ATP在Aβ1-42導(dǎo)致的突觸可塑性損害中的作用
周治平, 鄭 戈, 賴玉潔, 王年臻, 楊國(guó)帥, 王愛(ài)岳, 余 丹
?
ATP在Aβ1-42導(dǎo)致的突觸可塑性損害中的作用
周治平1,鄭戈2,賴玉潔1,王年臻1,楊國(guó)帥1,王愛(ài)岳1,余丹1
目的探討ATP在調(diào)節(jié)神經(jīng)元突觸可塑性中的作用。方法培養(yǎng)大鼠原代神經(jīng)元細(xì)胞,應(yīng)用Aβ1-42孵育原代培養(yǎng)的神經(jīng)元細(xì)胞48 h,或者在Aβ1-42孵育原代培養(yǎng)的神經(jīng)元細(xì)胞前30 min預(yù)先給予ATP處理細(xì)胞。應(yīng)用Alexa Fluor 488-phalloidin dye染色觀察不同處理后神經(jīng)元樹(shù)突棘的變化。同時(shí),應(yīng)用Western blot檢測(cè)Aβ1-42及ATP處理后對(duì)PDS-95蛋白表達(dá)的影響。結(jié)果ATP能減少Aβ1-42所導(dǎo)致的神經(jīng)元樹(shù)突棘丟失,Aβ1-42孵育原代培養(yǎng)的神經(jīng)元細(xì)胞48 h后,PSD-95蛋白水平降低;而經(jīng)過(guò)ATP預(yù)處理30 min后,神經(jīng)元PSD-95蛋白表達(dá)無(wú)顯著變化。結(jié)論ATP能夠調(diào)節(jié)神經(jīng)元突觸可塑性,從而發(fā)揮腦保護(hù)作用。
膠質(zhì)遞質(zhì);β淀粉樣蛋白;突觸可塑性
阿爾茨海默病(Alzheimer’s disease,AD)是一種進(jìn)行性神經(jīng)系統(tǒng)退行性變,也是最常見(jiàn)的一種癡呆類(lèi)型,其主要的病理特征包括老年斑、神經(jīng)原纖維纏結(jié)和神經(jīng)元死亡[1]。老年斑是由β淀粉樣蛋白(Amyloid-β,Aβ)沉積形成;Aβ的過(guò)度產(chǎn)生是AD發(fā)病的關(guān)鍵環(huán)節(jié)[2]。研究發(fā)現(xiàn),神經(jīng)元能夠釋放神經(jīng)遞質(zhì)以主動(dòng)響應(yīng)外界的刺激。這些遞質(zhì)包括谷氨酰胺、ATP、D-serine等小分子[3]。
谷氨酰胺、D-serine能通過(guò)多種途徑參與神經(jīng)元突觸可塑性調(diào)節(jié)已經(jīng)被證實(shí)[4,5]。同樣的,研究發(fā)現(xiàn)ATP通過(guò)激活嘌呤受體增加突觸后興奮性電流EPSCs的幅值,但在海馬GABA能神經(jīng)元突觸釋放中卻起抑制作用[6]。這些研究結(jié)果提示,神經(jīng)元可能通過(guò)釋放不同的神經(jīng)遞質(zhì)來(lái)參與神經(jīng)元興奮性的調(diào)節(jié),并且同一種神經(jīng)遞質(zhì)可能在不同神經(jīng)元種類(lèi)或突觸中發(fā)揮不同的作用[7,8]。然而,目前對(duì)ATP在阿爾茨海默病突觸功能障礙中作用機(jī)制的研究還遠(yuǎn)不及谷氨酰胺、D-serine等深入,但ATP產(chǎn)生障礙或不足所造成的損害作用是肯定的。因此,本實(shí)驗(yàn)在原代培養(yǎng)的神經(jīng)元內(nèi)觀察,補(bǔ)充ATP后對(duì)Aβ1-42導(dǎo)致的突觸功能障礙的影響,旨在探討神經(jīng)遞質(zhì)ATP參與AD突觸功能障礙的分子機(jī)制,為AD的治療尋找新的靶點(diǎn)。
1 實(shí)驗(yàn)材料
1.1實(shí)驗(yàn)動(dòng)物SPF級(jí)清潔SD孕鼠購(gòu)自重慶醫(yī)科大學(xué)實(shí)驗(yàn)動(dòng)物中心[許可證號(hào):SCXK(渝)2012-0001]。從孕18 d的SD母鼠子宮內(nèi)取胎鼠,雌雄不限。從胎鼠腦內(nèi)提取海馬神經(jīng)元進(jìn)行體外培養(yǎng)。
1.2實(shí)驗(yàn)試劑(1)Aβ1-42、MgATP、多聚賴氨酸均購(gòu)自美國(guó)Sigma公司;(2)Neurabasal培養(yǎng)基、DMEM-F12 培養(yǎng)液、B27、胎牛血清均是Gibco公司產(chǎn)品。L-谷氨酰胺購(gòu)自碧云天生物技術(shù)公司;(3)一抗:小鼠抗大鼠anti-PSD95 (santa,sc-32290);二抗:堿性磷酸酯酶標(biāo)記山羊抗小鼠IgG (H+L)(碧云天,A0258)、SDS-PAGE 凝膠配制試劑盒(碧云天,P0012A)、BCIP/NBT 堿性磷酸酯酶顯色試劑盒(碧云天,C3206)等。
1.3主要溶液配制種植液的配方:EMDM培養(yǎng)液90 ml、10%胎牛血清10 ml、 谷氨酰胺100 μl 、雙抗100 μg(1 μg/ml);培養(yǎng)液的配方:Neurobasal培養(yǎng)基98 ml、1%B27 2 ml、谷氨酰胺100 μl、雙抗100 μg(1 μg/ml)。
2 實(shí)驗(yàn)方法
2.1大鼠原代海馬神經(jīng)元細(xì)胞培養(yǎng)SD孕鼠腹腔注射麻醉后,開(kāi)腹取子宮獲取胎鼠,無(wú)菌條件下,斷頭至75%乙醇消毒,PBS清洗后開(kāi)顱分離胎鼠腦皮質(zhì)。將分離出的大腦皮質(zhì)置于無(wú)鈣鎂的D-hanks溶液中,將大腦皮質(zhì)剪碎,并加入0.25%的胰蛋白酶-EDTA消化液孵箱內(nèi)進(jìn)行消化15 min。消化完成后,將胰酶吸盡,加入含10%胎牛血清的種植液(DMED)終止消化。用巴氏吸管進(jìn)行吹打,然后用400目的網(wǎng)篩過(guò)濾,取細(xì)胞懸液進(jìn)行離心,以800 rpm離心5 min,留取沉淀物。將沉淀物用種植液進(jìn)行重懸。以5×105/ml接種到準(zhǔn)備好的多聚賴氨酸包被的六孔板內(nèi),置于37 ℃,5%CO2室溫培養(yǎng)箱里進(jìn)行培養(yǎng)。種植24 h后,給予全量換液,此后間隔1 d進(jìn)行半量換液。培養(yǎng)至7 d~9 d時(shí)給予相應(yīng)的處理,收集細(xì)胞勻漿后進(jìn)行免疫印跡檢測(cè)相應(yīng)指標(biāo)。
2.2細(xì)胞總蛋白提取(1)經(jīng)干預(yù)處理后的細(xì)胞,置于冰板上,吸盡培養(yǎng)基,用預(yù)冷的PBS漂洗兩次,每次2 min,加入蛋白裂解液60 μl/孔裂解細(xì)胞,用細(xì)胞刮快速刮取每組細(xì)胞,用標(biāo)記好的EP管收集各組細(xì)胞;(2)收集的各組細(xì)胞裂解液置于冰上,每5 min震蕩一次,重復(fù)3次后放入4 ℃離心機(jī),14000 rpm,15 min,吸取上清至新的標(biāo)記好的EP管;(3)留取一部分測(cè)蛋白濃度,剩余部分加入上樣緩沖液(loading buffer)后放入水浴鍋中煮沸5 min,-80 ℃冰箱保存。
2.3蛋白質(zhì)印跡法(Western blot)細(xì)胞蛋白提取液混合5×蛋白上樣緩沖液。煮沸5 min,上樣。等量的蛋白樣品經(jīng)8%的SDS- 聚丙烯酰胺凝膠電泳分離后,以濕轉(zhuǎn)法電轉(zhuǎn)移至PVDF 膜上,再經(jīng)5%BSA 封閉1 h后,加入適量稀釋的一抗(1∶1000),4 ℃過(guò)夜。用洗滌液(washing buffer)洗膜3遍,換入相應(yīng)的二抗(1∶4000),室溫孵育1 h,洗膜3遍,以BCIP/NBT顯色,并且進(jìn)行分析。
2.4海馬CA1神經(jīng)元圖像采集及樹(shù)突棘的形態(tài)定量分析采用LSM510 META激光掃描共聚焦顯微鏡(德國(guó)蔡司)掃描拍攝海馬CA1錐體細(xì)胞,掃描拍攝圖像經(jīng)過(guò)Neurolucida軟件繪制重塑神經(jīng)元的結(jié)構(gòu),并用Neuro Explorer軟件分別統(tǒng)計(jì)出二級(jí)樹(shù)突的樹(shù)突棘的密度。
3 結(jié) 果
3.1ATP能減少Aβ1-42所導(dǎo)致的神經(jīng)元樹(shù)突棘丟失Aβ1-42孵育原代培養(yǎng)的神經(jīng)元細(xì)胞48 h,或者在Aβ1-42孵育原代培養(yǎng)的神經(jīng)元細(xì)胞前30 min預(yù)先給予ATP處理細(xì)胞。應(yīng)用Alexa Fluor 488-phalloidin dye染色觀察不同處理后神經(jīng)元樹(shù)突棘的變化。經(jīng)Aβ1-42孵育后神經(jīng)元樹(shù)突棘顯著減少,而經(jīng)過(guò)ATP預(yù)處理后的神經(jīng)元樹(shù)突棘則無(wú)顯著變化(P=0.033,對(duì)照組 vs Aβ1-42;P=0.870,對(duì)照組 vs Aβ1-42+ATP;P=0.870,對(duì)照組 vs ATP,見(jiàn)表1)。
3.2ATP能拮抗Aβ1-42所導(dǎo)致的神經(jīng)元突觸蛋白PSD-95的表達(dá)減少Aβ1-42孵育原代培養(yǎng)的神經(jīng)元細(xì)胞48 h后,PSD-95蛋白表達(dá)水平降低;而經(jīng)過(guò)ATP預(yù)處理30 min后的神經(jīng)元PSD-95蛋白表達(dá)水平無(wú)顯著變化(P<0.001,對(duì)照組 vs Aβ1-42;P=0.509,對(duì)照組 vs Aβ1-42+ATP;P=0.835,對(duì)照組 vs ATP,見(jiàn)表2)。
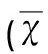
表1 各組熒光強(qiáng)度值比較±s)
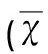
表2 各組蛋白印跡灰度值的比較±s)
4 討 論
我們的實(shí)驗(yàn)結(jié)果顯示,神經(jīng)遞質(zhì)ATP能夠抑制Aβ1-42所導(dǎo)致的突觸功能障礙。首先,我們?cè)谠囵B(yǎng)的大鼠神經(jīng)元里證實(shí)ATP能夠拮抗導(dǎo)致的神經(jīng)元樹(shù)突棘缺失。進(jìn)一步研究發(fā)現(xiàn),ATP不僅能改善Aβ1-42導(dǎo)致的突觸形態(tài)學(xué)改變,還能逆轉(zhuǎn)Aβ1-42所導(dǎo)致的突觸蛋白PSD-95的減少。這些結(jié)果表明ATP能夠拮抗Aβ1-42對(duì)神經(jīng)突觸可塑性的影響而發(fā)揮腦保護(hù)作用。
在中樞神經(jīng)系統(tǒng)中,ATP作為神經(jīng)遞質(zhì)對(duì)神經(jīng)元的興奮作用是普遍存在的[9]。突觸間隙中存在著ATP的特異性水解酶(ATP酶)和腺苷三磷酸雙磷酸酶。釋放到突觸間隙的ATP及其代謝產(chǎn)物具有信息傳遞的作用。在外周,ATP可使神經(jīng)元產(chǎn)生快速的興奮性突觸后電位(excitatory postsynaptic potential,EPSP)[10]。Nakazawa等報(bào)道,在大鼠交感神經(jīng)元和神經(jīng)肌肉接頭處ATP不僅能直接激活膽堿能受體使離子通道自發(fā)性開(kāi)發(fā)活動(dòng)的次數(shù)提高10倍,還能使其受體通道開(kāi)放的次數(shù)明顯增加[11]。
為了進(jìn)一步研究單純ATP對(duì)神經(jīng)元的影響,同時(shí)避免其他神經(jīng)遞質(zhì)如谷氨酰胺、細(xì)胞因子的影響。我們應(yīng)用ATP直接處理原代培養(yǎng)的神經(jīng)細(xì)胞,我們觀察到應(yīng)用ATP預(yù)處理神經(jīng)元30 min能夠有效逆轉(zhuǎn)Aβ1-42導(dǎo)致的神經(jīng)元樹(shù)突棘及突觸蛋白PSD-95的減少。PSD-95是突觸后致密物質(zhì)中的一種特殊蛋白,它能夠介導(dǎo)蛋白質(zhì)間的相互作用[12]。PSD-95在突觸上的數(shù)量變化是突觸功能活動(dòng)的重要結(jié)構(gòu)基礎(chǔ)[13]。研究表明,敲除PSD-95基因可引起小鼠長(zhǎng)時(shí)程增強(qiáng)(LTP)的改變和學(xué)習(xí)記憶功能障礙[14]。目前,LTP已被普遍認(rèn)為是學(xué)習(xí)記憶鞏固過(guò)程中神經(jīng)元生理活動(dòng)的客觀指標(biāo),反映了突觸水平上的信息儲(chǔ)存過(guò)程[15]。在我們的實(shí)驗(yàn)中,我們觀察到Aβ1-42能夠影響神經(jīng)元突觸可塑性,這表現(xiàn)在樹(shù)突棘數(shù)量及突觸蛋白PSD-95的減少。而ATP能夠抑制Aβ1-42對(duì)神經(jīng)元突觸可塑性的影響,表現(xiàn)在補(bǔ)充ATP后神經(jīng)元樹(shù)突棘數(shù)量及突觸蛋白PSD-95的表達(dá)增加。在神經(jīng)元樹(shù)突棘上,ATP及其偶聯(lián)的信號(hào)轉(zhuǎn)導(dǎo)通路,通過(guò)各種支架蛋白形成突觸后致密區(qū)(PSD),它含有幾百種蛋白質(zhì)。這種復(fù)雜而精巧的棘突結(jié)構(gòu),是接收突觸前信號(hào)并進(jìn)行生化加工的獨(dú)立單元。樹(shù)突棘能對(duì)接收的大量信號(hào)進(jìn)行神經(jīng)計(jì)算和整合,并依據(jù)刺激的方式做出反應(yīng),使突觸的結(jié)構(gòu)和功能發(fā)生相應(yīng)變化,即形成突觸的可塑性。而突觸可塑性被認(rèn)為是學(xué)習(xí)和記憶的分子機(jī)制與認(rèn)知功能相關(guān)。
5 結(jié) 論
我們的研究發(fā)現(xiàn)ATP能夠調(diào)節(jié)神經(jīng)元突觸可塑性。在AD中,由于Aβ所導(dǎo)致的樹(shù)突棘及突觸蛋白PSD-95的減少能夠被ATP所抑制,從而發(fā)揮腦保護(hù)作用。因此,有效控制ATP的釋放或糾正ATP生成不足可能成為治療AD的潛在靶點(diǎn)。
[1]Kam K,Duffy AM,Moretto J,et al.Interictal spikes during sleep are an early defect in the Tg2576 mouse model of beta-amyloid neuropathology[J].Scientific Reports,2016,6:201-219.
[2]Diaz M,Fabelo N,Casanas-Sanchez V,et al.Hippocampal lipid homeostasis in APP/PS1 mice is modulated by a complex interplay between dietary DHA and estrogens:relevance for Alzheimer’s disease[J].J Alzheimer’s Dis,2015,49(2):459-481.
[3]Li X,Zhang YY,Chen ZQ,et al.D-serine-induced inactivation of NMDA receptors in cultured rat hippocampal neurons expressing NR2A subunits is Ca2+-dependent[J].CNS Neuroscience & Therapeutics,2014,20(11):951-960.
[4]Natsubori T,Inoue H,Abe O,et al.Reduced frontal glutamate+glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia[J].Schizophrenia Bulletin,2014,40(5):1128-1139.
[5]Han H,Peng Y,Dong Z.D-Serine rescues the deficits of hippocampal long-term potentiation and learning and memory induced by sodium fluoroacetate[J].Pharmacology Biochemistry Behavior,2015,133:51-56.
[6]Khakh BS.ATP-gated P2X receptors on excitatory nerve terminals onto interneurons initiate a form of asynchronous glutamate release[J].Neuropharmacology,2009,56(1):216-222.
[7]Martin ED,Buno W.Stabilizing effects of extracellular ATP on synaptic efficacy and plasticity in hippocampal pyramidal neurons[J].Euro J Neurosci,2005,21(4):936-944.
[8]Willyerd FA,Empey PE,Philbrick A,et al.Expression of ATP-binding cassette transporters B1 and C1 after severe traumatic brain injury in humans[J].J Neurotrauma,2016,33(2):226-231.
[9]Pankratov Y,Lalo U,Verkhratsky A,et al.Vesicular release of ATP at central synapses[J].Euro J Physiol,2006,452(5):589-597.
[10]Suzuki T.Cellular mechanisms in taste buds[J].The Bulletin of Tokyo Dental College,2007,48(4):151-161.
[11]von Kugelgen I,Kurz K,Starke K.Axon terminal P2-purinoceptors in feedback control of sympathetic transmitter release[J].Neuroscience,1993,56(2):263-267.
[12]Sultana R,Banks WA,Butterfield DA.Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: Insights into their potential roles for loss of synapses and memory,accumulation of Abeta,and neurodegeneration in a prodromal stage of Alzheime’s disease[J].J Neurosci Res,2010,88(3):469-477.
[13]Greenwood IA.Intricate vascular architecture revealed after removing the scaffolding:PSD95 crucial for vascular Kv1 function[J].J Physiology,2011,589(24):5901.
[14]Huang YN,Tsai RY,Lin SL,et al.Amitriptyline attenuates astrocyte activation and morphine tolerance in rats: role of the PSD-95/NR1/nNOS/PKC gamma signaling pathway[J].Behavioural Brain Res,2012,229(2):401-411.
[15]Rammes G,Gravius A,Ruitenberg M,et al.MRZ-99030-A novel modulator of Abeta aggregation:II-Reversal of Abeta oligomer-induced deficits in long-term potentiation (LTP) and cognitive performance in rats and mice[J].Neuropharmacol,2015,92:170-182.
The effect of ATP on Aβ1-42mediated disruption of synaptic plasticity
ZHOUZhiping,ZHENGGe,LAIYujie,etal.
(DeparmentofNeurology,HaikouPeople’sHospital,Haikou570208,China)
ObjectiveThis study was conducted to investigate the effect of ATP in regulatory on synaptic plasticity.MethodsPrimary neurons were cultured successfully.Neurons were treated with Aβ1-42or ATP.The neuronal spine loss and PSD-95 expression were studied by using immunohistochemistry and Western blotting,respectively.ResultsWe found that exogenous ATP protected Aβ1-42mediated reduction in synaptic molecules,such as PSD-95 and prevented Aβ1-42induced spine reduction in cultured primary hippocampal neurons.ConclusionOur findings suggested that ATP plays a protective role against Aβ1-42mediated disruption of synaptic plasticity.
Neuron;Amyloid-β;Synaptic plasticity
1003-2754(2016)08-0724-03
2016-04-18;
2016-05-29
(1.海口市人民醫(yī)院神經(jīng)內(nèi)科,海南 海口 570208;2.吉林大學(xué)第二醫(yī)院肝膽胰外科,吉林 長(zhǎng)春 130041)
周治平,E-mail:zzping@yeah.net
R749.1
A

