5-6]。本課題組前期探討了PUFAs對不同齡人群學(xué)習(xí)記憶功能的影響和對大腦功能的保護作用。同時,近年來國內(nèi)外也大量開展PUFAs增強大腦學(xué)習(xí)記憶等大腦功能的細胞學(xué)機制研究,取得了一些重要的進展,包括PUFAs對大腦神經(jīng)細胞的形態(tài)和亞細胞結(jié)構(gòu)影響,對神經(jīng)細胞生成和神經(jīng)細胞變性及細胞凋亡的影響,以及對神經(jīng)細胞膜流動性、可塑性、端粒活性的調(diào)節(jié)作用等方面[3-5]。因此,細胞學(xué)機制研究已成為PUFAs增強大腦功能研究的重要內(nèi)容,并為進一步闡明PUFAs的營養(yǎng)干預(yù)機制,拓寬其應(yīng)用領(lǐng)域提供了理論依據(jù)和重要參考。
1 PUFAs促進神經(jīng)細胞生成
神經(jīng)細胞生成(或神經(jīng)發(fā)生)是神經(jīng)干細胞增殖、分裂成為定向祖細胞,并逐漸定向遷移、分化成為功能神經(jīng)細胞,構(gòu)成神經(jīng)系統(tǒng)的過程。腦組織中,通過神經(jīng)細胞生成作用,可增加大腦神經(jīng)元的數(shù)量,維護大腦的正常功能,而神經(jīng)細胞的生成與神經(jīng)干細胞(neural stem cell,NSC)的增殖、分化及遷移過程密不可分。神經(jīng)干細胞首先從成年小鼠側(cè)腦室膜下區(qū)分離獲得,隨后陸續(xù)在中樞神經(jīng)系統(tǒng)的多個部位(包括:大腦海馬區(qū)、大腦皮層和紋狀體等)分離獲得。例如,與大腦空間記憶功能有關(guān)的海馬神經(jīng)細胞,是由位于海馬顆粒細胞層和齒狀回門之間顆粒下層的海馬神經(jīng)干細胞經(jīng)歷增殖、分化,遷移至海馬CA1和CA3區(qū)形成[7]。盡管觀察到大腦的神經(jīng)生成能力可以持續(xù)到老年階段,但中年以后其生成能力急劇下降[8]。大量體內(nèi)和體外實驗均表明PUFAs可促進神經(jīng)細胞的生成,并能顯著延緩神經(jīng)細胞生成能力的衰退。例如,動物胚胎神經(jīng)發(fā)育期補充n-3 PUFAs可促進神經(jīng)細胞的生成,增加神經(jīng)干細胞的增殖、分化成熟,但是n-6 PUFAs無此作用[9-12]。Fat1轉(zhuǎn)基因小鼠(可將n-6 PUFAs轉(zhuǎn)化為n-3 PUFAs的轉(zhuǎn)基因鼠),由于二十二碳六烯酸(docosahexaenoic acid,DHA)合成的大量增加,增加了神經(jīng)細胞生成的能力,并伴隨實驗動物學(xué)習(xí)記憶能力的增強[13]。而且也發(fā)現(xiàn),即使是老年實驗動物,DHA仍具有促進神經(jīng)干細胞分化的功能[14],可通過促進神經(jīng)干細胞的增殖和成熟,對老年大腦功能發(fā)揮保護作用。盡管在動物實驗中觀察到n-3和n-6 PUFAs在保護大腦功能方面的顯著差異,但體外細胞培養(yǎng)實驗顯示:n-3 PUFAs中的DHA或n-6 PUFAs中的花生四烯酸(arachidonic acid,AA)都可以促進神經(jīng)干細胞的增殖[11,15]。這種體內(nèi)外作用的差異提示體內(nèi)微環(huán)境也是影響神經(jīng)細胞生成的重要因素。而且也有實驗觀察到n-3 PUFAs缺乏的大鼠,腦源性神經(jīng)營養(yǎng)因子(brain-drived neurotrophic factor,BDNF)合成減少,神經(jīng)細胞生成受到抑制的現(xiàn)象[16]。到目前為止,PUFAs影響神經(jīng)細胞生成的機制并不十分清楚,綜合來看,其機制主要涉及:1)神經(jīng)干細胞的磷脂脂肪酸組成及其影響的細胞膜功能和膜蛋白定位是重要影響因素[17];2)n-3 PUFAs及其活性衍生物通過相應(yīng)的信號轉(zhuǎn)導(dǎo)系統(tǒng)、膜相關(guān)途徑、核受體途徑等影響神經(jīng)細胞的功能[18]。
2 PUFAs促進神經(jīng)突生長
PUFAs可以影響神經(jīng)細胞的形態(tài),如增加細胞體積、促進突觸的生長等[19]。神經(jīng)細胞體積的增加,常伴隨著神經(jīng)原纖維合成的增加以及神經(jīng)元功能的改變,還通常伴隨著神經(jīng)末梢和突觸結(jié)數(shù)量的增加[19-20]。神經(jīng)元核周質(zhì)的體積與神經(jīng)末梢密度,與大腦學(xué)習(xí)記憶功能之間表現(xiàn)為明顯的正相關(guān)的關(guān)系[21]。n-3 PUFAs作為神經(jīng)細胞膜的重要組成部分,可對細胞膜形成某種外凸壓力,促進神經(jīng)細胞樹突棘形成,有助于神經(jīng)突起的生長和神經(jīng)突觸的生成[22]。神經(jīng)元形態(tài)變化,包括體積大小、神經(jīng)末梢分布以及突觸數(shù)量的改變是影響大腦神經(jīng)回路的關(guān)鍵因素,與大腦功能密切相關(guān)。如海馬神經(jīng)回路與大腦的學(xué)習(xí)記憶功能有著密切的聯(lián)系[23]。研究顯示,n-3 PUFAs中DHA的缺乏可導(dǎo)致海馬CA1區(qū)神經(jīng)元的萎縮,補充ɑ-亞麻酸(ɑ-linolenic acid,ALA)和DHA后,海馬CA1和CA3神經(jīng)元的體積增加[24]。其機制涉及蛋白激酶C(protein kinase C,PKC)的活化和海馬突觸小泡蛋白的表達增加[25-26]。
由于神經(jīng)突起和神經(jīng)細胞體積的變化往往聯(lián)系在一起,因此,當(dāng)神經(jīng)突生長時,總是首先表現(xiàn)為細胞體積和膜表面積的增加[27]。反過來也是這樣,當(dāng)海馬和下丘腦神經(jīng)元體積減小的同時,可以觀察到神經(jīng)元樹突分支的明顯減少[24]。研究表明DHA具有促進海馬組織和大腦皮質(zhì)神經(jīng)元神經(jīng)突生長的作用[28]。在胚胎發(fā)育期,n-3 PUFAs的缺乏可導(dǎo)致神經(jīng)細胞突起的發(fā)育不良(突起少而短),而補充DHA可使細胞的突起的長度增加、數(shù)量增多,并伴隨著突觸蛋白含量的增加[29];但n-6 PUFAs的AA在體內(nèi)沒有促進神經(jīng)突生長的作用[30]。然而在體外培養(yǎng)細胞中觀察到,不管是n-6 PUFAs的亞油酸(linoleic acid,LA)、AA,還是n-3 PUFAs的ALA和DHA等,都可以促進神經(jīng)突生長,只是飽和脂肪酸和單不飽和脂肪酸則無此作用[31-32]。這同樣提示,PUFAs對神經(jīng)突起和神經(jīng)細胞體積的影響與體內(nèi)微環(huán)境及代謝機制密切相關(guān)。有研究顯示DHA可能通過其代謝物N-二十二碳六烯乙醇胺(N-docosahexaenoylethanolamide,DEA)發(fā)揮作用,并通過G-蛋白受體促進神經(jīng)干細胞分化和神經(jīng)突起生長[33-34]。另外,PUFAs可能作用于神經(jīng)生長因子(nerve growth factor,NGF)[35],從而促進神經(jīng)細胞生長發(fā)育[24,36]。大腦n-3 PUFAs的缺乏,可導(dǎo)致海馬組織NGF含量顯著的下降。在NGF的刺激下,通過磷脂酶A2(phospholipase A2,PLA2)的作用,可導(dǎo)致AA和DHA從細胞膜的釋放。被釋放的AA和DHA可與突觸融合蛋白3結(jié)合,在可溶性N-乙酰基馬來酰亞胺敏感因子結(jié)合蛋白受體(soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor,SNAREs)的協(xié)助下,導(dǎo)致膜磷脂雙層結(jié)構(gòu)的融合,促進神經(jīng)突的生長。
雖然單獨補充DHA可增加神經(jīng)元樹突棘和突觸的數(shù)量[37]。但實驗觀察到DHA與尿苷、膽堿聯(lián)合干預(yù)具有明顯的協(xié)同效應(yīng),可顯著增加DHA對神經(jīng)細胞生長分化的作用[38]。由于尿苷、膽堿和PUFAs是合成磷脂的主要成分。因此,三者的合并使用可進一步促進大腦磷脂,以及多種突觸蛋白的合成。實驗顯示:在沙鼠的飼料中進行尿苷、膽堿和DHA的聯(lián)合干預(yù),可使突觸小泡突觸蛋白-1(synapsin-1)含量增加41%,突觸后蛋白質(zhì)psd-95增加38%,突觸的神經(jīng)纖維蛋白-M(neurofibrillar protein-M,NF-M)和神經(jīng)纖維蛋白-70(neurofibrillar protein-70,NF-70)分別增加48%和102%[38]。上述結(jié)果表明:尿苷、膽堿、AA和DHA的聯(lián)合干預(yù)是增加PUFAs功能的有效途徑。尿苷的食物來源常見的有:番茄和西蘭花。富含膽堿的食物包括:雞蛋、鱈魚、小麥胚芽、花椰菜、菠菜、藜麥等。母乳含有豐富的尿苷、膽堿、AA和DHA等成分[39],是嬰兒智力發(fā)育最好的食物來源。
3 PUFAs防止神經(jīng)變性
神經(jīng)變性(neurodegeneration)和壞死(necrosis)是造成神經(jīng)細胞數(shù)量減少的重要原因之一。PUFAs可通過抗神經(jīng)變性的作用,防止神經(jīng)細胞數(shù)量減少,維護正常的大腦學(xué)習(xí)記憶功能。影響神經(jīng)變性的因素眾多,主要包括常見的環(huán)境因素和機體自身的生理、病理性因素。前者包括化學(xué)(如酒精和環(huán)境污染)、物理(機械損傷、電離輻射和噪音等)、生物(病毒和細菌感染等)等因素;后者主要有衰老、精神因素(緊張和抑郁等)和神經(jīng)變性疾病或稱神經(jīng)退行性疾病等。在防范有害的環(huán)境因素基礎(chǔ)上,營養(yǎng)干預(yù)延緩生理或病理性神經(jīng)變性損傷對維護大腦功能尤為重要。大量研究表明[2-3,40-43]:PUFAs,特別是長鏈(long chain,LC)-PUFAs,在防止神經(jīng)細胞變性的過程中,發(fā)揮著重要的作用。
3.1 PUFAs防止衰老導(dǎo)致的神經(jīng)細胞變性
在生理特征上,衰老表現(xiàn)為感官、運動和認知功能的下降,是導(dǎo)致神經(jīng)變性重要生理因素[44]。隨著機體的衰老,血漿中n-3 PUFAs的含量逐漸下降,n-3 PUFAs含量的下降反過來又進一步促進機體的衰老[41]。研究發(fā)現(xiàn)大腦中的總脂質(zhì)的含量,特別是DHA的含量,與大腦的功能和神經(jīng)變性密切相關(guān)。在生命的開始20 a里,大腦脂質(zhì)的含量呈逐漸增加的趨勢,隨后保持基本的穩(wěn)定,老年后脂質(zhì)的含量快速下降[45]。衰老造成大腦功能損傷有兩個常見的原因:一是神經(jīng)細胞的喪失(包括神經(jīng)細胞的凋亡和壞死);二是神經(jīng)細胞結(jié)構(gòu)和功能的變化,即神經(jīng)變性。研究表明[46-47],在大腦衰老過程中實際死亡神經(jīng)細胞的數(shù)量并不多,造成大腦功能衰退的主要原因是由于年齡增長導(dǎo)致的神經(jīng)細胞結(jié)構(gòu)和功能的改變,表現(xiàn)為神經(jīng)遞質(zhì)釋放的減少和傳遞功能的下降等。而腦內(nèi)氧化物的增加,細胞解毒功能的下降是導(dǎo)致神經(jīng)變性的重要原因[48]。PUFAs則可以保護和防止這一過程,通過增加機體抗氧化能力,阻止活性氧(reactive oxygen species,ROS)的增加,促進樹突重塑,防止大腦興奮性中毒所致的大腦功能紊亂[45],從而對抗神經(jīng)變性,增加大腦學(xué)習(xí)記憶的能力[49-50]。進一步研究發(fā)現(xiàn)[51-52]:食物中的二十碳五烯酸(eicosapentaenoic acid,EPA)和DHA可以緩解衰老過程中大腦中PUFAs含量的下降,提高年齡相關(guān)的谷氨酸受體中的N-甲基-D-天冬氨酸受體2B(N-methyl-D-aspartate receptor 2B,NR2B)亞單位和α-氨基-3-羧基-5-甲基異唑-4-丙酸(alpha-amino-3-hydoxy-5-methyl-4-isoxazolepropionate,AMPA)受體的谷氨酸受體2(glutamate receptor 2,GluR2)亞單位的表達,并增加大腦的學(xué)習(xí)記憶功能。這些受體在神經(jīng)變性的過程中起著重要的作用,除直接參與突觸的傳遞功能外,GluR2還在促進樹突增長和維持樹突棘穩(wěn)定中扮演著重要的角色[53]。
3.2 PUFAs改善細胞膜的功能,防止神經(jīng)變性
突觸的可塑性被廣泛認為是大腦功能最重要的細胞結(jié)構(gòu)基礎(chǔ)[45]。許多研究探討了與年齡相關(guān)的突觸可塑性變化和大腦認知功能障礙之間的相關(guān)性,研究發(fā)現(xiàn)影響記憶形成的主要因素可能是突觸可塑性的變化,而影響記憶喪失(遺忘)的主要因素是突觸功能衰退[54-55]。年齡的增長可導(dǎo)致記憶形成的閾值增加,記憶喪失的閾值減小[56]。AA和DHA是神經(jīng)細胞質(zhì)膜(包括細胞膜、線粒體膜、蘘泡膜等)的重要組成部分,在突觸部位其含量可達摩爾水平[18]。AA和DHA在神經(jīng)細胞質(zhì)膜水平的改變可直接影響到細胞膜的特性(包括膜的流動性、曲率和脂質(zhì)筏的形成等),進一步影響鑲嵌在脂質(zhì)雙層中蛋白質(zhì)的功能(包括受體、通道和酶類等),因此PUFAs在維持突觸可塑性中發(fā)揮了關(guān)鍵的作用。食物中的PUFAs,有利于緩解由衰老導(dǎo)致的大腦AA和DHA水平的下降,對抗由衰老導(dǎo)致的神經(jīng)細胞結(jié)構(gòu)和功能的變化,增強大腦學(xué)習(xí)記憶的功能[57]。
膜的流動性受多方面因素的影響。飲食習(xí)慣可以直接影響神經(jīng)元膜脂肪酸組成。實驗證明通過補充PUFAs可以改善膜流動性,進而影響突觸的可塑性,神經(jīng)傳遞和突觸生成,增強學(xué)習(xí)記憶的能力[55,57]。膜流動性也受到年齡和神經(jīng)變性疾病的影響,如阿爾茨海默病(Alzheimer’s disease,AD)和帕金森病(Parknson’s disease,PD)。影響膜流動性的其他原因包括:膽固醇和活性氧含量的增加[58]。活性氧可導(dǎo)致膜蛋白和脂質(zhì)化學(xué)交聯(lián),減少細胞膜的不飽和性。這除了可降低膜流動性外,還可導(dǎo)致膜中酶、離子通道和受體的抑制[59]。增加PUFAs的攝入量可以部分對抗ROS的作用,但大劑量的使用仍然值得注意[58]。n-3 PUFAs結(jié)合入神經(jīng)膜,可降低細胞膜膽固醇的含量、改善神經(jīng)細胞膜的特性,例如流動性、滲透性和黏度等,同時改善神經(jīng)傳遞、突觸可塑性、學(xué)習(xí)記憶及其他復(fù)雜的認知過程[2,6,40]。
PUFAs影響膜流動性的機制之一,是通過抑制脂質(zhì)筏(膜質(zhì)雙層含中由特殊的脂質(zhì)和蛋白質(zhì)組成的微區(qū),該區(qū)域結(jié)構(gòu)致密,屬抗去垢劑的膜組分[60],富含膽固醇、鞘磷脂、糖脂,是蛋白質(zhì)停泊的平臺,與膜的信號轉(zhuǎn)導(dǎo)、蛋白質(zhì)的分選有關(guān)系密切)的形成和跨膜受體蛋白的定位和表達來完成的。脂質(zhì)雙層的磷脂中DHA的含量增加,導(dǎo)致脂質(zhì)雙層更加松散,更多的流動性和可壓縮性以及更強的滲透性。此外DHA還具有排斥膽固醇的作用。DHA取代膜內(nèi)的飽和脂肪酸后,造成該區(qū)域內(nèi)膽固醇的濃度降低,從而干擾脂筏的形成[61]。實驗證明膳食中補充ALA并不能改變膜磷脂分布,然而補充DHA可以明顯提高細胞膜磷脂的水平[62]。雖然DHA可迅速結(jié)合入脂質(zhì)雙層結(jié)構(gòu)中的磷脂,但在各類磷脂之間分布是不均勻的。DHA優(yōu)先結(jié)合入sn-2位置磷脂酰乙醇胺(phosphatidylethanolamine,PE)以及少量的磷脂酰膽堿(phosphatidyl choline,PC)或磷脂酰絲氨酸(phosphatidylserine,PS)[63-65]。在大腦,DHA結(jié)合在磷脂酰絲氨酸磷脂中可促進信號轉(zhuǎn)導(dǎo)分子蛋白激酶B(protein kinase B,PKB)(也稱AKT)向細胞核的易位,調(diào)節(jié)凋亡基因的轉(zhuǎn)錄[66]。另外,PUFAs還可調(diào)節(jié)細胞膜的硬度,導(dǎo)致巨噬細胞黏度增加和對凋亡細胞的吞噬作用的下降[62,67]。
3.3 PUFAs調(diào)節(jié)端粒的長度,延緩大腦功能衰退
神經(jīng)細胞的衰老死亡可導(dǎo)致神經(jīng)元的數(shù)量減少,影響大腦的功能。關(guān)于細胞的衰老目前有多種學(xué)說,最重要是內(nèi)置時鐘說、氧化應(yīng)激說和端粒學(xué)說[45]。端粒學(xué)說提出細胞的衰老源于端粒縮短。端粒是真核細胞染色體兩臂末端由5′TTAGGG-3′重復(fù)序列構(gòu)成的結(jié)構(gòu),具有保護染色體末端,維持染色體結(jié)構(gòu)的穩(wěn)定和完整、避免其發(fā)生融合、降解、重組等功能。端粒的長度與衰老、認知能力(包括學(xué)習(xí)記憶能力)、以及老年性疾病的發(fā)生(如癡呆和神經(jīng)退行性疾病)有著密切的關(guān)系[68-69],并受多種因素的影響,如:炎性反應(yīng)和氧化應(yīng)激反應(yīng)等[70-72]。體育鍛煉、他汀類藥物(用于治療高膽固醇血癥)、補充n-3 PUFAs等可增加端粒的長度,延緩衰老,其機制可能與端粒酶的活性變化有關(guān)[73]。
端粒的長度受到飲食中脂肪酸組成的影響。最近一項研究將實驗分為飽和脂肪酸組、低脂高糖組和地中海飲食組(富含單不飽和脂肪酸)三組,通過隨機交叉分析發(fā)現(xiàn)地中海飲食組細胞內(nèi)活性氧的水平、細胞凋亡數(shù)和端粒的縮短都明顯低于脂肪酸組和低脂高糖組[71]。PUFAs對端粒的長度也有影響,且發(fā)現(xiàn)n-6和n-3 PUFAs對端粒的作用是不同的。n-3 PUFAs可延緩端粒縮短,而n-6 PUFAs可能會加速端粒的縮短,因此在實驗中應(yīng)區(qū)分n-3和n-6脂肪酸的作用[74]。最近采用前瞻性隊列研究對冠狀動脈疾病的患者追蹤調(diào)查后發(fā)現(xiàn),血液中n-3 PUFAs的含量與端粒縮短的速率成負相關(guān)的關(guān)系[75]。O’Callaghan等[68]在對輕度認知障礙(mild cognitive impairment,MCI)老年人補充n-3 PUFAs時發(fā)現(xiàn),雖然干預(yù)與治療組端粒長度沒有顯著的變化,但血液中的紅細胞DHA的水平與端粒縮短密切相關(guān),即紅細胞DHA含量增加端粒縮短的速率降低,提示n-3 PUFAs可能延緩端粒的縮短,且n-3脂肪酸對端粒的影響與n-6/n-3的比例有關(guān)。Kiecolt-glaser等[72]研究采用隨機對照實驗探討了n-3 PUFAs的干預(yù),與白細胞端粒長度、促炎細胞因子和氧化應(yīng)激的關(guān)系,發(fā)現(xiàn)n-3 PUFAs可降低血液中促炎細胞因子的濃度和氧化應(yīng)激的水平,降低n-6/n-3 PUFAs的比值可導(dǎo)致端粒長度增加,提示飲食中n-6/n-3 PUFAs比值對延緩大腦功能衰退的重要作用,表明n-3 PUFAs與n-6 PUFAs對炎性應(yīng)激和氧化應(yīng)激存在作用差異,從而對端粒長度的影響不一致。雖然PUFAs對端粒的作用以及與大腦功能的關(guān)系目前仍缺乏足夠的資料,但為PUFAs的功能作用與機理研究提供了新的方向[76]。
3.4 PUFAs干預(yù)疾病所致的神經(jīng)變性
神經(jīng)變性疾病是影響神經(jīng)變性的重要的病理因素。神經(jīng)變性疾病是一組原因不明的,以神經(jīng)細胞變性為主要的病理特征的中樞神經(jīng)系統(tǒng)疾病,典型的如AD和PD[41]。
AD是以進行性癡呆為主要臨床表現(xiàn)的大腦變性疾病,俗稱老年癡呆癥。研究發(fā)現(xiàn)AD的發(fā)病與腦內(nèi)β淀粉樣蛋白(Aβ)異常沉淀有關(guān)。Aβ的前體是前β淀粉樣蛋白,由于三級結(jié)構(gòu)中的β折疊,形成了不可溶的特性。研究發(fā)現(xiàn)Aβ對周圍的突觸和神經(jīng)元具有毒性作用,可導(dǎo)致神經(jīng)細胞變性死亡[77]。隨著神經(jīng)元的變性死亡,導(dǎo)致腦內(nèi)相應(yīng)的神經(jīng)遞質(zhì)水平下降,其中最重要的是乙酰膽堿。由于AD患者中腦內(nèi)的乙酰膽堿的水平下降得最早和最為明顯,因此產(chǎn)生了AD的膽堿能學(xué)說:即AD患者乙酰膽堿的缺乏是導(dǎo)致認知功能障礙的主要因素。增加腦內(nèi)乙酰膽堿的水平是目前AD藥物治療的重要手段。例如,采用膽堿酯酶抑制劑,通過抑制乙酰膽堿的酶解,增加膽堿能神經(jīng)元的傳遞功能。但這些擬膽堿類藥物,雖然能緩解患者認知能力的下降,但并不能延緩AD的病程的發(fā)展。
研究表明[78-80],AD的發(fā)病與機體能量代謝失衡有關(guān),且氧化應(yīng)激和線粒體功能障礙在AD的發(fā)病機制中扮演著重要的角色,是藥物治療AD新的潛在的靶標(biāo)。線粒體功能障礙是導(dǎo)致β樣淀粉樣變的重要病因[81-84]。DHA可抑制前淀粉樣蛋白轉(zhuǎn)化為Aβ,并阻止Aβ的累積[85-86]。DHA可對抗Aβ引起的神經(jīng)毒性反應(yīng),對大腦產(chǎn)生保護作用[87]。在人胚腎-淀粉酶前體蛋白(human embryonic kidney-amyloid precursor protein,HEK-APP)細胞,DHA可顯著提高膜流動性、增加可溶性淀粉酶前體蛋白α(soluble amyloid precursor protein α,sAPPα)的分泌。后者具有對線粒體的保護作用以及抗凋亡的功能[88]。動物實驗表明[86,89-90]:富含DHA的飲食可以減少老年癡呆癥小鼠模型中Aβ的含量。對老年3hTg-AD小鼠(大腦中含有大量Aβ的模型鼠)補充DHA,可導(dǎo)致膜磷脂內(nèi)DHA水平增加、AA含量降低以及心磷脂水平增加;并明顯改善大腦的認知能力和內(nèi)嗅皮層神經(jīng)元的功能障礙[91-92]。大量研究顯示[85],減少n-3 PUFAs的攝入量或魚類食品的消費,可增加與年齡相關(guān)的認知能力的下降或患呆癡癥的風(fēng)險。
PD又稱為振顫麻痹癥,是一種與年齡相關(guān)的慢性和進行性的神經(jīng)變性疾病。主要的臨床特征是運動障礙,伴隨著大腦認知能力的下降和精神癥狀,病理特征為大腦黑質(zhì)致密部(substantia nigra pars compacta,SNPC)多巴胺能神經(jīng)元的廣泛的變性死亡[93]。在尸檢報告中,大腦SNPC勻漿DHA水平明顯下降[94]。尸檢結(jié)果還表明PD患者額葉皮層脂質(zhì)筏的分離體中DHA和AA的含量大幅下降[95]。氧化應(yīng)激是PD重要的發(fā)病機制。在PD腦內(nèi)發(fā)現(xiàn),脂質(zhì)過氧化物水平,黑質(zhì)和紋狀體中8-OHdG(8-羥脫氧鳥苷,DNA氧化損傷的標(biāo)志物)的含量以及亞硝酰基和蛋白質(zhì)碳酰基水平均明顯的增加。進一步可導(dǎo)致線粒體功能障礙,誘導(dǎo)細胞變性和凋亡,最終導(dǎo)致多巴胺能神經(jīng)元的死亡。
攝入DHA膠囊可選擇性地增加小鼠額葉皮層DHA的水平,阻斷由1-甲基-4-苯基吡啶離子(1-methyl-4-phenyl-pyridinium,MPP+)導(dǎo)致黑質(zhì)細胞數(shù)量的下降以及核受體相關(guān)因子1(nuclear receptor-related factor 1,Nurr1)mRNA和多巴胺轉(zhuǎn)運體mRNA水平的下降[96]。Nurr1是多巴胺能神經(jīng)元重要轉(zhuǎn)錄因子[97]。飲食中的DHA可對抗由MPP+導(dǎo)致的紋狀體多巴胺及其代謝物二羥基苯乙酸(dihydroxyphenylacetic acid)含量的下降[96]。在MPP+誘導(dǎo)的PD模型中,DHA治療可緩解PD部分神經(jīng)癥狀[98-99]。采用MPP+PD模型,實驗探討了LC-PUFAs對左旋多巴(levodopa,LD)誘導(dǎo)運動障礙的影響,DHA可對抗LD誘導(dǎo)運動障礙或延遲運動障礙的發(fā)展[100]。在1-甲基-4-苯基-1,2,3,6-四氫吡啶(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine,MPTP)-丙磺舒(probenecid)PD小鼠的模型中,飲食補充乙基-EPA后,可預(yù)防運動功能減退和記憶力的改善,但不能阻止黑質(zhì)紋狀體多巴胺的下降[101]。在小鼠和大鼠PD模型中,腦室注射6-羥多巴胺(6-hydroxydopamine,6-OHDA)可引起紋狀體病變,包括多巴胺的水平下降[102-103]。口服DHA可對抗6-OHDA的作用,部分恢復(fù)多巴胺能神經(jīng)傳遞功能[103]。老鼠腹腔內(nèi)注射乙基-DNA,也可對抗6-OHDA導(dǎo)致小鼠紋狀體多巴胺的水平下降[102]。大量文獻均表明高n-3 LC-PUFAs的飲食對預(yù)防或緩解PD具有顯著作用[96,98,100,104]。
4 PUFAs對神經(jīng)細胞凋亡的影響
PUFAs對細胞凋亡的影響是目前營養(yǎng)生物學(xué)研究的重要領(lǐng)域[22,88,105-106]。PUFAs的抗凋亡功能與n-3 PUFAs的抗炎、抗氧化有關(guān),并對大腦具有保護作用,包括嬰幼兒大腦的發(fā)育、老年大腦的衰老以及神經(jīng)變性疾病等[22,106]。n-3 PUFAs可通過抗凋亡的作用,保持一定神經(jīng)元的數(shù)量,維持神經(jīng)系統(tǒng)的正常功能。
PUFAs的抗凋亡功能目前已有許多文獻的報道。最新研究表明孕婦和產(chǎn)婦飲食中的DHA有助于預(yù)防由產(chǎn)前精神壓力導(dǎo)致新生兒記憶功能的障礙,氧化標(biāo)記物含量的增加,線粒體代謝功能紊亂以及海馬細胞的凋亡[107]。同時,產(chǎn)前n-3 PUFAs的干預(yù)可對抗高氧血癥誘導(dǎo)大鼠大腦細胞的凋亡[108]。懷孕期間食用富含DHA的食物,可以通過抑制氧化應(yīng)激和細胞的凋亡,減少新生兒大腦的損傷[109]。新生兒的高血膽紅素血癥所致的血清未結(jié)合膽紅素(unconjugated bilirubin,UCB)水平升高,可對中樞神經(jīng)系統(tǒng)造成各種副作用。DHA的干預(yù)可對抗UCB導(dǎo)致的超氧化物歧化酶(superoxide dismutase,SOD)、過氧化氫酶以及谷胱甘肽過氧化物酶(glutathione peroxidase,GPX)活性下降;以及UCB誘導(dǎo)的星形細胞的凋亡,表明DHA可通過抑制細胞的凋亡和增加抗氧化酶的活性,對大腦細胞產(chǎn)生保護作用[110]。在大腦的生長發(fā)育期間,充足的n-3 PUFAs也可以通過抑制感光細胞凋亡和視網(wǎng)膜的變性,對視網(wǎng)膜產(chǎn)生保護作用[111-113]。氧化損傷和線粒體功能失調(diào)是導(dǎo)致神經(jīng)細胞凋亡的重要因素,DHA可激活細胞內(nèi)多種調(diào)節(jié)機制、抑制氧化應(yīng)激反應(yīng)、維護線粒體的功能、提高抗凋亡蛋白-B淋巴細胞瘤-2(B-cell lymphoma-2,Bcl-2)的含量,保護神經(jīng)細胞免受到傷害[111-112]。
PUFAs的抗凋亡作用是一個復(fù)雜的過程,多種信號轉(zhuǎn)導(dǎo)系統(tǒng)參與了PUFAs的抗凋亡過程,如細胞內(nèi)Ca2+信號系統(tǒng)、磷脂酰肌醇(-3)激酶/蛋白激酶B(phosphatidylinositol (-3) kinase/protein kinase B,PI3K/Akt)途徑、細胞外調(diào)節(jié)蛋白激酶(extracellular regulated protein kinases,ERK)/絲裂原活化蛋白激酶(mitogenactivated protein kinases,MAPKs)途徑和P38蛋白激酶(mitogen-activated protein kinases p38,P38MAPKs)途徑等。機理涉及docosanoid類脂質(zhì)信號分子的作用、線粒體在抗凋亡過程中的作用以及表觀遺傳學(xué)效應(yīng)等。
綜上所述,近年來開展的PUFAs對神經(jīng)細胞保護作用研究取得了很多成果,并注意到不同類型PUFAs對神經(jīng)細胞結(jié)構(gòu)與功能影響存在差異。其中n-3 PUFAs展現(xiàn)了較為全面的有益作用和獨特的細胞學(xué)機制,歸納起來包括:通過促進神經(jīng)細胞增殖分化、增加神經(jīng)細胞的數(shù)量;通過抗氧化、抗凋亡及阻止端粒縮短等機制防止生理或病理條件下的神經(jīng)細胞變性;以及通過影響神經(jīng)細胞的膜可塑性和突觸功能,維護神經(jīng)細胞的正常結(jié)構(gòu)與功能,實現(xiàn)對大腦的保護作用(圖1)。這些機制研究為PUFAs尤其是其中的n-3 PUFAs保護大腦功能的營養(yǎng)干預(yù)奠定了重要的細胞生物學(xué)基礎(chǔ)。
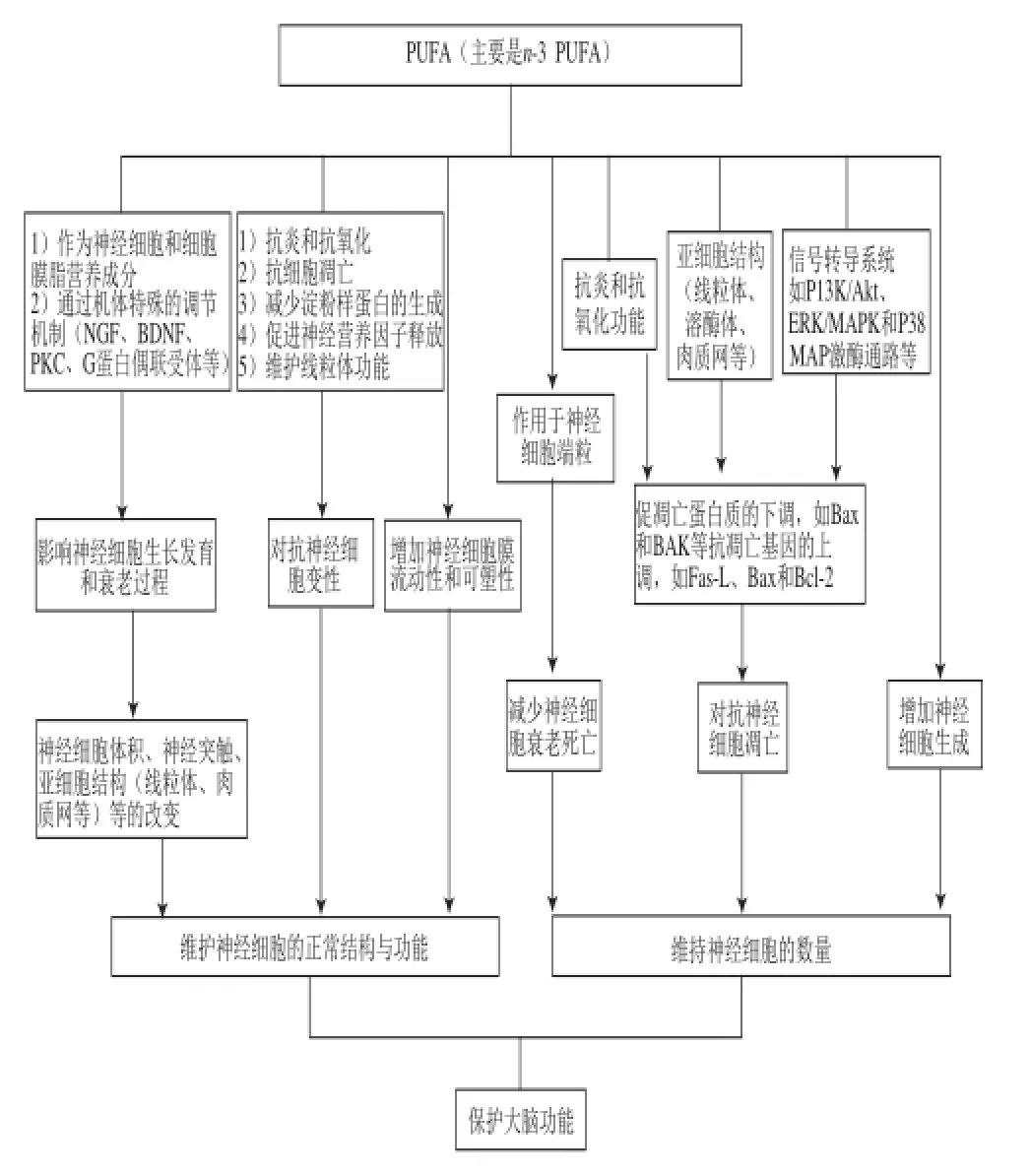
圖1 PUFAs保護大腦功能的細胞生物學(xué)機制示意圖Fig.1 Schematic diagram of the mechanism of action of PUFAs in protecting brain function
5 結(jié) 語
近年來,有關(guān)PUFAs對大腦功能的保護作用研究已取得了豐碩成果,發(fā)現(xiàn)PUFAs不僅可以通過抗炎、抗氧化、維護心腦血管功能等全身性功能,改善大腦的微環(huán)境而發(fā)揮對大腦的保護作用;而且揭示了其直接作用于神經(jīng)細胞,促進神經(jīng)細胞生長和突觸的形成,以及通過調(diào)節(jié)細胞膜流動性和可塑性,保護神經(jīng)細胞正常功能。展望未來,研究將不斷深入到細胞與分子水平,從細胞生物學(xué)與分子生物學(xué)角度揭示其對神經(jīng)細胞作用的分子機制。特別是關(guān)于PUFAs及其活性代謝物調(diào)節(jié)細胞信號轉(zhuǎn)導(dǎo)系統(tǒng)、膜相關(guān)途徑、核受體途徑,促進神經(jīng)細胞生成、突觸生長,阻止細胞變性和凋亡的細胞內(nèi)途徑與作用機制,從而發(fā)揮其延緩大腦功能衰退與神經(jīng)變性類疾病發(fā)展的有益作用,為PUFAs的營養(yǎng)功能及其對大腦功能的保護作用研究奠定基礎(chǔ)。
[1] DENIS I, POTIER B, VANCASSEL S, et al. Omega-3 fatty acids and brain resistance to ageing and stress: body of evidence and possible mechanisms[J]. Ageing Research Reviews, 2013, 12(2): 579-594. DOI:10.1016/j.arr.2013.01.007.
[2] LUCHTMAN D W, SONG C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies[J]. Neuropharmacology, 2013, 64: 550-565. DOI:10.1016/j.neuropharm.2012.07.019.
[3] JANSSEN C I, KILIAAN A J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration[J]. Progress in Lipid Research, 2014, 53: 1-17. DOI:10.1016/j.plipres.2013.10.002.
[4] SWANSON D, BLOCK R, MOUSA S A. Omega-3 fatty acids EPA and DHA: health benefits throughout life[J]. Advance Nutrition, 2012,3(1): 1-7. DOI:10.3945/an.111.000893.
[5] HENNEBELLE M, CHAMPEIL-POTOKAR G, LAVIALLE M, et al. Omega-3 polyunsaturated fatty acids and chronic stress-induced modulations of glutamatergic neurotransmission in the hippocampus[J]. Nutrition Reviews, 2014, 72(2): 99-112. DOI:10.1111/nure.12088.
[6] ROGERS L K, VALENTINE C J, KEIM S A. DHA supplementation: current implications in pregnancy and childhood[J]. Pharmacological Research, 2013, 70(1): 13-19. DOI:10.1016/j.phrs.2012.12.003.
[7] TROUCHE S, BONTEMPI B, ROULLET P, et al. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(14): 5919-5924. DOI:10.1073/ pnas.0811054106.
[8] LEUNER B, YEVGENIA K, CHARLES G G, et al. Diminished adult neurogenesis in the marmoset brain precedes old age[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(43): 17169-17173. DOI:10.1073/pnas.0708228104.
[9] COTII B P, O’KUSKY J R, INNIS S M. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain[J]. Journal of Nutrition, 2006, 136(6): 1570-1575. DOI:10.1111/j.1471-4159.2005.03513.x.
[10] YAVIN E, HIMOVICHI E, EILAM R. Delayed cell migration in the developing rat brain following maternal omega-3 alpha linolenic acid dietary deficiency[J]. Neuroscience, 2009, 162(4): 1011-1022. DOI:10.1016/j.neuroscience.2009.05.012.
[11] KAWAKITA E, HASHIMOTO M, SHIDO O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo[J]. Neuroscience, 2006, 139(3): 991-997. DOI:10.1016/j.neuroscience.2006.01.021.
[12] DYALL S C, MICHAEL G J, MICHAEL-TITUS A T. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats[J]. Journal of Neuroscience Research, 2010, 88(10): 2091-2102. DOI:10.1002/jnr.22390.
[13] HE C W, QU X Y, CUI L B, et al. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid[J]. Proceedings of theNational Academy of Sciences of the United States of America, 2009, 106(27): 11370-111375. DOI:10.1073/pnas.0904835106.
[14] ROBSON L G, DYALL S, SIDLOFF D, et al. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurones throughout development and in aged animals[J]. Neurobiol Aging, 2010, 31(4): 678-687. DOI:10.1016/ j.neurobiolaging.2008.05.027.
[15] KAN I, MELAMED E, OFFEN D, et al. Docosahexaenoic acid and arachidonic acid are fundamental supplements for the induction of neuronal differentiation[J]. Journal of Lipid Research, 2007, 48(3): 513-517. DOI:10.1194/jlr.C600022-JLR200.
[16] RAO J S, HYUN J L, STALEY I. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex[J]. Molecular Psychiatry, 2007, 12(2): 151-157. DOI:10.1038/sj.mp.4001887.
[17] LANGELIER B, LINARD A, BORDAT C, et al. Long chainpolyunsaturated fatty acids modulate membrane phospholipid composition and protein localization in lipid rafts of neural stem cell cultures[J]. Journal of Cellular Biochemistry, 2010, 110(6): 1356-1364. DOI:10.1002/jcb.22652.
[18] PIOMELLI D, ASTARITA G, RAPAKA R. A neuroscientist’s guide to lipidomics[J]. Nature Reviews Neuroscience, 2007, 8(10): 743-754. DOI:10.1002/jcb.22652.
[19] HAJJAR T, GOH Y M, RAJION M A, et al. Alterations in neuronal morphology and synaptophysin expression in the rat brain as a result of changes in dietary n-6: n-3 fatty acid ratios[J]. Lipids in Health and Disease, 2013, 12: 113. DOI:10.1186/1476-511X-12-113.
[20] PURVES D, SNIDER W D, VOYYODIC J T. Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system[J]. Nature, 1988, 336: 123-128. DOI:10.1038/336123a0.
[21] GUSTILO M C, MARKOWSKA A L, BRECKLER S J, et al. Evidence that nerve growth factor influences recent memory through structural changes in septohippocampal cholinergic neurons[J]. Journal of Comparative Neurology, 1999, 405(4): 491-507. DOI:10.1002/ (SICI)1096-9861(19990322)405:4<491::AID-CNE4>3.0.CO;2-N.
[22] SU H M. Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance[J]. Journal of Nutrition Biochemistry, 2010, 21(5): 364-373. DOI:10.1016/ j.jnutbio.2009.11.003.
[23] SMITH T D, ADAMS M M, GALLAGHER M, et al. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats[J]. Journal Neuroscience, 2000, 20(17): 6587-6593.
[24] AHMAD A, MORIGUCHI T, SALEM N. Decrease in neuron size in docosahexaenoic acid-deficient brain[J]. Pediatric Neurology, 2002, 26(3): 210-218. DOI:10.1016/S0887-8994(01)00383-6.
[25] VENNA V R, DEPLANQUE D, ALLET C, et al. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus[J]. Psychoneuroendocrinology, 2009, 34(2): 199-211. DOI:10.1016/j.psyneuen.2008.08.025.
[26] HAMA H, HARA C, MIYAWAKI A. PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes[J]. Neuron, 2004, 41(3): 405-415. DOI:10.1016/S0896-6273(04)00007-8.
[27] FUTERMAN A H, Banker G A. The economics of neurite outgrowth-the addition of new membrane to growing axons[J]. Trends in Neuroscience, 1996, 19(4): 144-149. DOI:10.1016/S0166-2236(96)80025-7.
[28] CAO D H, XU J F, XUE R H, et al. Protective effect of chronic ethyl docosahexaenoate administration on brain injury in ischemic gerbils[J]. Pharmacology, Biochemistry and Behavior, 2004, 79(4): 651-659. DOI:10.1016/j.pbb.2004.09.016.
[29] CAO D H, KEVALA K, KIM J, et al. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function[J]. Journal of Neurochemistry, 2009, 111(2): 510-521. DOI:10.1111/j.1471-4159.2009.06335.x.
[30] CANSEV M, WURTMAN R J. Chronic administration of docosahexaenoic acid or eicosapentaenoic acid, but not arachidonic acid, alone or in combination with uridine, increases brain phosphatide and synaptic protein levels in gerbils[J]. Neuroscience, 2007, 148(2): 421-431. DOI:10.1016/j.neuroscience.2007.06.016.
[31] DARIOS F, DAVLETOV B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3[J]. Nature, 2006, 440(7085): 813-817. DOI:10.1038/nature04598.
[32] HAAG M. Essential fatty acids and the brain[J]. The Canadian Journal of Psychiatry, 2003, 48(3): 195-203.
[33] MA D, ZHANG M, LARSEN C P, et al. DHA promotes the neuronal differentiation of rat neural stem cells transfected with GPR40 gene[J]. Brain Research, 2010, 1330: 1-8. DOI:10.1016/j.brainres.2010.03.002. [34] IKEMOTO A, ATSUMI N, FURUKAWA S, et al. Dietary n-3 fatty acid deficiency decreases nerve growth factor content in rat hippocampus[J]. Neuroscience Letter, 2000, 285(2): 99-102. DOI:10.1016/S0304-3940(00)01035-1.
[35] YAMASHIMA T. A putative link of PUFA, GPR40 and adult-born hippocampal neurons for memory[J]. Progress in Neurobiology, 2008, 84(2): 105-115. DOI:10.1016/j.pneurobio.2007.11.002.
[36] KIM H Y, MOON H S, CAO D H, et al. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons[J]. Biochemical Journal, 2011, 435(2): 327-336. DOI:10.1042/BJ20102118.
[37] SALAMOTO T, CANSEV M, WURTMAN R J. Oral supplementation with docosahexaenoic acid and uridine-5’-monophosphate increases dendritic spine density in adult gerbil hippocampus[J]. Brain Research, 2007, 1182: 50-59. DOI:10.1016/j.brainres.2007.08.089.
[38] WURTMAN R J, ULUS I H, CANSEV M, et al. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally[J]. Brain Research, 2006, 1088(1): 83-92. DOI:10.1016/j.brainres.2006.03.019.
[39] THORELL L, SJOBERG L B, HEMELL O. Nucleotides in human milk: sources and metabolism by the newborn infant[J]. Pediatric Research, 1996, 40(6): 845-852. DOI:10.1203/00006450-199612000-00012.
[40] PARLETTA N, MILTE C M, MEYER B J. Nutritional modulation of cognitive function and mental health[J]. Journal of Nutrition Biochemisty, 2013, 24(5): 725-743. DOI:10.1016/ j.jnutbio.2013.01.002.
[41] ECKERT G P, LIPKA U, MULLER W E. Omega-3 fatty acids in neurodegenerative diseases: focus on mitochondria[J]. Prostaglandins Leukot Essent Fatty Acids, 2013, 88(1): 105-114. DOI:10.1016/ j.plefa.2012.05.006.
[42] BORRE Y E, PANAGAKI T, KOELINK P J, et al. Neuroprotective and cognitive enhancing effects of a multi-targeted food intervention in an animal model of neurodegeneration and depression[J]. Neuropharmacology, 2014, 79: 738-749. DOI:10.1016/ j.neuropharm.2013.11.009.
[43] TORRES M, PRICE S L, FIOL-DEROQUE M A, et al. Membrane lipid modifications and therapeutic effects mediated by hydroxydocosahexaenoic acid on Alzheimer’s disease[J]. Biochimica Et Biophysica Acta, 2014, 1838(6): 1680-1692. DOI:10.1016/ j.bbamem.2013.12.016.
[44] MATTSON M P, MAGNUS T. Ageing and neuronal vulnerability[J]. Nature Reviews Neuroscience, 2006, 7(4): 278-294. DOI:10.1038/ nrn1886.
[45] LEDESMA M D, MARTIN M G, DOTTI C G. Lipid changes in the aged brain: effect on synaptic function and neuronal survival[J]. Progress in Lipid Research, 2012, 51(1): 23-35. DOI:10.1016/ j.plipres.2011.11.004.
[46] MORRISON J H, HOF P R. Life and death of neurons in the aging brain[J]. Science, 1997, 278: 412-419. DOI:10.1126/ science.278.5337.412.
[47] APRIKYAN G V, GEKCHYAN K G. Release of neurotransmitter amino acids from rat brain synaptosomes and its regulation in aging[J]. Gerontology, 1988, 34(1/2): 35-40. DOI:10.1159/000212928.
[48] MCEEEN B S. Physiology and neurobiology of stress and adaptation: central role of the brain[J]. Physiological Reviews, 2007, 87(3): 873-904. DOI:10.1152/physrev.00041.2006.
[49] LEE L K, SHAHAR S, RAJAB N F, et al. The role of long chain omega-3 polyunsaturated fatty acids in reducing lipid peroxidation among elderly patients with mild cognitive impairment: a case-control study[J]. Journal of Nutrition Biochemistry, 2013, 24(5): 803-808. DOI:10.1016/j.jnutbio.2012.04.014.
[50] GIORDANO E, VISIOLI F. Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions[J]. Prostaglandins Leukot Essential Fatty Acids, 2014, 90(1): 1-4. DOI:10.1016/ j.plefa.2013.11.002.
[51] FOSTER T C, KUMAR A. Calcium dysregulation in the aging brain[J]. Neuroscientist, 2002, 8(4): 297-301. DOI:10.1177/107385840200800404.
[52] DYALL S C, MICHAEL G J, WHEIPTON R, et al. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain[J]. Neurobiol Aging, 2007, 28(3): 424-439. DOI:10.1016/j.neurobiolaging.2006.01.002.
[53] PASSAFARO M, NAKAGAWA T, SALA C, et al. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2[J]. Nature, 2003, 424: 677-681. DOI:10.1038/nature01781.
[54] BARNES C A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat[J]. Journal of Comparative and Physiological, 1979, 93(1): 74-104. DOI:10.1037/ h0077579.
[55] ROSENZWEIG E S, BARNES C A. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition[J]. Progress in Neurobiology, 2003, 69(3): 143-179. DOI:10.1016/S0301-0082(02)00126-0.
[56] BARNES C A, MCNAUGHTON B L. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence[J]. The Journal of Physiology, 1980, 309: 473-485. DOI:10.1113/jphysiol.1980.sp013521.
[57] MCGAHON B M, MARTIN D S D, HORROBIN D F, et al. Agerelated changes in synaptic function: analysis of the effect of dietary supplementation with omega-3 fatty acids[J]. Neuroscience, 1999, 94(1): 305-314. DOI:10.1016/S0306-4522(99)00219-5.
[58] YEHUDA S, RABINOVITZ S, CARASSO R, et al. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane[J]. Neurobiol Aging, 2002, 23(5): 843-853. DOI:10.1016/ S0197-4580(02)00074-X.
[59] FRISARDI V, PANZA F, SERIPA D, et al. Glycerophospholipids and glycerophospholipid-derived lipid mediators: a complex meshwork in Alzheimer’s disease pathology[J]. Progress in Lipid Research, 2011, 50(4): 313-330. DOI:10.1016/j.plipres.2011.06.001.
[60] SIMONS K, IKONEN E. Functional rafts in cell membranes[J]. Nature, 1997, 387: 569-572. DOI:10.1038/42408.
[61] HUBER T, RAJAMOORTHI K, KURZE V F, et al. Structure of docosahexaenoic acid-containing phospholipid bilayers as studied by (2)H NMR and molecular dynamics simulations[J]. Journal of the American Chemical Society, 2002, 124(2): 298-309. DOI:10.1021/ ja011383j.
[62] KEW S, BANERJEE T, MINIHANE A M, et al. Relation between the fatty acid composition of peripheral blood mononuclear cells and measures of immune cell function in healthy, free-living subjects aged 25-72 y[J]. The American Journal of Clinical Nutrition, 2003, 77(5): 1278-1286.
[63] WASSALL S R, STILLWELL W. Polyunsaturated fatty acidcholesterol interactions: domain formation in membranes[J]. Biochim Biochimica Et Biophysica Acta, 2009, 1788(1): 24-32. DOI:10.1016/ j.bbamem.2008.10.011.
[64] VANKUIJK F J, BUCK P. Fatty acid composition of the human macula and peripheral retina[J]. Investigative Ophthalmology & Visual Science, 1992, 33(13): 3493-3496.
[65] KISHIMOTO Y, AGRANOFF B W, RADIN N S, et al. Comparison of the fatty acids of lipids of subcellular brain fractions[J]. Journal of Neurochemistry, 1969, 16(3): 397-404. DOI:10.1111/j.1471-4159.1969.tb10380.x.
[66] AKBAR M, CALDERON F, WEN Z, et al. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(31): 10858-10863. DOI:10.1073/ pnas.0502903102.
[67] CALDER P C, BOND J A, HARVEY D J, et al. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis[J]. Biochemical Journal, 1990, 269(3): 807-814. DOI:10.1042/bj2690807.
[68] O’CALLAGHAN N, PARLETTA N, MILTE C M, et al. Telomere shortening in elderly individuals with mild cognitive impairment may be attenuated with omega-3 fatty acid supplementation: a randomized controlled pilot study[J]. Nutrition, 2014, 30(4): 489-491. DOI:10.1016/j.nut.2013.09.013.
[69] MARTIN-RUIZ C, DICKINSON H O, KEYS B, et al. Telomere length predicts poststroke mortality, dementia, and cognitive decline[J]. Annals of Neurology, 2006, 60(2): 174-180. DOI:10.1002/ ana.20869.
[70] VANADAL M, ALATA W, TREMBLAY C, et al. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2[J]. Journal of Neurochemistry, 2014, 129(3): 516-526. DOI:10.1111/jnc.12640.
[71] MARIN C, DELQADO-LISTA J, RAMIREZ R, et al. Mediterranean diet reduces senescence-associated stress in endothelial cells[J]. Age, 2012, 34(6): 1309-1316. DOI:10.1007/s11357-011-9305-6.
[72] KIECOLT-GLASER J K, EPEL E S, BELURY M A, et al. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial[J]. Brain Behavior and Immunity, 2013, 28: 16-24. DOI:10.1016/j.bbi.2012.09.004.
[73] GLEIHMANN U, GLEICHMANN U S, GLEICHMANN S. From cardiovascular prevention to anti-aging medicine: influence on telomere and cell aging[J]. Deutsche Medizinische Wochenschrift, 2011, 136(38): 1913-1916. DOI:10.1055/s-0031-1286363.
[74] KANG J X. Differential effects of omega-6 and omega-3 fatty acids on telomere length[J]. The American Journal of Clinical Nutrition, 2010, 92(5): 1276-1277. DOI:10.3945/ajcn.110.000463.
[75] FARZANEH-FAR R, LIIN J, EPEL E S, et al. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease[J]. The Japan Automobile Manufacturers Association, 2010, 303(3): 250-257. DOI:10.1001/jama.2009.2008.
[76] LAYE S. What do you eat dietary omega 3 can help to slow the aging process[J]. Brain, Behavior, and Immunity, 2013, 28: 14-15. DOI:10.1016/j.bbi.2012.11.002.
[77] VALLA J, BERMDT J D, GONZALEZ-LIMA F. Energy hypometabolism in posterior cingulate cortex of Alzheimer’s patients: superficial laminar cytochrome oxidase associated with disease duration[J]. The Journal of Neuroscience, 2001, 21(13): 4923-4930.
[78] SCHIOTH H B, CRAFT S, BROOK S J, et al. Brain insulin signaling and Alzheimer’s disease: current evidence and future directions[J]. Molecular Neurobiology, 2012, 46(1): 4-10. DOI:10.1007/s12035-011-8229-6.
[79] MATTSON M P, GLEICHMANN M, CHENG A. Mitochondria in neuroplasticity and neurological disorders[J]. Neuron, 2008, 60(5): 748-766. DOI:10.1016/j.neuron.2008.10.010.
[80] ECKERT G P, RENNER K, ECKERT S H, et al. Mitochondrial dysfunction-a pharmacological target in Alzheimer’s disease[J]. Molecular Neurobiology, 2012, 46(1): 136-150. DOI:10.1007/s12035-012-8271-z.
[81] DU H, GUO L, YAN S Q, et al. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(43): 18670-18675. DOI:10.1073/pnas.1006586107.
[82] BORGER E. Mitochondrial beta-amyloid in Alzheimer’s disease[J]. Biochemical Society Transactions, 2011, 39(4): 868-873. DOI:10.1042/BST0390868.
[83] PAVLOV P F, WIEHAGER B, SAKAI J, et al. Mitochondrial gammasecretase participates in the metabolism of mitochondria-associated amyloid precursor protein[J]. The FASEB Journal, 2011, 25(1): 78-88. DOI:10.1096/fj.10-157230.
[84] LEUNER K, SCHUTT T, KURZ C, et al. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation[J]. Antioxidants and Redox Signaling, 2012, 16(12): 1421-1433. DOI:10.1089/ars.2011.4173.
[85] COLE G M, MA Q L, FRAUTSCHY S A. Omega-3 fatty acids and dementia[J]. Prostaglandins Leukot Essent Fatty Acids, 2009, 81(2/3): 213-221. DOI:10.1016/j.plefa.2009.05.015.
[86] GREEN K N, MARTINEZ-CORIA H, KHASHWJI H, et al. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloidbeta and tau pathology via a mechanism involving presenilin 1 levels[J]. The Journal of Neuroscience, 2007, 27(16): 4385-4495. DOI:10.1523/JNEUROSCI.0055-07.2007.
[87] GRIMM M O, CHENBECKER J, GROSQEN S, et al. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms[J]. Journal of Biological Chemistry, 2011, 286(16): 14028-14039. DOI:10.1074/jbc.M110.182329.
[88] ECKERT G P, CHANG S, ECKMANN J, et al. Liposomeincorporated DHA increases neuronal survival by enhancing nonamyloidogenic APP processing[J]. Biochimica Et Biophysica Acta, 2011, 1808(1): 236-243. DOI:10.1016/j.bbamem.2010.10.014.
[89] PEREZ S E, BERG B M, MOORE K A, et al. DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: possible gender effects[J]. Journal of Neuroscience Research, 2010, 88(5): 1026-1040. DOI:10.1002/jnr.22266.
[90] OSTER T, PILLOT T. Docosahexaenoic acid and synaptic protection in Alzheimer’s disease mice[J]. Biochimica Et Biophysica Acta, 2010, 1801(8): 791-798. DOI:10.1016/j.bbalip.2010.02.011.
[91] ARSENAULT D, JULIEN C, TREMBLAY C, et al. DHA improves cognition and prevents dysfunction of entorhinal cortex neurons in 3xTg-AD mice[J]. PLoS One, 2011, 6(2): 17397. DOI:10.1371/ journal.pone.0017397.
[92] STANLEY W C, KHAIRALLAH R J, DABKOWSKI E R. Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids[J]. Current Opinion in Clinical Nutrition and Metabolic Care, 2012, 15(2): 122-126. DOI:10.1097/ MCO.0b013e32834fdaf7.
[93] KONES R. Parkinson’s disease: mitochondrial molecular pathology, inflammation, statins, and therapeutic neuroprotective nutrition[J]. Nutrition in Clinical Practice, 2010, 25(4): 371-389. DOI:10.1177/0884533610373932.
[94] DALFO E, PORTERO-OTIN M, AYALA V, et al. Evidence of oxidative stress in the neocortex in incidental Lewy body disease[J]. The Journal of Neuropathology & Experimental Neurology, 2005, 64(9): 816-830. DOI:10.1097/01.jnen.0000179050.54522.5a.
[95] FABELO N, MARTIN V, SANTPERE G, et al. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease[J]. Molecular Medicine, 2011, 17(9/10): 1107-1118. DOI:10.2119/molmed.2011.00119.
[96] BOUSQUET M, SAINT-PIERRE M, JULIEN C, et al. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson’s disease[J]. The Faseb Journal, 2008, 22(4): 1213-1225. DOI:10.1096/fj.07-9677com.
[97] HERMANSON E, PERLMANN T, OLSON L E, et al. Nurr1 regulates dopamine synthesis and storage in MN9D dopamine cells[J]. Experimental Cell Research, 2003, 288(2): 324-334. DOI:10.1016/ S0014-4827(03)00216-7.
[98] OZSOY O, SEVAL-CELIK Y, HACIOGLU G, et al. The influence and the mechanism of docosahexaenoic acid on a mouse model of Parkinson’s disease[J]. Neurochemistry International, 2011, 59(5): 664-670. DOI:10.1016/j.neuint.2011.06.012.
[99] TANRIOVER G, SEVAL-CELIK Y, OZSOY O, et al. The effects of docosahexaenoic acid on glial derived neurotrophic factor and neurturin in bilateral rat model of Parkinson’s disease[J]. Folia Histochemica et Cytobiologica, 2010, 48(3): 434-441. DOI:10.2478/ v10042-010-0047-6.
[100] SAMADI P, GREQOIRE L, ROUILLARD C, et al. Docosahexaenoic acid reduces levodopa-induced dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys[J]. Annals of Neurology, 2006, 59(2): 282-288. DOI:10.1002/ana.20738.
[101] LUCHTMAN D W, MENG Q, SONG C. Ethyl-eicosapentaenoate(E-EPA) attenuates motor impairments and inflammation in the MPTP-probenecid mouse model of Parkinson’s disease[J]. Behavioural Brain Research, 2012, 226(2): 386-396. DOI:10.1016/j.bbr.2011.09.033.
[102] KABUTO H, AMAKAWA M, MANKURA M, et al. Docosahexaenoic acid ethyl ester enhances 6-hydroxydopamineinduced neuronal damage by induction of lipid peroxidation in mouse striatum[J]. Neurochemical Research, 2009, 34(7): 1299-1303. DOI:10.1007/s11064-008-9909-0.
[103] CANSEV M, ULUS I H, WANG L, et al. Restorative effects of uridine plus docosahexaenoic acid in a rat model of Parkinson’s disease[J].Neuroscience Research, 2008, 62(3): 206-209. DOI:10.1016/ j.neures.2008.07.005.
[104] BOUSQUET M, GUE K, EMOND V, et al. Transgenic conversion of omega-6 into omega-3 fatty acids in a mouse model of Parkinson’s disease[J]. Journal of Lipid Research, 2011, 52(2): 263-271. DOI:10.1194/jlr.M011692.
[105] LUKIW W J, BAZAN N G. Inflammatory, apoptotic, and survival gene signaling in Alzheimer’s disease: a review on the bioactivity of neuroprotectin D1 and apoptosis[J]. Molecular Neurobiology, 2010, 42(1): 10-16. DOI:10.1007/s12035-010-8126-4.
[106] MAYURASAKORM K, WILLIAMS J J, TEN V S, et al. Docosahexaenoic acid: brain accretion and roles in neuroprotection after brain hypoxia and ischemia[J]. Current Opinion in Clinical Nutrition and Metabolic Care, 2011, 14(2): 158-167. DOI:10.1097/ MCO.0b013e328342cba5.
[107] FENG Z H, ZOU, X, JIA H Q, et al. Maternal docosahexaenoic acid feeding protects against impairment of learning and memory and oxidative stress in prenatally stressed rats: possible role of neuronal mitochondria metabolism[J]. Antioxidants & Redox Signaling, 2012, 16(3): 275-289. DOI:10.1089/ars.2010.3750.
[108] TUZUN F, KUMRAL A, OZBAL S, et al. Maternal prenatal omega-3 fatty acid supplementation attenuates hyperoxia-induced apoptosis in the developing rat brain[J]. International Journal of Developmental Neuroscience, 2012, 30(4): 315-523. DOI:10.1016/ j.ijdevneu.2012.01.007.
[109] SUGANUMA H, ARAI Y, KITAMURA Y, et al. Maternal docosahexaenoic acid-enriched diet prevents neonatal brain injury[J]. Neuropathology, 2010, 30(6): 597-605. DOI:10.1111/j.1440-1789.2010.01114.x.
[110] BECERIR C, KILICI I, SAHIN O, et al. The protective effect of docosahexaenoic acid on the bilirubin neurotoxicity[J]. Journal of Enzyme Inhibition and Medicinal Chemistry, 2013, 28(4): 801-807. DOI:10.3109/14756366.2012.684053.
[111] ROTSEIN N P, AVELDANO M, FRANCISCO B J, et al. Apoptosis of retinal photoreceptors during development in vitro: protective effect of docosahexaenoic acid[J]. Journal of Neurochemistry, 1997, 69(2): 504-513. DOI:10.1046/j.1471-4159.1997.69020504.x.
[112] ROTSEIN N P, POLITI L E, GERMAN O L, et al. Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors[J]. Investigative Ophthalmology & Visual Science, 2003, 44(5): 2252-2259. DOI:10.1167/iovs.02-0901.
[113] YOSHIZAWA K, SASAKI T, KURO M, et al. Arachidonic acid supplementation during gestational, lactational and post-weaning periods prevents retinal degeneration induced in a rodent model[J]. The British Journal of Nutrition, 2013, 109(8): 1424-1432. DOI:10.1017/ S0007114512003327.
Advances in Research on Neuron-Protective Role of Polyunsaturated Fatty Acids
LIU Zhiguo, WANG Hualin, WANG Limei, LIU Lieju*
(School of Biology and Pharmaceutical Engineering, Wuhan Polytechnic University, Wuhan 430023, China)
The protective effects of polyunsaturated fatty acids (PUFAs) on brain function and the nervous system have attracted a lot of attention. Numerous studies have illustrated the anti-inflammation, anti-oxidative and cardiovascular protective effects of PUFAs, as well as the protective effects on brain function, particularly nerve cells. In this article, we review the mechanisms of brain-protective effects of PUFAs in following fields: how PUFAs promote neurogenesis,maintain nerve cell morphology and function, improve neurite growth; prevent neurodegeneration, depress nerve cell apoptosis and regulate membrane fluidity, plasticity and telomere activity in neurons. This review provides a prospective insight on nutritional studies of PUFAs against aging and/or diseases (such as Alzheimer’s disease and Parkinson’s disease) inducing brain disorders.
polyunsaturated fatty acid; neurodegeneration; nerve cells; neurogenesis; brain function
10.7506/spkx1002-6630-201607043
TS201.4
A
1002-6630(2016)07-0239-10
劉志國, 王華林, 王麗梅, 等. 多不飽和脂肪酸對神經(jīng)細胞保護作用的研究進展[J]. 食品科學(xué), 2016, 37(7): 239-248. DOI:10.7506/spkx1002-6630-201607043. http://www.spkx.net.cn
LIU Zhiguo, WANG Hualin, WANG Limei, et al. Advances in research on neuron-protective role of polyunsaturated fatty acids[J]. Food Science, 2016, 37(7): 239-248. (in Chinese with English abstract) DOI:10.7506/spkx1002-6630-201607043. http://www.spkx.net.cn
2015-03-26
國家自然科學(xué)基金面上項目(31271855);國家自然科學(xué)基金青年科學(xué)基金項目(31000772);湖北省自然科學(xué)基金項目(2014CFB887);湖北省教育廳科技計劃項目(D20141705)
劉志國(1963—),男,教授,博士,研究方向為營養(yǎng)與食品安全。E-mail:zhiguo_l@126.com
*通信作者:劉烈炬(1952—),男,教授,碩士,研究方向為營養(yǎng)生物學(xué)。E-mail:liulieju@qq.com

