輔助配體導向合成兩個鋅金屬配位聚合物:結構,穩定性和熒光性質
郭培英 呂利霞 馬德運 郭海福*,(內蒙古化工職業學院化學工程系,呼和浩特 00070)(肇慶學院化學化工學院,肇慶 5606)
?
輔助配體導向合成兩個鋅金屬配位聚合物:結構,穩定性和熒光性質
郭培英1呂利霞1馬德運2郭海福*,2
(1內蒙古化工職業學院化學工程系,呼和浩特010070)
(2肇慶學院化學化工學院,肇慶526061)
摘要:以二苯-4,4′-二羧酸和六水硝酸鋅為原料,在相同反應條件下,通過改變不同的輔助配體(2,2′-二甲基-4,4′-聯吡啶和三乙烯二胺),合成出2個金屬有機配位聚合物:{[Zn(DBA)(dmbpy)0.5]·2H2O}n(1)和[Zn2(DBA)2(dabco)]n(2)(H2DBA=二苯-4,4′-二羧酸dmbpy=2,2′-二甲基-4,4′-聯吡啶,dabco=三乙烯二胺)。通過元素分析、紅外光譜、差熱分析、X射線粉末衍射和X射線單晶衍射等手段對配合物進行了結構表征。結果顯示,化合物1為二維(4,4)網絡結構,2個二維結構在平行方向上通過互鎖進一步形成2D→2D的互鎖網絡結構。化合物2為一維帶狀結構。熱穩定性表明化合物1能夠穩定到370℃;而化合物2穩定性差。熒光分析表明常溫固態下配合物1和2均發射藍色熒光,熒光壽命分別為231.4 ns和2.33 ns。
關鍵詞:配位聚合物;晶體結構;二苯-4,4′-二羧酸;熒光性質
粵西林產化工技術開發與應用研究中心項目基金(No.2014KTSPT040)資助項目。
*通信聯系人。E-mail:guohaifu@sina.com
0 Introduction
Metal-organic coordination polymers(CPs),have received widespread attention over the past decade owing to their modular assembly,structural diversity and fascinating topology,chemical tailorability and tenability,as well as their excellent properties with promisingapplicationssuchasgasstorageand separation,nonlinearoptics,catalysis,magnetism,luminescence,drug delivery,sensing,and detection[1-7]. During the attainment of CPs,many factors can influence the construction progress,e.g.,metal ions,organic ligands,solvents,pH values,reaction temperatures,and so on[8-9].Among many on-going efforts to develop high-performance CPs materials,selection and utilization of different organic ligands is considered to be the most significant factors that affect the structures and properties of final products[10-11].Up to date,more attention has been paid to complicated organic ligands because of their various coordinated modesandabilitytoobtainnovelstructures[12]. However,it is fussy to obtain these ligands,which requires much more effort to achieve industrialization or practical application.As part of an on-going study related to functional CPs,4,4′-methylenedibenzoic acid(H2DBA),2,2′-dimethyl-4,4′-bipyridine(dmbpy)and triethylenediamine(dabco)were chosen to afford two transition metal-based coordination polymers,{[Zn (DBA)(dmbpy)0.5]·2H2O}n(1)and[Zn2(DBA)2(dabco)]n(2),which are characterized by elemental analyses,IR spectrum,thermogravimetric analyses,powder X-ray diffraction and single-crystal X-ray crystallography. Furthermore,the stability and luminescence properties of 1 and 2 were also investigated.
1 Experimental
1.1Materials and measurements
All chemicals purchased were of reagent grade and used without further purification.2,2′-dimethyl-4,4′-bipyridine(dmbpy)was isolated based on the reported procedures[6].All syntheses were carried out in 23 mL Teflon-lined autoclaves under autogenous pressure.Elemental analyses(C,H and N)were performed on a Perkin-Elmer 240 CHN elemental analyzer.Infrared spectra were taken on a Shimadzu IR-440 spectrometer with a KBr disk in the 4000~400 cm-1region.Thermogravimetric analyses(TGA)were carried out on an automatic simultaneous thermal analyzer(DTG-60,Shimadzu)under N2atmosphere at a heating rate of 10℃·min-1within a temperature range of 25~800℃.Powder XRD investigations were carried out on a Bruker AXS D8-Advanced diffractometer at 40 kV and 40 mA with Cu Kα(λ=0.154 06 nm)radiation.Luminescence spectra and lifetime for crystal solid samples were recorded at room temperature on an Edinburgh FLS920 phosphorimeter.
1.2Synthesis of 1
A mixture of Zn(NO3)2·6H2O(0.15 g,0.5 mmol),H2DBA(0.128 g,0.5 mmol)and dmbpy(0.046,0.25 mmol)in 12 mL DMF.The resulting solution was sealed in a 23 mL Teflon-lined stainless steel autoclave and heated at 130℃ for 3 days under autogenous pressure.Colorlesssinglecrystalswereobtained (Yield:68%,based on DBA)upon cooling the solution to room temperature at 5℃·h-1.Anal.Calcd.for C42H31N2O8Zn2(%):C,61.28;H,3.77;N,3.40.Found (%):C,62.51;H,3.53;N,3.31.IR(KBr,cm-1):3 442 (s),3 063(w),2 933(m),1 674(m),1 613(vs),1 565(s),1 535(m),1 383(vs),1 303(s),1 172(s),1 107(m),1 016 (m),967(w),886(m),854(m),813(m),762(vs),743(m)m,711(s),699(m),645(w),637(w),566(m),512(m),477(w),428(s).
1.3Synthesis of 2
The synthetic procedure of 2 was same as that of 1 except that dmbpy was replaced by dabco(Yield: 45%,based on DBA).Anal.Calcd.for C36H32N2O8Zn2(%):C,57.49;H,4.26;N,3.73.Found(%):C,57.61;H,4.13;N,3.80.IR(KBr,cm-1):3 423(s),2 981(w),2 935(w),1 608(vs),1 555(s),1 504(m),1 447(m),1 367 (vs),1 315(w),1 268(w),1 187(w),1 150(w),1 084 (m),1 046(m),857(m),745(s),659(m),579(m),517 (w),442(w).
1.4Crystal structure analysis
SinglecrystalX-raydiffractionanalysesof compounds 1~2 were performed on a Bruker ApexⅡCCD diffractometer operating at 50 kV and 30 mAusing Mo Kα radiation(λ=0.071 073 nm).Data collection and reduction were performed using the APEX Ⅱsoftware[18].Multi-scan absorption corrections were applied for all the data sets using SADABS,as included in the APEXⅡprogram[18].The structures were solved by direct methods and refined by least squares on F2using the SHELXTL program package[19]. The routine SQUEEZE(PLATON)[20]were applied to remove diffuse electron density caused by badly disordered water molecules of 1.The formula unit of 1 was arrived at through combination of elemental analyses,IR spectra and thermogravimetric characteri zation.All non-hydrogen atoms were refined with anisotropic displacement parameters.Hydrogen atoms attachedtocarbonandoxygenwereplacedin geometrically idealized positions and refined using a riding model.Crystallographic data and structura refinement detail of compounds 1~2 can be found in Table 1.Selected bond lengths and bond angles are given in Table 2.
CCDC:1450687,1;1450688,2.
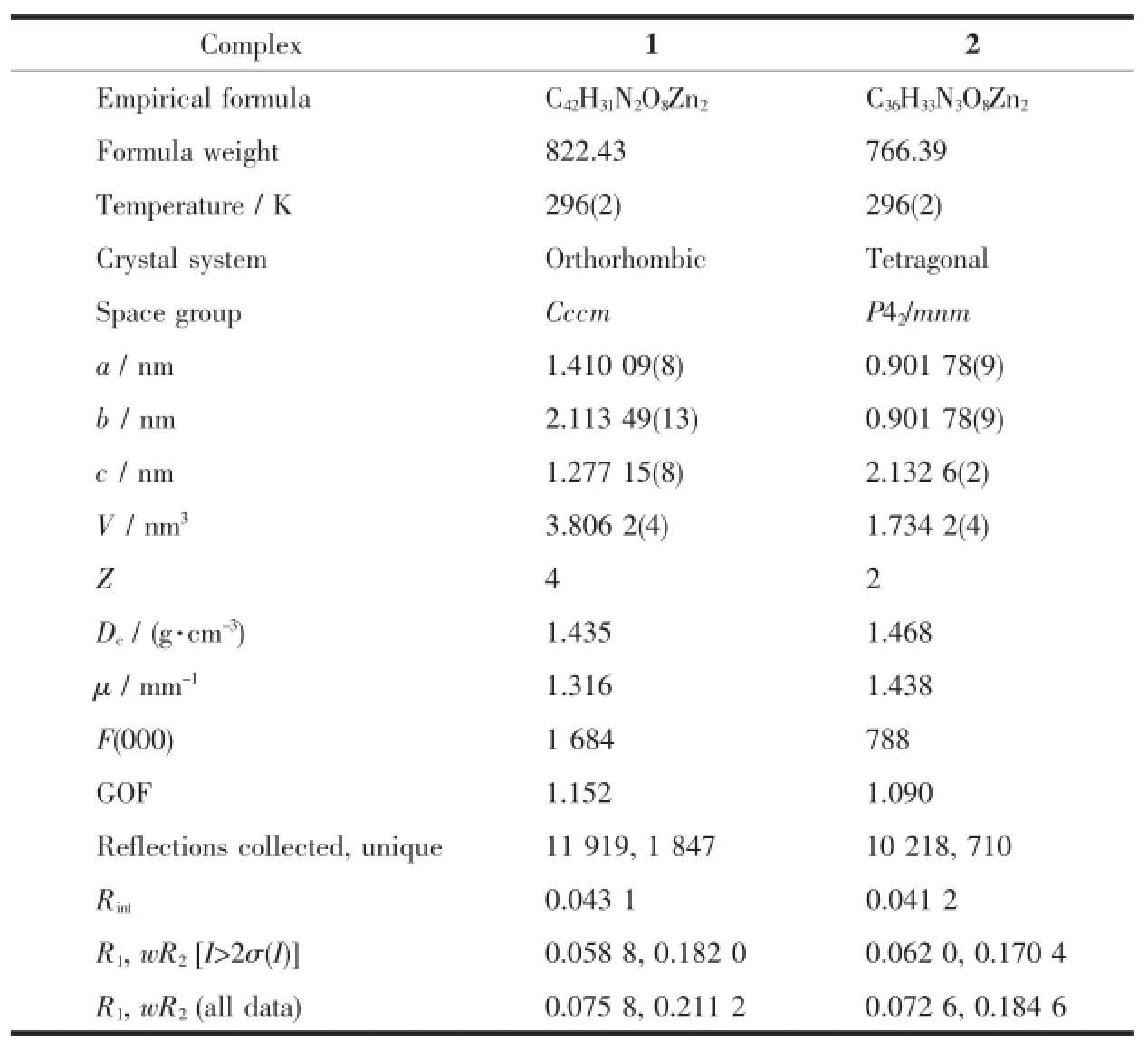
Table 1 Crystal data and structure refinement information for compounds 1~2
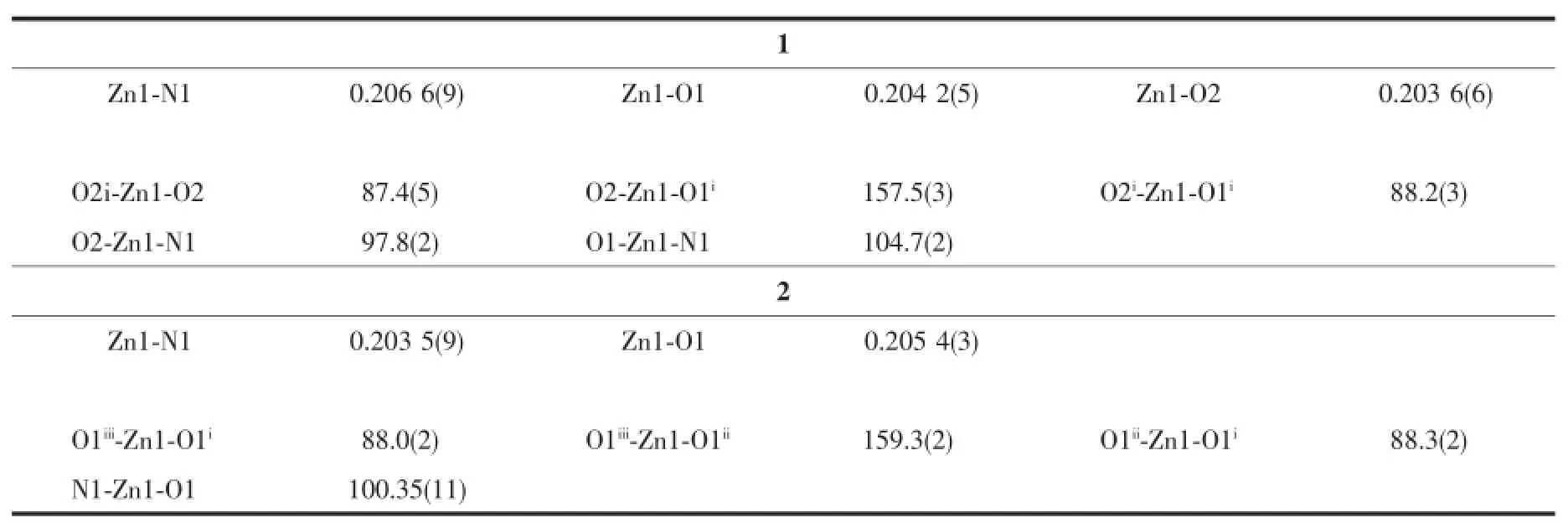
Table 2 Selected bond distances(nm)and angles(°)of 1 and 2
2 Results and discussion
2.1Structure description
Single-crystal X-ray diffraction analysis reveals that compound 1 has 2D(4,4)layered structure and crystallizesinorthorhombicCccmspacegroup. Coordination environment of Zn(Ⅱ) atom is shown in Fig.1a.In the asymmetric unit of 1,there is one Zn(Ⅱ)cation,half a DBA anion and one quarter dmbpy ligand.The Zn(Ⅱ) center is five-coordinated by four oxygen atoms from four DBA ligands,one nitrogen atom from one dmbpy ligand,and displays a distorted trigonal bipyramidal geometry.The Zn-O,Zn-N bond lengths and O-Zn-O,O-Zn-N bond angles range from 0.202 9(4)to 0.205 6(4)nm and 87.7(3)°to 157.55(18)°,respectively,which is within the reasonable range of observed values for other five-coordinated Zn(Ⅱ) complexes with oxygen and nitrogen donating ligands[10,17]. In the crystal structure of 1,the DBA ligands adopt one μ4bridging mode to link four Zn(Ⅱ)ions,whereas dmbpy ligand acts as a trans bridging ligand to link two Zn(Ⅱ)ions(Scheme 1:modesⅠandⅡ).In this manner,the dinuclear zinc units are linked into a linear chain through DBA anions with the separation of 1.277 2 nm between two dinuclear zinc cores(Fig. 1b).The dmbpy ligands also bridge the dinuclear zinc units to form a linear chain with the dinuclear zinc cores separation of 1.410 1 nm.Such two types of parallel linear chains are perpendicularly intersected by the dinuclear zinc nodes,resulting in a 2D(4,4)network(Fig.1c)when the dinuclear zinc unit and organic ligands are regarded as a node and linkers,respectively.Each 4-membered ring consists of four DBA ligands,two dmbpy ligands and eight Zn(Ⅱ)atoms.The large voids with dimensions of 0.75 nm× 1.04 nm for each layer allow the formation of catenation between adjacent two layers in a parallel manner.As shown in Fig.1d,two(4,4)networks interlock in parallel and give rise to a polycatenated layer(2D→2D).Such parallel(4,4)layered interlocking modes are well know to belonging to the parallel-parallel(p-p)class[21].

Scheme 1 Coordination modes of DBA,dmbpy and dabco ligands in 1 and 2
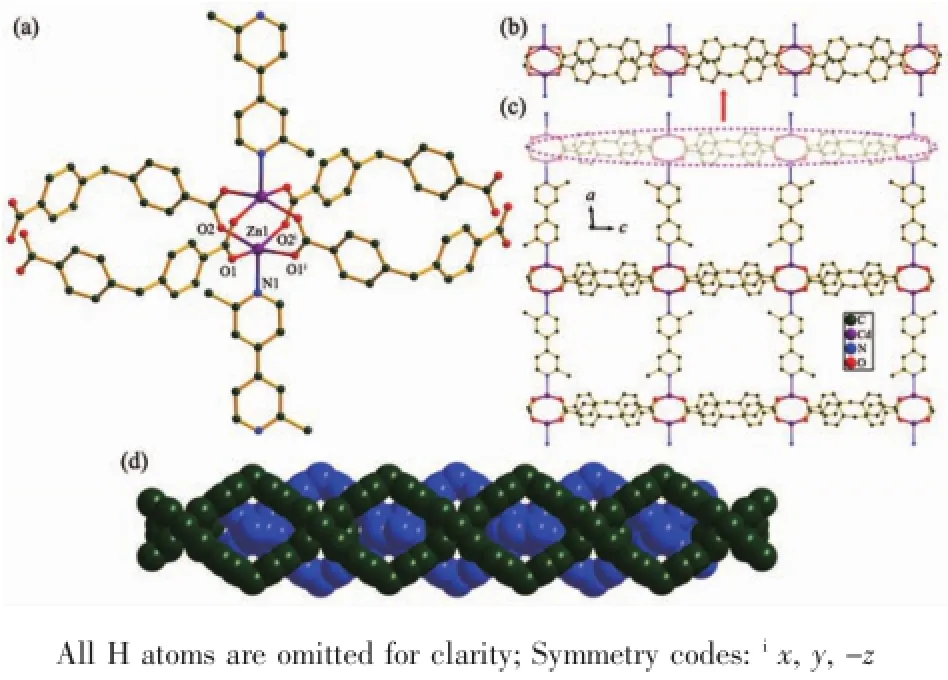
Fig.1 (a)Coordination environment of Zn(Ⅱ)in 1;(b)View of a linear chain of 1;(c)View of the 2D layer structure of 1;(d)View of the 2D polycatenation in 1 along the a-axis
Compound 2 crystallizes in the tetragonal space group P42/mnm,with one Zn atom,one DBA anion and a dabco ligand in the asymmetric unit.The fivecoordinated Zn(Ⅱ)center is surrounded by four oxygen atomsfromfourdifferentDBAanionsandone nitrogen atoms from a dabco ligand,which exhibits a distorted trigonal bipyramidal geometry(Fig.2a),with Zn-O,Zn-N distances and O-Zn-O,O-Zn-N bond angles range from 0.203 5(9)to 0.205 4(3)nm and from 88.0(2)°to 159.3(2)°,respectively.All of within the range of those found in other five-coordinated Zn(Ⅱ)complexes with oxygen and nitrogen donating ligands[10,17].In the polymeric structure of 2,the DBA and dabco ligands act in μ4bridging and monodentate modes,respectively(Scheme 1:modesⅠ andⅢ).Inthis manner,the μ4-DBA ligands link dinuclear zinc units to result in a ribbon-like infinite chain with dabco ligands point alternately up and down with respect to the chain(Fig.2b).The separation of two neighboring dinuclear zinc cores is 1.275 3 nm.
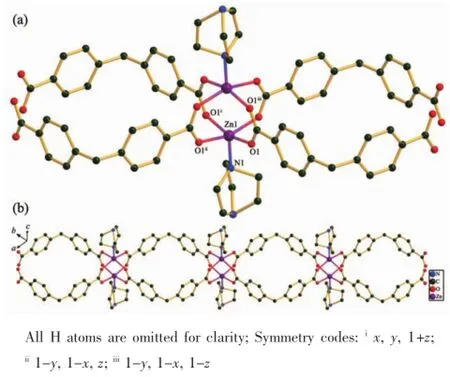
Fig.2 (a)Coordination environment of Zn(Ⅱ)in 2;(b)View of a ribbon-like infinite chain of 2
2.2IR spectra and TGA
The IR spectra shows broad bands in the 3 442 cm-1for 1,3 423 cm-1for 2,which may be assigned to the ν(O-H)stretching vibrations of the adsorbed water (impurity)and the free water molecules,respectively (Fig.S1).The absorption band observed at 2 933 cm-1for 1,and 2 981,2 935 cm-1for 2 are attributed to the ν(Cmethyl-H)vibration of dmbpy ligands.The features at 1 613 and 1 383 cm-1for 1,1 608 and 1 367 cm-1for 2,are associated with the asymmetric ν(COO)and symmetric ν(COO)stretching vibrations.
Thermal gravimetric analyses(TGA)were carried out to examine the thermal stability of 1 and 2.The samples were heated up in flowing N2with a heating rate of 10℃·min-1.The TG curve for 1 shows a weight loss in the temperature range of 80~200℃ corresponding to the removal of two lattice water molecules (Calcd.7.29%,Obsd.7.52%).Upon further heating,the framework was stable up to 370℃ and then a sharp weight loss was observed above 370℃ due to the collapse of the framework(Fig.3).The TGA curve for 2 shows a weight loss between 80~200℃,which corresponds to the loss of one dabco ligand(Calcd. 14.93%,Obsd.15.01%).Upon further heating,a sharp weight loss was observed above 200℃ due to th collapse of the framework.

Fig.3 TGA curves of compounds 1 and 2
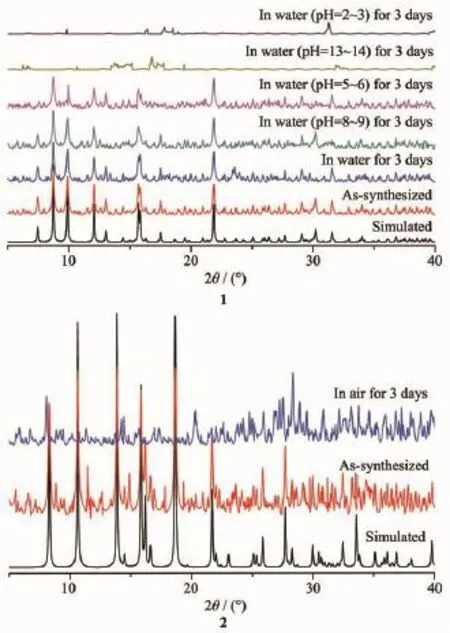
Fig.4 PXRD patterns of 1 and 2
2.3Stability properties
It has demonstrated that the incorporation o hydrophobic functional groups(e.g.-CH3)within frameworks might largely enhance the M-O bonds and thus improve the water resistance of the CPs[10].Th pH-dependent stability of 1 in aqueous solutions were investigated by PXRD(Fig.4).For these tests,50 mg of as-synthesized compounds were soaked in aqueoussolutions with different pH values and stirred for 3 day at room temperature.Compared to 1,compound 2 is unstable even in air(Fig.4).Stirring under more basic(pH=13~14)or acidic(pH=2~3)conditions,1 shows a complete decomposition of its framework.The stabilities of 1 are similar to several aluminumisophthalate-based MOFs(CAU-10-X,where X=H,CH3,OCH3,NO2,NH2,OH)[22],but lower than the series of carboxylate-based MOFs involving zirconium ions[23-24].However,the result is very remarkable,especially when compared with other CPs constructed fromaromaticcarboxylate,N-containingauxiliary ligands and zinc/cadmium ions[25-27].Moreover,the peak positions of the experimental patterns are in a good agreement with the simulated patterns,which clearly indicates the good purity of the complexes(Fig.4).
2.4Luminescent properties
Luminescent properties of coordination polymers with d10metal centers have attracted intense interest due to their potential applications[28-29].The photoluminescenct spectrum of H2DBA ligand shows emission maxima at 391 nm(λex=314 nm)[30](Fig.5).The emission band of the free ligand is probably attributable to the π*-π transition.Upon complexation of the ligands with Zn(Ⅱ) ions,the emission peak occur at 416 nm (λex=322 nm)for 1 and 405 nm(λex=313 nm)for 2,respectively.The short wavelength band of 1 and 2 are almost same as that of the H2DBA ligand,which indicates that the emissions of 1 and 2 probably origins from the H2DBA ligands.The luminescent lifetimes of solid 1 and 2 using an Edinburgh FLS920 phosphorimeter with 450 W xenon lamp as excitation source show lifetimes of 231.4 ns and 2.33 ns,respectively(Fig.6).
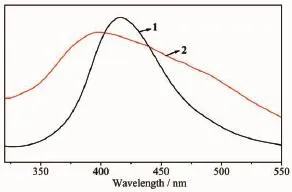
Fig.5 Emission spectra for 1 and 2 in solid state at room temperature
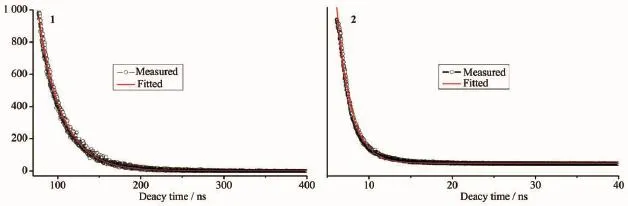
Fig.6 Luminescent lifetimes of 1 and 2 in solid state at room temperature
3 Conclusions
In summary,by changing the auxiliary ligands,two zinc-based CPs have been prepared based on the reaction of 4,4′-methylenedibenzoic acid and Zn(NO3)2· 6H2O under the same reaction conditions.1 performs a polycatenated layer(2D→2D)structure and is stable in aqueous solutions in the pH value range of 5~9,whereas 2 shows a ribbon-like infinite chain with dabco ligands point alternately up and down with respect to the chain and is unstable in air.Both 1 and 2 emit bright blue fluorescence with the lifetimes of 231.4 ns and 2.33 ns,respectively.
Supporting information is available at http://www.wjhxxb.cn
References:
[1]Ma D,Wang W,Li Y,et al.CrystEngComm,2010,12:4372-4377
[2]Wu H,Gong Q,Olson D H,Li J.Chem.Rev.,2012,112:836 -868
[3]Zhang W,Xiong R G.Chem.Rev.,2012,112:1163-1195
[4]Tsukube H,Juanes S.Chem.Rev.,2002,102:2389-2403
[5]Bünzli J C G.Chem.Rev.,2010,110:2729-2755
[6]MaDY,LiZ,XiaoJX,etal.Inorg.Chem.,2015,54:6719-6726
[7]Hu Y,Liu Z,Xu J,et al.J.Am.Chem.Soc.,2013,135:9287 -9290
[8]Rao A S,Pal A,Ghosh R,et al.Inorg.Chem.,2009,48:1802 -1804
[9]Liu L,Huang S P,Yang G D,et al.Cryst.Growth Des.,2010,10:930-936
[10]Liu H,Zhao Y,Zhang Z,et al.Adv.Funct.Mater.,2011,21: 4754-4762
[11]Eddaoudi M,Kim J,Rosi N,et al.Science,2002,295:469-472
[12]Wang Z J,Qin L,Zhang X,et al.Cryst.Growth Des.,2015,15:1303-1310
[13]Couck S,Denayer J F M,Baron G V,et al.J.Am.Chem. Soc.,2009,131:6326-6327
[14]Yuan B,Ma D,Wang X,et al.Chem.Commun.,2012,48: 1135-1137
[15]Liu H,Zhao Y,Zhang Z,et al.Chem.Asian J.,2013,8:778-785
[16]Ma D Y,Li X,Wu X G,et al.J.Mol.Struct.,2015,1083: 421-425
[17]Ma D,Li Y,Li Z.Chem.Commun.,2011,47:7377-7379
[18]APEXⅡSoftware,Ver.6.3.1,Bruker AXS Inc,Madison,Wisconsin,USA,2004.
[19]Sheldrick G M.Acta Crystallogr.,2008,A64:112-122
[20]Spek A L.PLATON:A Multipurpose Crystallographic Tool Utrecht University,Utrecht,The Netherlands,2008.
[21]Carlucci L,Ciani G,Proserpio D M.Coord.Chem.Rev. 2003,246:247-289
[22]Reinsch H,Veen M A,Gil B,et al.Chem.Mater.,2013,25 17-26
[23]Lalioti N,Raptopoulou C P,Terzis A,et al.Angew.Chem Int.Ed.,2001,40:3211-3214
[24]Kanoo P,Ghosh A C,Cyriac S T,et al.Chem.Eur.J.,2012 18:237-244
[25]Zhang J W,Zhao C C,Zhao Y P,et al.CrystEngComm 2014,16:6635-6644
[26]Jiang H L,Tatsu Y,Lu Z H,et al.J.Am.Chem.Soc.,2010 132:5586-5587
[27]Guo J,Sun S,Zhang L,et al.Cryst.Growth Des.,2012,12 5649-5654
[28]An J,Shade C M,Chengelis-Czegan D A,et al.J.Am.Chem Soc.,2011,133:1220-1223
[29]Gole B,Bar A K,Mukherjee P S.Chem.Commun.,2011,47 12137-12139
[30]Liu G X,Wang X F,Zhou H.J.Solid State Chem.,2013 199:305-316
中圖分類號:O614.24+1
文獻標識碼:A
文章編號:1001-4861(2016)05-0921-07
DOI:10.11862/CJIC.2016.104
收稿日期:2016-01-31。收修改稿日期:2016-03-16。
Auxiliary Ligands Controlled Assembly of Two Zinc Coordination Polymers:Structures,Stability and Luminescence
GUO Pei-Ying1Lü Li-Xia1MA De-Yun2GUO Hai-Fu*,2
(1Department of Chemical Engineering,Inner Mongolia Vocational College of Chemical Engineering,Hohhot 010070,China)
(2School of Chemistry and Chemical Engineering,Zhaoqing University,Zhaoqing,Guangdong 526061,China)
Abstract:By changing the auxilary ligands,two zinc-based coordination polymers(CPs),{[Zn(DBA)(dmbpy)0.5]· 2H2O}n(1)and[Zn2(DBA)2(dabco)]n(2)(DBA=4,4′-methylenedibenzoate,dmbpy=2,2′-dimethyl-4,4′-bipyridine dabco=triethylenediamine)have been prepared based on 4,4′-methylenedibenzoate and Zn(NO3)2·6H2O under th same reaction conditions and were structurally characterized by elemental analysis,IR,TGA,powder X-ra diffraction and single-crystal X-ray diffraction.1 is a 2D(4,4)network,and two nets interlock in parallel and give rise to a polycatenated layer(2D→2D).2 is a ribbon-like infinite chain with dabco ligands point alternately up and down with respect to the chain.The results of thermal analysis indicate that 1 is quite stable up to 370℃whereas 2 shows low thermal stability.Finally,both of 1 and 2 emit the intensely blue characteristic luminescence a room temperature,with lifetimes of up to 231.4 ns and 2.33 ns,respectively.CCDC:1450687,1;1450688,2.
Keywords:coordination polymers;crystal structure;4,4′-methylenedibenzoate;luminescent

