Pharmacokinetic study of dl-tetrahydropalmatinepatchesby UPLC–MS/MSin rabbits
Wei-Jie Xie,Yong-Ping Zhang?,Jian Xu
1School of Pharmacy,Guiyang College of Traditional Chinese Medicine,Guiyang 550002,China.
1.Introduction
Dl-tetrahydropalmatine is a very effective monoamine depletor in brain.It is extracted from Chinese herbal medicine corydalis [1]. DL-tetrahydropalmatine possesses analgesic,sedative,tranquilizing,hypnotic,antihypertensive and hypo-locomotion actions[2-3].Dl-tetrahydropalmatine presents a good effect with chronic lasting pain and viscera blunt pain,such as headache,chest pain,hypochondriac pain,epigastric pain, abdominal pain, backache, arthralgia,dysmenorrheal and trauma[4].It is effective not only for the effect of abirritation but also for sedative-hypnotic [5].The results suggest that dl-tetrahydropalmatine at defined low dosages acts as anxiolytics in mice[6].With lower toxicity,greater safety and no addiction,dl-tetrahydropalmatine is widely used asa non-narcotic analgesics.
Considering its smaller molecular,it should be absorbed easily by the skin.Since 2006,experts from Guiyang College of Traditional Chinese Medicine devoted themselves to research transdermal patch..Many researches were finished based on the matrixes of dl-tetrahydropalmatine transdermal patch.Finally they determined the optimum ingredients of formulation was PVA-PVP-glycerolv-CMC-Na
laurocapram-propanediol=14.44∶5.87∶11.53 ∶3.00∶4.97∶4.62.Experimental data suggest the transdermal patch offers better effect in penetrating the skin[7].Based on the previous study,we observed the dl-tetrahydropalmatine patch on pharmacokinetics mode,and established a measuring method to supervise the blood drug concentration,which would promote the clinical applications of dl-tetrahydropalmatine.
Many test methods have been used to determine the content of dl-tetrahydropalmatine,such as Spectrophotometry[8],Thin Layer Chromatography Scanning[9,10],Fluorescence[11]and HPLC[12].UPLC-MS/MS was employed in this experiment,with its feature of precision,stability,and the lowest test line.
2.Methods
2.1 Chemicalsand reagents
Acetonitrile and methanol(HPLC grade)were purchased from American Tedia Company.BEH Shield C18(solid-phase extraction)cartridge columns were purchased from American Waters Corporation.Standard substance of dl-tetrahydropalmatine was purchased from the National Institute for the Control of Pharmaceutical and Biological Products(Beijing,China),and dl-tetrahydropalmatinepharmaceutical raw materials were purchased from Sha anxi Jingtai Biological Corporation(the Purity more than 98%).The dl-tetrahydropalmatine transdermal patches were prepared in the Key Laboratory of Modern Materia Medica Preparation(Guiyang,China).
2.2 Instrumental analysis
Technology of UPLC-MS/MS[13-14]was employed to detect dl-tetrahydropalmatine concentration in plasma.It consisted of the ACQUITY UPLC instrument,the triple quadrupole mass spectrometer of WATERSXevo TQseriesand the Waters Empower 2.0 chromatography data software(American Waters Company,U.S).The mobile phase consisted of 70%acetonitrile(B)and 30%water(A).The flow rate was kept at 0.2mL/min for a total run time of two minutes.The samples were introduced into the electrospray ion source of the triple quadrupole mass spectrometer of WATERSXevo TQ with ESI ion source.Electrospray ionization interface parameters were as follows:spray voltage:electrospray ionization interface parameters were as follows:Ionization(ESI)in positive ion mode;capillary voltage 4000V,atomizing air pressure:30psi;drying gasflow rate(N2):8L/min;drying temperature:350℃;debris voltage:165eV;mass scan range:m/z 200~1500.The MSwas operated in multiple reaction monitoring mode (MRM) with the dl-tetrahydropalmatine quantitative ion:Parent[m/z]356.27,Daughter[m/z]165.14,Daughter[m/z]192.12,which wasasfollows:Table1.
2.3Animals
New Zealand white rabbits,weighing 2-2.5kg,were purchased from Guiyang Medical College Animals Center(Guiyang,China).All the rabbits were divided into 2 groups(group A and group B).They were raised in standard environmental conditionsfor two dayswith free access to food and water.And then rabbits were administered percutaneously with dl-tetrahydropalmatine patches(group A,n=3)and dl-tetrahydropalmatine drugs (group B, n=3)respectively.
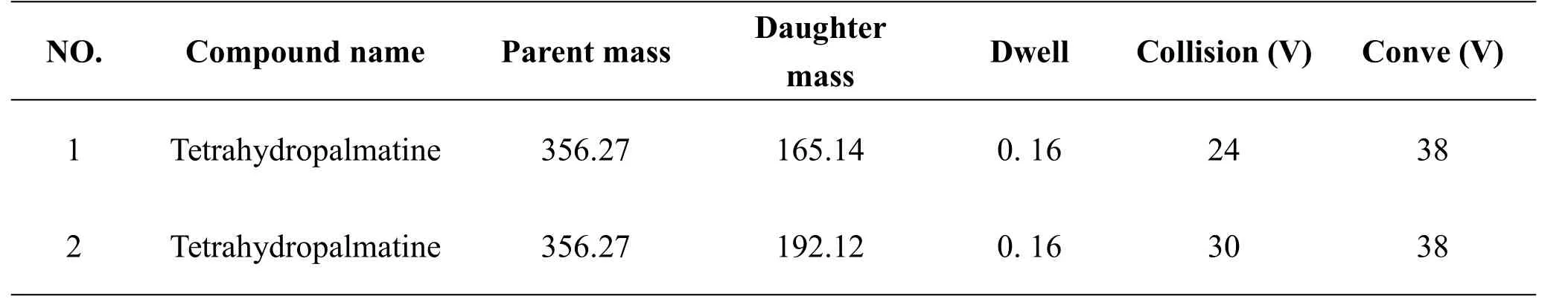
Table1 Quantitativeion spectrometry conditions
2.4 Samplepreparation
The dl-tetrahydropalmatine patch was prepared on the basis of previous formulation and preparation process.The determination of thearea was 5.83cm2;the content of dl-tetrahydropalmatine was 45 mg approximately.Rabbits had been fasting but had free access to water for 18 hours prior to the experiment.Each blood sample(2ml)was taken in the marginal ear vein at 0hr,1hr,2hr,4hr,6hr,8hr,12hr,17hr,20hr and 24hr after administration,and was transferred to a heparinized glasstubeimmediately.
Then 2ml of rabbit blood were mixed with concentrated ammonia to be alkaline,then centrifuged at 6000 rpm for 10 min.The precise amount of the upper plasma0.7ml wasvortexed for 3 min with 3mL chloroform,and then centrifuged at 4000 rpm for 15 min.The 2.7ml upper solution was taken out;the raffinate was extracted two times as the first one.The extract was dried with nitrogen;0.7mL acetonitrilewas drawn precisely,which was used to redissolve,vortex for 3 minutes,ultrasonic for 15 minutes in the tube;after the sample was via 0.22μm microporous membrane,it was injected into the UPLC-MS/MS system asa tested sample.
2.5 Method validation
The appropriate dl-tetrahydropalmatine reference substance was weighed accurately,and was formulated into standard solutions with acetonitrile(1.9μg.ml-1),as the reserve liquid.Blank plasma and standard solutions were mixed and then were processed,as the reference solution.Blank plasma(2ml)was taken and processed,as a blank solution;the reference solution,blank solution and the test solution was injected.The result showed that measurement method of plasma componentswasnon-intrusiveand specific(Figure1).
2.5.1 Calibration curveestablishment
Precisely the dl-tetrahydropalmatine reference solution amount were placed in a centrifuge tube,then 2mL blank plasma was added into each tube;which was made into the standard samples; the sample concentrations contained 10.45ng/ml,52.25ng/ml,104.50ng/ml, 522.50ng/ml, 1045.00 ng/ml and 2090.00ng/ml.According to the sample processing method,the control standard samples were prepared,then the standard sample solutions of different concentrations,respectively,were injected in 3μl.The linear regression on peak area(A)and the reference samples concentration(C)was that:A=5425C+213362,showing dl-tetrahydropalmatine in the control standard sampleat 0.0104~2.090μg/ml.
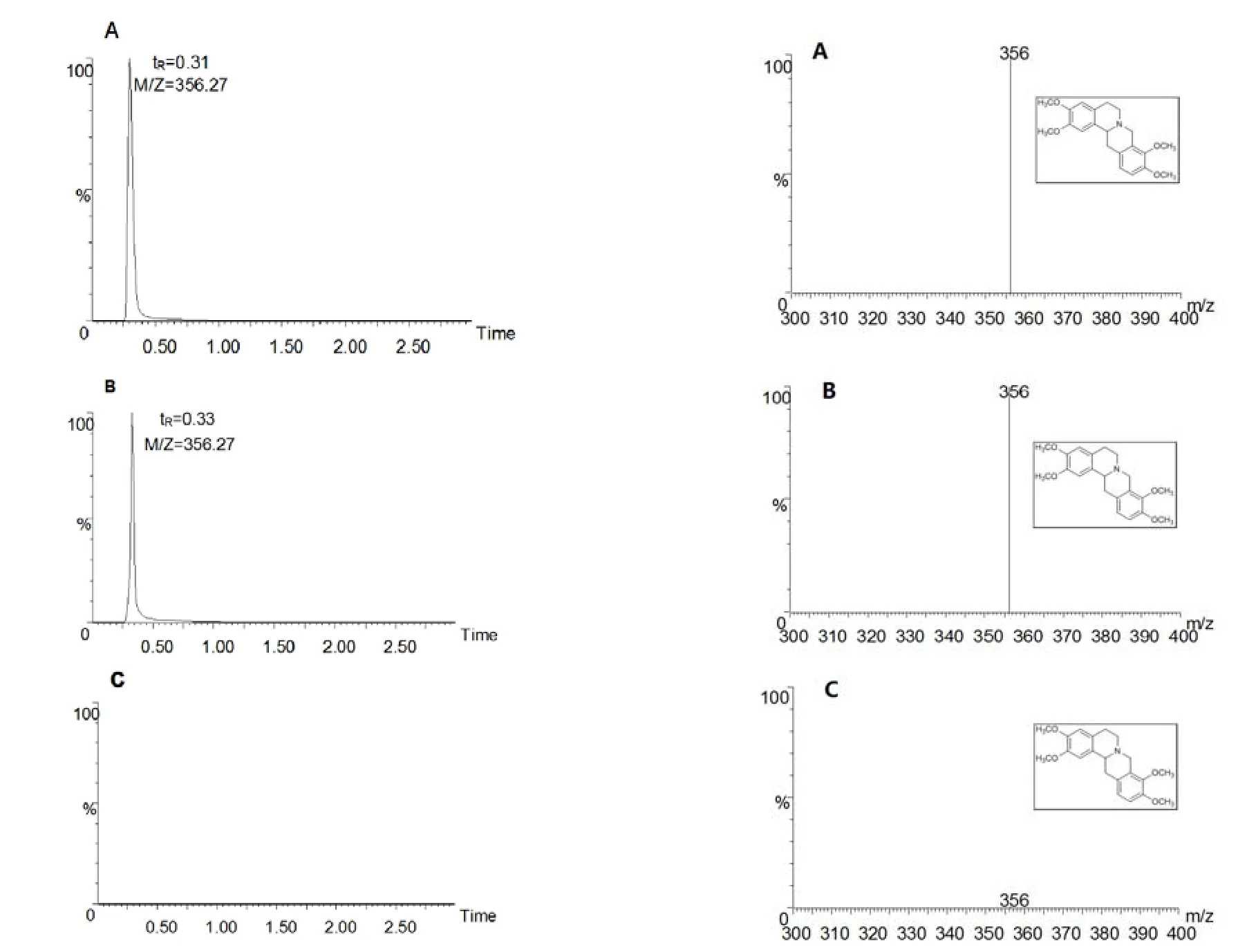
Figure 1 Dl-tetrahydropalmatineTIC chromatograms(L)and mass spectrum(R)A.Reference solution B.Plasma solution C.Blank solution.
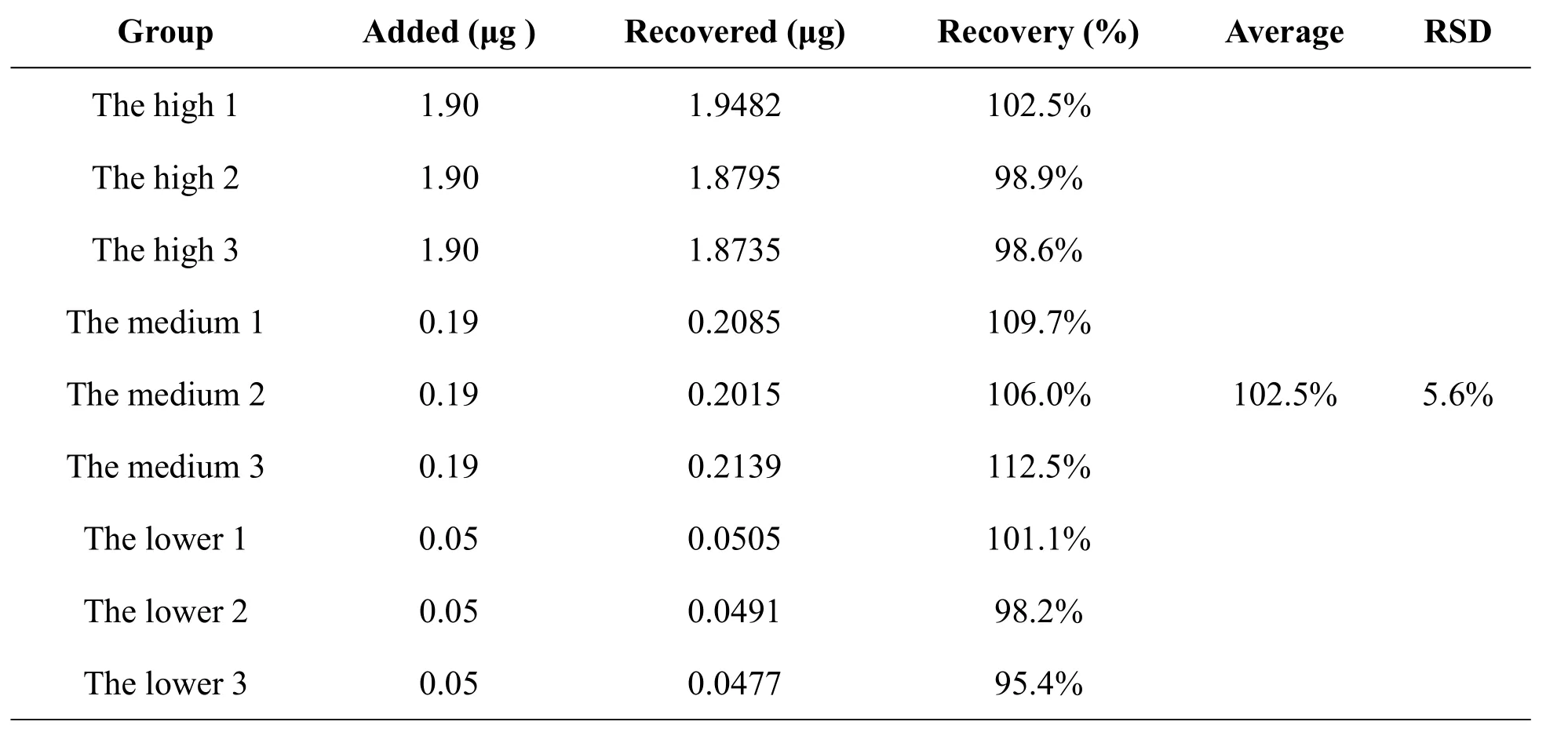
Table 2 Recovery of dl-tetrahydropalmatine(mean,n=3).
2.5.2Accuracy and precision
Three parts the reference solution of three kinds of concentration contained 1.9μg,0.19μg,0.05μg;after been dried with nitrogen,they were added blank plasma 0.7ml,mixed,processed and were prepared the high,the medium and the lower samples of the recovery test.As a result,the recovery was 102.5%(Table2).
According to the sample processing method above,the test sample was well processed.The test sample and 190ng/ml standard solution was detected to determine the precision.This result,RSD 1.1%and 1.2%(n=6),fully met therequirements.
2.5.3 Stability study
In accordance with the processing method above,blood samples away from light were handled and stored under the condition of 38℃,which was detected once every 4 hours to study the stability within 24 hours.This result,RSD 4.3%,showed that at body temperature,the sample remained stable without no significant difference,meeting therequirements.
In the light of the processing method above,blood samples were handled and then stored in the refrigerator at 4℃,which was detected once every 24 hours to study the stability within a week.The result,RSD1.1%(n=7),indicated that the sample remained stablein 7 days.
3.Results
3.1 Pharmacokinetic parameters
The pharmacokinetic data processing software PKSolver2.0 was applied to process the data[15-16].The concentration-time curves of group A and group B were shown in Figure 2 and Figure 3.The analysis showed that the absorption, distribution and metabolism of the two groups matched one compartment model(Table 3),and pharmacokinetic parameterswereshown in Table3.
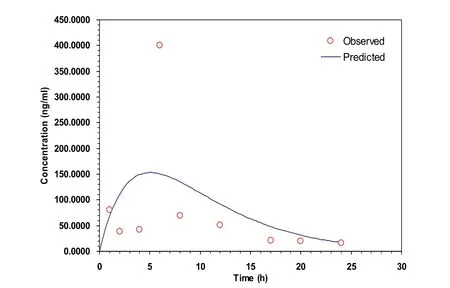
Figure 2 Tansdermal administration C-t curve of the A group(mean,n=3).
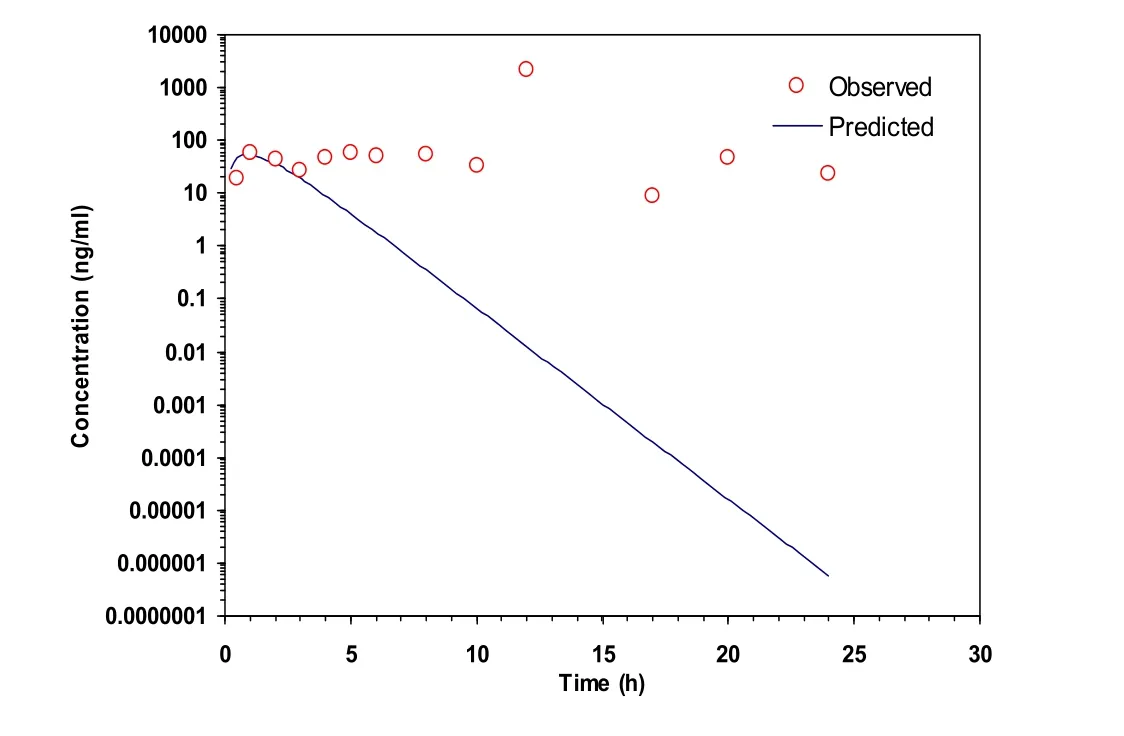
Figure 3 Transdermal administration C-t curve of the B group(mean,n=3).
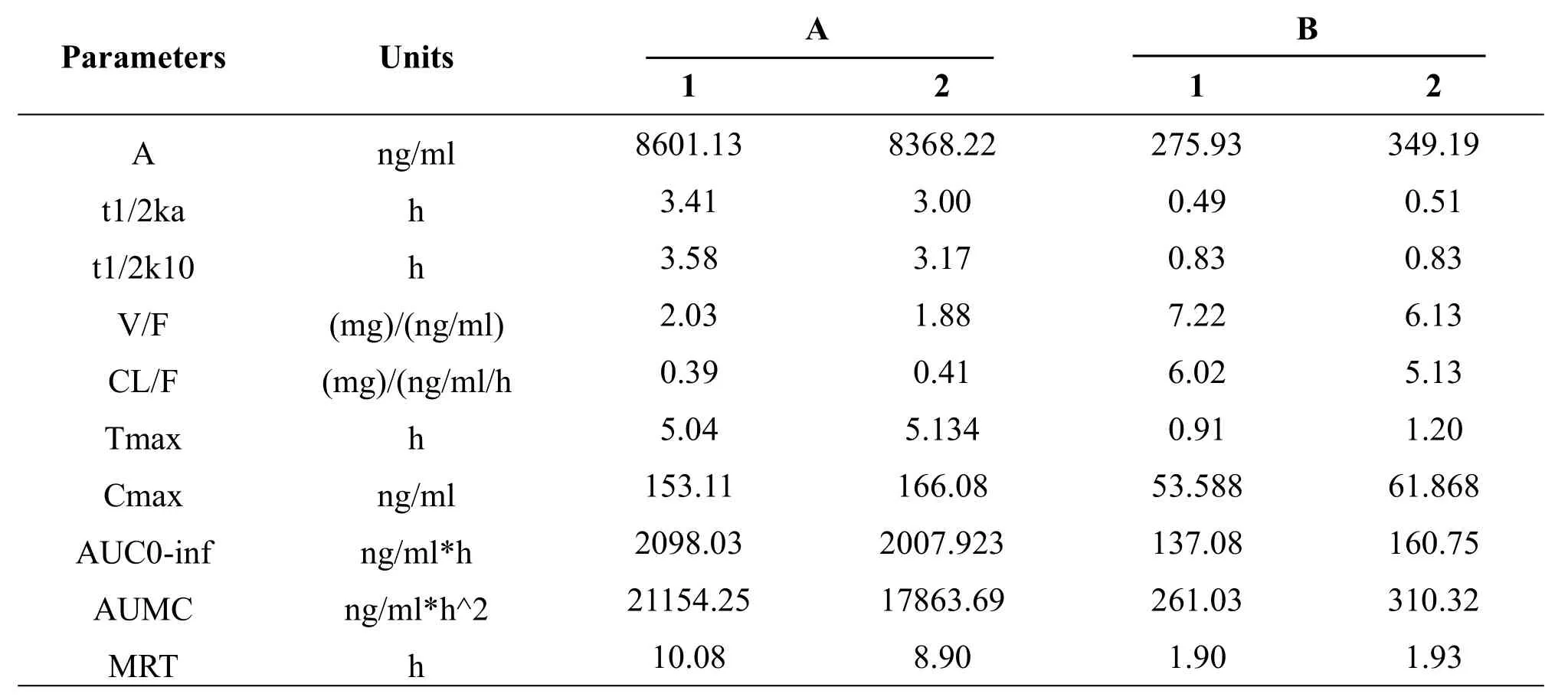
Table 3 Pharmacokineticsparametersof two groups(mean,n=3)
4.Discussion
The paper established the determination method of the plasma concentration of dl-tetrahydropalmatine patch via rabbit skin.According to the authentication and related data,it was indicated that it is simple,convenient and fast to determine plasma concentrations of the dl-tetrahydropalmatine patch via rabbit skin by UPLC-MS/MS.For precision,stability,absolute recovery,the lowest test line(0.14ng/ml)is investigated,and the results show that the related method validation are fully meeting the determined requirements.
Meanwhile, the measurement conditions of dl-tetrahydropalmatine are established, using UPLC-MS/MS:Parent[m/z]356.27,Daughter[m/z]165.14 and Daughter[m/z]192.12,which is showing as Table 1,and the experimental section gives its collision energy and cone voltage parameters.When treating a blood sample,the study mainly researches chloroform and ethyl acetate as the purified extraction solvent.By comparison,it is found that chloroform dissolution rate and solubility of dl-tetrahydropalmatine are higher than ethyl acetate,and could be extracted completely within a few seconds.Whereas extracted dl-tetrahydropalmatine using chloroform,theresidual degreein the vessel wall is far less than the ethyl acetate;through the average recovery test,it can be found that extraction and purification using chloroform could fully meet the test requirements.
For the dl-tetrahydropalmatine patch,it can be found that the water content is a great impact factor on the transdermal efficiency,so it must be controlled more than 33%in the experiment,and primarily with the stratum corneum(SC)water-related.Appropriately increasing the humidity can affect skin SC hydration and then the skin SC cells loosely arranged.The transdermal effect wasenhanced[17].
After dl-tetrahydropalmatine patch transdermal administration,the pharmacokinetics model of the rabbit in vivo is consistent with one compartment model, and obtained relevant pharmacokinetics parameters.The specific data can be seen in the above tables.Compared with the pure dl-tetrahydropalmatine transdermal(group B),the transdermal effect of dl-tetrahydropalmatine patches are better than the pure dl-tetrahydropalmatine:the Cmaxincreased about 3 times to group B and the maximum plasma concentration is significantly improved.The MRT of group A is more than five times to the group B.The residence time in vivo is significantly longer.It indicates that the patch can decrease the releasing rate and also significantly improvethebioavailability.
Acknowledgements
Supported by the Guizhou Province Science and Technology Agency Fund Project(NO.2011 KZ7048).
Conflict of interest
Theauthorsreport no declarationsof interest.
1.Liu GQ, Algeri S, Garattini S.D-L-tetrahydropalmatine as monoamine depletor.Arch Int Pharmacodyn Thér 1982,258(1):39-50.
2.Lin MT,Chueh FY,Hsieh MT,et al.Antihypertensive Antihypertensive effects of dl-tetrahydropalmatine:an active principle isolated from corydalis,Clin Exp Pharmacol Physiol,1996,23(8):738-742.
3.Lin MT,Chueh FY,Hsieh MT.The hypothermic effects of dl-tetrahydropalmatine in rats.Neurosci Lett 2001,315(1–2):53-56.
4.Huang TK.Handbook of the composition and pharmacology of common Chinese drugs.Beijing:Chinese medicine science and technology press,1994.
5.Hsu B,Kin KC.Pharmacological study of tetrahydropalmatineand itsanalogs.A new type of central depressants.Arch Int Pharmacodyn Thér 1962,139:318-327.
6.Leung WC,Zheng H,Huen M,et al.Anxiolytic-like action of orally administered dltetrahydropalmatine in elevated plus-maze.Prog Neuropsychopharmacol Biol Psychiatry 2003,27(5):775-779.
7.Xu J,Zhang ZH,Zhang YP.Formula of tetrahydropalmatine patch.Central South Ph armacy 2008,6(2):148-150.
8.Meng QG,Lu XR,Wang QY.Discrimination and Determination of Rotuntine in Zhitong Powder.Acta Academiae Med Weifang1998,20(2):101-102.
9.Yang LX,Wang LX,Zhang BC.Determination of Tetrahydropalmatine in Yuanhuzhitong Dropping Pills by TLC-scanning.Chin Tradit Pat Med 2001,23(12):869-871.
10.Zhang M,Wu YH,Shao GZ.Determination of Tetrahydropalmatine in Anwei Tablets by Dual Wavelength TLC-scanning.Chin Tradit Pat Med 2000,22(12):868-870.
11.Pang ZG,Wang BQ,Huang H.Study on the content of Tetrahydropalmatine in Corydalis Yanhusuo by SLS-micellar Enhanced Fluorometry.Journal of Chin Pharm Univ 1993,24(6):345-347.
12.Gong Q,Zhou D,Wang BJ.Assay of tetrahydropalmatine in yanhusuo by HPLC.Chin J Mod Appl Pharm 2000,17(4):315-317.
13.Xu HQ,Xu LQ,Xu JW.Common natural extract quality standard reference.Beijing:Chemical Industry Press,2003:40-41.
14.Zeng CX,Zhang SH,Fang Y.The preparation and pharmacokinetics research of Cetirizine transdermal patch.Chin J Mod Drug Appl 2011,11(5):1-2.
15.Fang SP,Yang BY.Pharmacy promote transdermal absorption mechanism of Technology[J].Chin J Hosp Pharm 2000,20(12):750-752.
16.Wang Y,Xie H,Pan SL.Pharmacokinetics of laetispicine and its brain distribution in rats.Am J Chin Med 2010,38(5):895-907.
17.Cutsuridis V, Wennekers T. Wennekers.Hippocampus, microcircuits and associative memory.Neural Netw 2009,22(8):1120-1128.
 Traditional Medicine Research2016年4期
Traditional Medicine Research2016年4期
- Traditional Medicine Research的其它文章
- Dynamic changesof circulating Th1 and Th17 cellsin psoriasispatients:a report of 3 cases treated by Chineseherbal medicine
- A prospective,randomized controlled trial of sanguisorba oil in the treatment of tamoxifen-related vaginitisin breast cancer patients
- A Literature Review of the Acupoint Plaster Therapy for Asthma in Summer
- Effectsof acupuncture treatment for irritablebowel syndrome:a systematic review and meta-analysis
- A new idea of electro-acupuncturetreatment for peripheral facial paralysis and thenerve-endocrinehypothesis
- Challengesand opportunitiesof applying P4 medicineand traditional Chinese medicinefor cancer treatment and prevention in the21st century:A medical oncologist’sperspectives
