PNUTS在D-半乳糖誘導老化小鼠耳蝸中的表達
吳喜迪,張俏,李文靜,劉雙月
PNUTS在D-半乳糖誘導老化小鼠耳蝸中的表達
吳喜迪,張俏,李文靜,劉雙月△
目的觀察D-半乳糖誘導的老化小鼠耳蝸中蛋白磷酸酶1核目標亞基(PNUTS)的表達。方法6周齡清潔級昆明小鼠20只隨機分為對照組和D-半乳糖組,每組10只。D-半乳糖組小鼠頸背部皮下注射D-半乳糖800 mg/(kg·d);對照組頸背部注射等量生理鹽水,均為每日1次,連續8周。造模完成后,進行聽腦干反應(ABR)測試檢測小鼠聽力變化;取2組小鼠耳蝸,采用Western blot檢測PNUTS及p53蛋白表達;免疫組織化學觀察PNUTS蛋白在耳蝸毛細胞、螺旋神經節細胞及血管紋中的表達與分布情況。結果D-半乳糖組小鼠在8、12、24 kHz這3個頻率下的ABR閾值與對照組差異均無統計學意義。PNUTS蛋白在小鼠耳蝸毛細胞、螺旋神經節細胞及血管紋細胞中有表達,且D-半乳糖組小鼠耳蝸中PNUTS蛋白的陽性表達水平較對照組顯著降低(P<0.05);而p53蛋白表達水平則顯著升高(P<0.01)。結論PNUTS在小鼠耳蝸中有表達,且D-半乳糖能夠誘導老化小鼠耳蝸中PNUTS表達下調。
蛋白質磷酸酶1;半乳糖;老年性聾;耳蝸;蛋白磷酸酶1核目標亞基
老年性耳聾(age-related hearing loss,ARHL)是伴隨衰老的過程中主要由內耳的退行性變所引起的自然聽力損失。實驗動物慢性注射大劑量D-半乳糖可以建立理想的聽覺系統自然衰老模型[1]。蛋白磷酸酶1核目標亞基(protein phosphates 1 nuclear targeting subunit,PNUTS)是蛋白磷酸酶1(protein phosphates 1,PP1)結合蛋白。PNUTS這種無催化活性的核定位蛋白具有降低端粒縮短,抑制PP1調節的細胞凋亡,參與DNA修復等生物活性作用[2-4]。已有研究發現,PNUTS在衰老心肌細胞的凋亡過程中發揮重要作用[5],那么PNUTS是否在內耳中表達,PNUTS在老年性耳聾的發生發展中是否起作用,目前鮮見報道。本研究旨在應用D-半乳糖誘導老化模型小鼠,觀察PNUTS在內耳的表達,初步探討PNUTS在老年性耳聾中的作用機制。
1 材料與方法
1.1材料
1.1.1實驗動物6周齡清潔級昆明小鼠20只,雌雄不限,體質量18~22 g,耳廓反射靈敏,無中耳炎,由錦州醫科大學實驗動物中心提供。小鼠于安靜狀態下飼養,給予常規飲食。建立老年性耳聾動物模型[1]:昆明小鼠隨機進行編號,然后令其雙數為對照組,單數為D-半乳糖組,每組10只,適應性喂養1周。D-半乳糖組小鼠頸背部皮下注射D-半乳糖[800 mg/(kg·d)];對照組頸背部注射等量生理鹽水,每日1次,連續8周。每日監測小鼠體質量以調整藥量。
1.1.2主要試劑D-半乳糖(G5388)購自美國Sigma公司;兔抗鼠PNUTS多克隆抗體(bs-11666R)購自博奧森公司;兔抗鼠p53多克隆抗體(ab1431)購于Abcam公司;DAB顯色劑及生物素-鏈霉卵白素免疫組化檢測試劑盒(SP-9001)購于北京中杉金橋生物技術有限公司。
1.2方法
1.2.1聽腦干反應(auditory brainstem response,ABR)測試分別于給藥前和停藥后24 h內對2組小鼠進行ABR測試。測聽在隔音屏蔽室內進行。1%戊巴比妥鈉90 mg/kg對小鼠腹腔麻醉,將電極正極置于小鼠顱頂正中皮下,負極及接地電極分別埋于測聲側及對側的耳廓后下。聽覺誘發電位-耳聲發射記錄系統給予小鼠短純音(tone burst)刺激,依次選取8、12、24 kHz 3個頻率測試后記錄ABR閾值(聽閾)。聲強從95 dB聲壓級(sound pressure level,SPL)開始,以5 dB逐次遞減,以ABRⅢ波剛出現時判定聽閾,并至少重復2次。
1.2.2Western blot檢測PNUTS和p53蛋白的表達耳蝸加入RIPA裂解液后,于0℃下超聲粉碎,12 000 r/min 4℃低溫離心25 min。上清經BCA法測定蛋白含量。上樣、電泳,轉膜,封閉,4℃下搖床孵育1 h之后,加一抗(1∶1 000稀釋),隨即4℃冰箱過夜。TBST緩沖液沖洗,5 min×3次。辣根過氧化物酶標記二抗(1∶1 000稀釋),4℃搖床孵育1 h。再次TBST緩沖液沖洗5 min×3次。滴加BCIP/NBT顯色后,可見成像條帶。全自動凝膠成像系統對條帶進行半定量分析,βactin為內參照物。
1.2.3免疫組織化學SABC法檢測PNUTS蛋白的表達于體視顯微鏡下充分暴露耳蝸,刺破卵圓窗和圓窗,蝸尖鉆孔,用含有4%多聚甲醛的0.01 mol/L PBS緩沖液(pH 7.4)緩慢灌流,于4℃下固定2 h。PBS沖洗后,放入4%EDTA溶液中,4℃下脫鈣5~7 d。標本經梯度乙醇脫水、二甲苯透明、常規石蠟包埋。蠟塊沿著蝸軸正中行5 μm連續切片。切片常規脫蠟至水;PBS中浸泡5 min,于枸櫞酸鹽緩沖液(pH 6.0)中微波修復8 min;3%H2O2浸泡15 min;室溫下山羊血清封閉20 min,滴加抗PNUTS抗體(1∶100稀釋),4℃過夜。切片甩干,生物素標記IgG 37℃孵育30 min;37℃下SABC復合物孵育30 min;DAB顯色,流水終止反應。以上每一步都需用PBS沖洗3次,每次5 min。蘇木素復染,脫水、透明、封片,顯微鏡下觀察結果、照相。陰性對照染色切片用PBS代替一抗,其余步驟不變。利用Image J 1.48軟件進行分析。每組隨機抽取10張切片,同等條件下測量各組耳蝸毛細胞、螺旋神經節及血管紋3個部位PNUTS陽性反應產物的光密度值。其數值越大,代表陽性反應越強烈。
1.3統計學方法采用SPSS 16.0軟件進行統計學分析,計量資料以均數±標準差(x ±s)表示。2組間比較采用t檢驗,P<0.05為差異有統計學意義。
2 結果
2.1D-半乳糖對小鼠ABR閾值的影響應用D-半乳糖后,在8、12和24 kHz這3個刺激頻率下,D-半乳糖組小鼠的ABR閾值與對照組比較差異無統計學意義(均P>0.05),見表1。
Tab.1The ABR threshold in two groups表1 小鼠ABR閾值(dB SPL,)
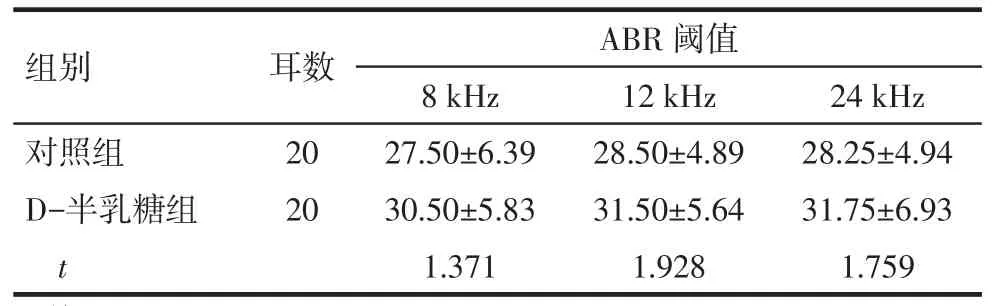
Tab.1The ABR threshold in two groups表1 小鼠ABR閾值(dB SPL,)
均P>0.05
組別對照組D-半乳糖組t耳數20 20 ABR閾值8 kHz 27.50±6.39 30.50±5.83 1.371 12 kHz 28.50±4.89 31.50±5.64 1.928 24 kHz 28.25±4.94 31.75±6.93 1.759
2.2PNUTS及p53蛋白在小鼠耳蝸的表達情況Western blot結果顯示,對照組小鼠耳蝸中有PNUTS蛋白的表達,與對照組相比,D-半乳糖組小鼠耳蝸中PNUTS蛋白的表達水平顯著降低(P<0.05),p53蛋白表達水平顯著升高(P<0.01),見圖1、表2。免疫組織化學結果顯示,PNUTS在對照組小鼠耳蝸毛細胞、螺旋神經節及血管紋3個部位均有表達,給予D-半乳糖誘導老化后,PNUTS在上述3個部位的表達均顯著減少(P<0.01),陰性對照未見陽性表達(P<0.01),見圖2、表3。
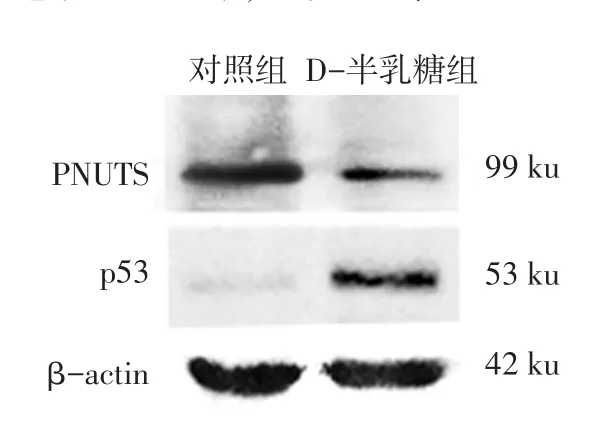
Fig.1Expressions of PNUTS and p53 protein in cochlear of mice圖1 PNUTS和p53蛋白在小鼠耳蝸中的表達
Tab.2Comparison of expression levels of PNUTS and p53 protein in cochlear between two groups表2 PNUTS和p53蛋白在小鼠耳蝸中表達水平比較(%,)
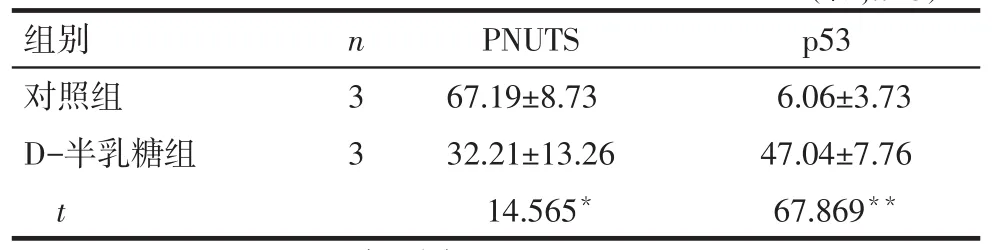
Tab.2Comparison of expression levels of PNUTS and p53 protein in cochlear between two groups表2 PNUTS和p53蛋白在小鼠耳蝸中表達水平比較(%,)
*P<0.05,**P<0.01,表3同
組別對照組D-半乳糖組t p53 6.06±3.73 47.04±7.76 67.869**n33 PNUTS 67.19±8.73 32.21±13.26 14.565*
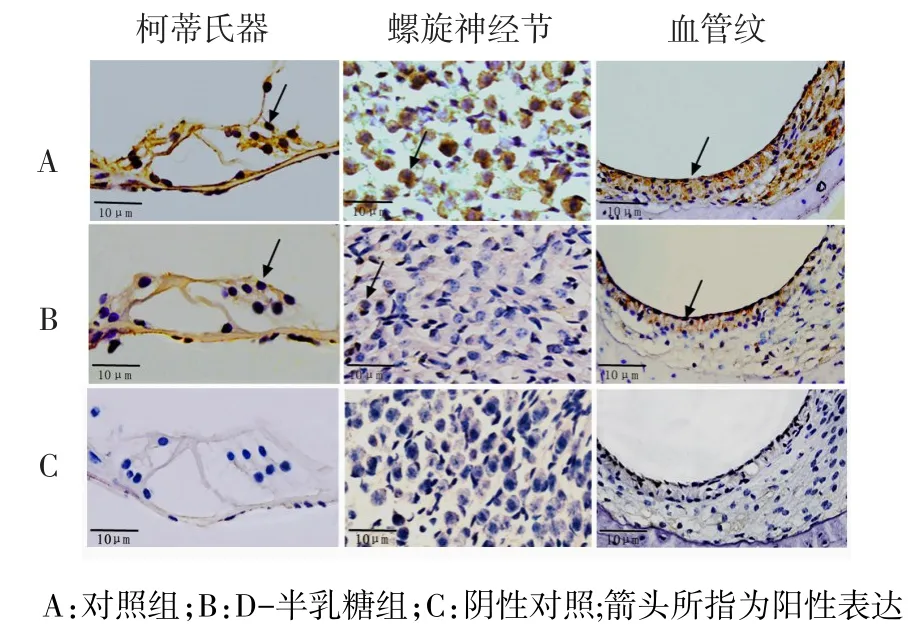
Fig.2Expressions of PNUTS in the cochlear Corti,spiral ganglion and striavascularis(DAB staining,×400)圖2 PNUTS蛋白在柯蒂氏器、螺旋神經節及血管紋的表達(DAB染色,×400)
Tab.3Expressions of PNUTS in cochlear hair cells,spiral ganglion cells and striavascularis in two groups表3 PNUTS蛋白在耳蝸毛細胞、螺旋神經節及血管紋的表達情況
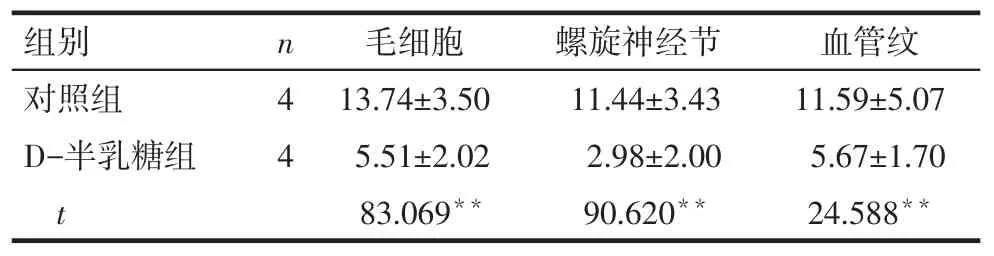
Tab.3Expressions of PNUTS in cochlear hair cells,spiral ganglion cells and striavascularis in two groups表3 PNUTS蛋白在耳蝸毛細胞、螺旋神經節及血管紋的表達情況
組別對照組D-半乳糖組t n44毛細胞13.74±3.50 5.51±2.02 83.069**螺旋神經節11.44±3.43 2.98±2.00 90.620**血管紋11.59±5.07 5.67±1.70 24.588**
3 討論
隨著世界人口老齡化的加劇,老年性耳聾現已引起醫學界越來越多的關注。D-半乳糖可被半乳糖氧化酶轉化成醛糖及氫過氧化物,產生過多的活性氧(ROS),導致細胞和機體的老化[6]。而高濃度的ROS可破壞DNA的結構,進而引發DNA損傷應答,或可通過直接調控衰老相關的信號通路,促進細胞衰老[7]。故國內學者常用D-半乳糖來構建老年性聾動物模型[8-9]。本研究參考Wu等[1]的研究劑量,給予小鼠高劑量D-半乳糖來建立小鼠老化模型。ABR結果顯示D-半乳糖組小鼠在各頻率下的ABR閾值與對照組比較差異均無統計學意義,聽力改變與以往的報道一致,老年性耳聾小鼠模型建模成功。
Boon等[5]發現在老年化心臟中,PNUTS的表達相較于未老化的心肌細胞顯著下調;而PNUTS的下調可以誘導心肌細胞DNA的損傷反應和端粒的磨損,繼而引起心肌細胞的凋亡。本研究發現PNUTS蛋白在小鼠耳蝸中的毛細胞、血管紋和螺旋神經節細胞處均有表達,且在老年性耳聾模型小鼠耳蝸的上述3個部位中PNUTS的表達均較對照組顯著下調。以上結果表明D-半乳糖誘導了小鼠耳蝸細胞中PNUTS的下調表達,進而導致內耳細胞老化。這與Boon等[5]在衰老心肌細胞中的發現基本一致,提示PNUTS與耳蝸細胞尤其是毛細胞、血管紋和螺旋神經節細胞的衰老關系密切。
最新研究表明,p53在細胞衰老的啟動和維持中起著重要作用。生理狀態下,p53具有抗癌和抗衰老的作用;而p53的過度激活則具有抗癌的作用,但同時也會促進衰老[7]。眾多研究也證實了這一觀點,在順鉑誘導的細胞衰老過程中,p53的表達水平持續升高[10]。研究人員發現,在D-半乳糖誘導的大鼠衰老模型的骨髓間充質干細胞中,p53蛋白表達同樣顯著增加[11]。而在衰老的C57BL/6小鼠耳蝸中,大量乙酰化的p53誘發了耳蝸毛細胞的凋亡增多,最終導致了小鼠老年性耳聾的發生[12]。本研究結果顯示,D-半乳糖可以誘導小鼠耳蝸中PNUTS蛋白表達下調和p53蛋白表達上調。由此筆者推測,在老年性耳聾模型小鼠中,下調的衰老相關蛋白PNUTS可能通過上調凋亡相關因子p53,引起耳蝸毛細胞、螺旋神經節細胞及血管紋細胞發生凋亡,繼而誘發聽覺損傷,最終導致老年性耳聾的發生發展,提示PNUTS是D-半乳糖誘導的老年性耳聾的重要作用靶點。因此,PNUTS是小鼠耳蝸細胞老化中的重要因子,PNUTS可能通過上調p53導致小鼠耳蝸細胞老化從而引起老年性耳聾。這為臨床防治老年性耳聾提供了理論依據和可能的治療靶點。當然,由于本實驗應用的是D-半乳糖誘導老化模型小鼠,與自然衰老動物還有一定的差異。本課題組將在自然衰老動物中近一步證實耳蝸中PNUTS的表達與老年性耳聾的關系及其致老年性耳聾的相關機制。
[1]Wu L,Sun Y,Hu YJ,et al.Increased p66Shc in the inner ear of D-galactose-induced aging mice with accumulation of mitochondrial DNA 3873-bp deletion:p66Shc and mtDNA damage in the inner ear during aging[J].PLoS One,2012,7(11):e50483.doi:10.1371/journal.pone. 0050483.
[2]Choy MS,Hieke M,Kumar GS,et al.Understanding the antagonism of retinoblastoma protein dephosphorylation by PNUTS provides insights into the PP1 regulatory code[J].Proc Natl Acad Sci U S A,2014,111(11):4097-4102.doi:10.1073/pnas.1317395111.
[3]Li DW,Liu JP,Schmid PC,et al.Protein serine/threoninephosphatase-1 dephosphorylates p53 at Ser-15 and Ser-37 to modulate its transcriptional and apoptotic activities[J].Oncogene,2006,25(21):3006-3022.doi:10.1038/sj.onc.1209334.
[4]Lee SJ,Lim CJ,Min JK,et al.Protein phosphatase 1 nuclear targeting subunit is a hypoxia inducible gene:its role in posttranslational modification of p53 and MDM2[J].Cell Death Differ,2007,14(6):1106-1116.doi:10.1038/sj.cdd.4402111.
[5]Boon RA,Iekushi K,Lechner S,et al.MicroRNA-34a regulates cardiac ageing and function[J].Nature,2013,495(7439):107-110.doi:10.1038/nature11919.
[6]Giorgio M,Migliaccio E,Orsini F,et al.Electron transfer between cytochrome and p66Shc generates reactive oxygen species that trigger mitochondria apoptosis[J].Cell,2005,122(2):221-233. doi:10.1016/j.cell.2005.05.011.
[7]Rufini A,Tucci P,Celardo I,et al.Senescence and aging:the critical roles of p53[J].Oncogene,2013,32(43):5129-5143.doi:10.1038/onc.2012.640.
[8]Du Z,Yang Q,Liu L,et al.NADPH oxidase 2-dependent oxidative stress,mitochondrial damage and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging rats[J].Neuroscience,2015, 286:281-292.doi:10.1016/j.neuroscience.2014.11.061.
[9]Zeng L,Yang Y,Hu Y,et al.Age-related decrease in the mitochondrial sirtuindeacetylase Sirt3 expression associated with ROS accumulation in the auditory cortex of the mimetic aging rat model[J].PLoS One,2014,9(2):e88019.doi:10.1371/journal. pone.0088019.
[10]Qu K,Lin T,Wei J,et al.Cisplatin induces cell cycle arrest and senescence via upregulating p53 and p21 expression in HepG2 cells[J].Nan Fang Yi Ke Da Xue Xue Bao,2013,33(9):1253-1259.doi:10.3969/j.issn.1673-4254.2013.09.01.
[11]Yan BX,Yu SS,Feng X,et al.Effects of D-galactose on ageing of rat mesenchymal stem cells[J].J Zhejiang Univ(Medical Sci),2013,42(6):625-631.[顏冰希,余姍姍,馮曉,等.D-半乳糖對大鼠骨髓間充質干細胞衰老的影響[J].浙江大學學報(醫學版),2013,42(6):625-631.doi:10.3785/j.issn.1008-9292.2013.06.006.
[12]Xiong H,Pang J,Yang H,et al.Activation of miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis:implications for age-related hearing loss[J].Neurobiol Aging,2015,36(4):1692-1701.doi:10.1016/j.neurobiolaging.2014.12.034.
(2015-12-30收稿2016-04-04修回)
(本文編輯魏杰)
The expression of PNUTS in the cochlea of D-galactose induced ageing mice
WU Xidi,ZHANG Qiao,LI Wenjing,LIU Shuangyue△
Department of Physiology,Jinzhou Medical University,Jinzhou 121000,China△
E-mail:dongfangyue5@sina.com
ObjectiveTo observe the expression of protein phosphates 1 nuclear targeting subunit(PNUTS)in the cochlea of D-galactose induced ageing mice.MethodsTwenty Kunming mice,six weeks old,cleaning degree,were randomly divided into two groups,control group and D-galactose group,ten mice for each group.Mice in D-galactose group were administrated with D-galactose at a dose of 800 mg/(kg·d)by subcutaneous injection for eight weeks.Mice in control group were injected with the same volume of saline.After eight weeks,auditory brainstem responses(ABR)were collected to test the hearing thresholds of mice.Western blot assay was used to detect expressions of PNUTS and p53 protein.The expression and distribution of PNUTS in the cochlear Corti,spiral ganglion and striavascularis cells were observed by immunohistochemical(IHC)staining.ResultsThere were no significant differences in ABRs at 8,12 and 24 kHz between two groups.Protein expressions of PNUTS were located in the cochlear hair cells,spiral ganglion cells and striavascularis cells,and the expression level of cochlea was significantly decreased in D-galactose group than that in control group(P<0.05).The expression level of p53 protein was significantly increased in D-galactose group than that in control group(P<0.01).ConclusionPNUTS is expressed in the normal mouse cochlea,and which is down-regulated in the cochlea of ageing mice induced by D-galactose.
protein phosphatase 1;galactose;presbycusis;cochlea;protein phosphates 1 nuclear targeting subunit
R764.43
A
10.11958/20150438
遼寧省教育廳基金資助項目(201410160023,L2015316)
錦州醫科大學生理學教研室(郵編121000)
吳喜迪(1992),男,本科在讀,主要從事老年性耳聾發病機制的研究
E-mail:dongfangyue5@sina.com

