Applicability of tooth derived stem cells in neural regeneration
Ludovica Parisi, Edoardo ManfrediCentro Universitario di Odontoiatria, University of Parma, Parma, Italy; Dip. Scienze Biomediche, Biotecnologiche e Traslazionali, University of Parma, Parma, Italy
Applicability of tooth derived stem cells in neural regeneration
Ludovica Parisi*, Edoardo Manfredi
Centro Universitario di Odontoiatria, University of Parma, Parma, Italy; Dip. Scienze Biomediche, Biotecnologiche e Traslazionali, University of Parma, Parma, Italy
How to cite this article:Parisi L, Manfredi E (2016) Applicability of tooth derived stem cells in neural regeneration. Neural Regen Res 11(11):1704-1707.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Within the nervous system, regeneration is limited, and this is due to the small amount of neural stem cells, the inhibitory origin of the stem cell niche and often to the development of a scar which constitutes a mechanical barrier for the regeneration. Regarding these aspects, many efforts have been done in the research of a cell component that combined with scaffolds and growth factors could be suitable for nervous regeneration in regenerative medicine approaches. Autologous mesenchymal stem cells represent nowadays the ideal candidate for this aim, thank to their multipotency and to their amount inside adult tissues. However, issues in their harvesting, through the use of invasive techniques, and problems involved in their ageing, require the research of new autologous sources. To this purpose, the recent discovery of a stem cells component in teeth, and which derive from neural crest cells, has came to the light the possibility of using dental stem cells in nervous system regeneration. In this work, in order to give guidelines on the use of dental stem cells for neural regeneration, we briefly introduce the concepts of regeneration and regenerative medicine, we then focus the attention on odontogenesis, which involves the formation and the presence of a stem component in different parts of teeth, and finally we describe some experimental approaches which are exploiting dental stem cells for neural studies.
multipotent stem cells; odontogenesis; regeneration; brain; spinal cord; teeth
Introduction
Regeneration and reparation are two processes that lead to wound healing, with distinctive features,e.g., they occur with a different frequency. Reparation is a common phenomenon in the majority of wounded tissues, which promotes healing through the replacement of the damaged tissue with an aspecific tissue, produced by fibroblasts and commonly called scar. When scar is formed, the wound gap is filled up and the integrity of the tissue is reconstructed, albeit not the architecture, with consequent loss of tissue function. If reparation is an aspecific phenomenon, on the other hand, regeneration is a completely specific process for wound healing. Regeneration entails the replacement of lost tissue with the same tissue, which is produced by specific cells and which, being equal to the original one, allows for the restoration of the architectural and functional integrity of the tissue (Robbins et al., 2010).
In this respect, tissues have commonly different abilities to regenerate. The amount of adult stem cells in a tissue, which are able to self-renew and to differentiate, is the first element to consider about the regeneration. However, the mere presence of a stem cell component in a tissue is not sufficient for regeneration, because cells require to be activated to induce regeneration. The stem cell niche, the milieu in which stem cells are entrapped and quiescent and which supplies a trophic, mechanical and geometric environment for cell surviving, plays a pivotal role in their activation. Niches can be inhibitory, allowing the activation of stem cells only in the presence of inflammatory signals, or of expansion,i.e., niches that continuously support the generation of new stem cells (Jones and Wagers, 2008). Moreover, beside the amount of stem cells and the available type of niche in which cells are contained, the type of damage is often an additional obstacle to regeneration: tissue damage may spontaneously evolve indeed into a scar tissue, which represents a mechanical, physical and chemical barrier to regeneration.
Hystorically, it is evident that central nervous system do not regenerate after injuries. However, as reported by Cajal (1928) on the base of Tello’s observations, a permissive environment could enhance neurons regrowths in mammalians nervous system. Tello’s observations confirm our previous argumentation on regeneration and explain why inside the nervous system neurons lack of spontaneous regeneration. Indeed, disorders, both neurological and traumatic, associated to the nervous system lead to neuron loss or degeneration, and to a compromised environment. This, togetherwith the amount of available neural stem cells (NSCs) and the inhibitory nature of the neural stem cells niche explains the lack of spontaneous regeneration in the nervous system.
Regenration inside the nervous system requires a multistep process, which involves the recruitment of new cells, the delivery of neurotrophic factors and scaffolding for regeneration guidance. To this purpose regenerative medicine (RM) approaches can be thought to induce regeneration inside the nervous system (Horner and Gage, 2000).
RM represents a valid interdisciplinary approach to restore structure and function of damaged tissues and organs, and in context of the nervous system, RM aims to restore a suitable micro-environment to induce tissue regeneration through the use of molecular factors, cells and scaffolds (Langer and Vacanti, 1993).
As for the cell components used in nervous system RM approaches, these can be used alone as in cellular therapies or in combination with scaffolds in tissue engineering approaches. Among alternative cell sources, adult stem cells, afterin vitrodifferentiation, have obtained extensive attention. At the present moment, NSCs clearly represent the ideal cell source for this approach, because they are able to differentiate into neurons, astrocytes and oligodendrocytes; however, on the other hand, they are difficult to be isolated from the brain and, consequently, other sources are needed (Martens et al., 2013). In the last two decades, mesenchymal stem cells (MSCs) have been widely studied too, both those isolated from bone marrow and from adipose tissue. MSCs are multipotent cells able to differentiate into a wide array of cell types, included osteogenic, chondrogenic, adipogenic, myogenic and neurogenic lineages. Although MSCs seem to be a promising source of mature cells, the difficulties with their harvesting, which requires invasive techniques like bone marrow aspiration or liposuction, and problems with aging cell populations, limit their use in the practice (Kim et al., 2012).
Recently, the presence of different mesenchymal stem components has been discovered in teeth. These cells origin from the neural crest during embryogenesis, and it is thus theoretically possible to exploit them in RM applied to the nervous system (Kim et al., 2012; Martens et al., 2013; Park et al., 2016). Now we proceed to discuss teeth formation events, to highlight the origin of dental stem cells and consequently their capability to be used for neural regeneration.
Odontogenesis and Dental Stem Cells
Embryogenesis of teeth is directed by a sequence of events and interactions between two layers of cells, both arising from the migrating neural crest cells: the oral epithelium and the mesenchymal tissue. Tooth morphogenesis involves five steps (Figure 1). The first stage is called the “Thickening Stage”; it involves the proliferation of epithelial cells and the consequent thickening of oral epithelium in the site of the future tooth. This phenomenon leads to the activation of the underlying mesenchymal cells, which during the second step, the “Bud Stage”, differentiate into two distinct lineages, peripheral basal and stellate reticulum cells. These cells limit the area around the niche of the developing tooth. During the third and the fourth stage, the “Cap” and the “Bell” stages respectively, the inner enamel epithelium paves the way to the formation of tooth follicle and to the morphogenesis of the entire dental structure. The fifth and last step is the “Secretory Stage”, during which the mature tooth makes its way through the gingiva and erupts (Tucker and Sharpe, 2004).
During teeth development, a quiescent population of stem cells deriving from neural crest cells remains entrapped into dental structures, becoming a useful source of stem cells (Kim et al., 2012; Martens et al., 2013; Park et al., 2016). Dental stem cells are classified on the basis of their location into tooth structure, as reported inTable 1. We will now discuss potentials of dental pulp stem cells (DPSCs), periodontal ligament stem cells (PLDSCs), stem cells from the apical papilla (SCAPs) and of cells isolated from child deciduous teeth (SHEDs) or from dental follicle of developing teeth (DFSCs).
DPSCs were the first type of dental stem cells discovered, and were isolated from the dental pulp tissue by Gronthos et al. (2000). Physiologically, these cells forms new odontoblasts and produces new dentin in case of severe lesions of the teeth. Under opportune conditions, DPSCs are able to differentiate into neurogenic lineages, to express neural markers and to produce and secrete neurotrophic factors. At the present moment, DPSCs are the most investigated and promising dental stem cells for neural regeneration (Martens et al., 2013). PDLSCs are isolated from the periodontal ligament, a specialized connective tissue that connects cementum to the alveolar bone in order to maintain tooth stability. It has been demonstrated that PDLSCs are involved in cementum new formation, but they are able to produce and secrete markers typical of neuron, oligodendrocytes and astrocytesin vitro(Martens et al., 2013). DPSCs and PDLSCs represent the two cell populations that can be isolated from mature adult teeth. SCAPs are isolated from developing teeth roots, so they are highly osteo/odontogenic-committed. It has been shown thatin vitroSCAPs can express markers of glial cells (Martens et al., 2013). DFSCs can be isolated both from child developing teeth or from developing adult third molars. DFSCs are highly undifferentiated cells, andin vitrohave shown the ability to differentiate into neuron-like cells (Martens et al., 2013). Finally, a component of stem cells can be isolated also from the child deciduous teeth. SHEDs are isolated from dental pulp of the deciduous teeth and possess osteo-inductive capacity. SHEDs are yet less studied, but they are gainingattention for their capacity to differentiate into neurons (Martens et al., 2013).
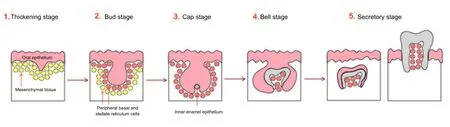
Figure 1 Schematic representation of teeth development.

Table 1 Dental stem cells
The embryonic derivation of tooth stem cells from neural crest cell and they ability to differentiate into neuron-like cells under opportune conditions, make these cells a new suitable source for neural regeneration.
In vitro Neural Differentiation of Dental Stem Cells
To date, extensive studies have demonstrated that stem cells isolated from teeth express several neural markers upon appropriate conditions.
Takeyasu et al. (2006) showed that Stro-1 and Nestin positive DPSCs are able to form neurospheres in suspension in the presence of FGF2 factor and to became neuron-like cells when plated on a laminin-coated disk in the presence of cAMP, GDNF, SHH and FGF8. Shortly afterward Widera et al. (2007) were able to obtain neurospheres expressing Nestin and Sox-2 from PDLSCs, and to differentiate them into neuron-like cells on laminin-coated plates without the use of cytokines. Moreover, SCAPs isolated from human immature permanent teeth showed high proliferation rates and the expression of important neuronal markers, included GFAP, BIII-tubulin and neurofilament M after the exposure to bFGF and to EGF (Sonoyama et al., 2008). In addiction, Wang et al. (2010) indicated the formation of neurospheres also from SHEDs, if cultivated in medium optimized for NSCs and the possibility of further differentiating them into dopaminergic neurons.
These data show the possibility of direct differentiation into neural lineage of stem cells isolated from dental tissue. Hence, it is necessary to thoroughly investigate the possibility of using these cells in clinical practice.
In vivo Applicability of Dental Stem Cells
Although all the cells isolated from teeth have shown the capacity to differentiate into nervous system cells, only DPSCs and SHEDs have been yet tested as an innovative cell source for nervous system regenerative therapies. Pre-differentiated DPSCs and SHEDs have proved successful both at the central nervous system (CNS) and the peripheral nervous system (PNS) levels, and seem to play a pivotal role in the secretion of neurotrophic factors useful for regeneration.
Concerning the CNS, Kiraly et al. (2011) demonstrated the ability of DPSCs to integrate into a mouse traumatic brain injury, while de Almeida et al. (2011) showed the possibility ofusing these cells in a model of spinal cord injury. Moreover, the neuroprotective effect of DPSCs were reported by several groups to be effective in animal models of CNS neurological disorders such as Alzheimer’s and Parkinson’s disease (Nosrat et al., 2001; Martens et al., 2013). As for SHEDs, SHED neurospheres obtainedin vitrowere transplantedin vivoand were able to survive and differentiate into dopaminergic neurons in a rat model of Parkinson’s disease. Moreover these structures allowed partial improvements of the functional impairment in treated animals (Wang et al., 2010).
DPSCs showed their applicability also at PNS level for peripheral nerve injury treatment: they were loaded on PLGA-collagen and the scaffold was inserted in a model of facial nerve injury. The system allowed the reconnection of damaged axons (Sasaki et al., 2011).
Conclusions
The use of dental stem cells for cell-based therapies have gained increasingly interest in the last two decades. Promising results have already been obtained bothin vitroand in animal modelsin vivo, and firmly pave the way to the applicability of these cells for the treatment of nervous system disorders. However, dental stem cells are not free from issues of concern, first of all possible problems with tumorigenicity, like other types of adult stem cells, and, secondly, more animal studies have to be done before their applicability can be convincingly established at a human level. In spite of this, dental stem cells represent today a valid alternative to other sources of MSCs and the research in this field will probably obtain interesting results in the future.
Acknowledgments:The authors would like to thank Prof. Guido Maria Macaluso and Dr. Carlo Galli (Dip. Scienze Biomediche, Biotecnologiche e Traslazionali), for the support to the work.
Author contributions:LP wrote the manuscript, and EM revised the manuscript before the submission.
Conflicts of interest:The authors have no conflicts of interest to disclose.
de Almeida FM, Marques SA, Ramalho Bdos S, Rodrigues RF, Cadilhe DV, Furtado D, Kermis I, Pereira LV, Rehen SK, Martinez AM (2011) Human dental pulp cells: a new source of cell therapy in a mouse model of compressive spinal cord injury. J Neurotrauma 28:1939-1949.
Gronthos S, Mangani M, Brahim J, Robe PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625-13630.
Horner PJ, Gage FH (2000) Regenerating the damaged central nervous system. Nature 407:963-970.
Jones DL, Wagers AJ (2008) No place like home: anatomy and function of the stem cell niche. Nature 9:11-21.
Kim BC, Bae H, Kwon IK, Lee EJ, Park JH, Khademhosseini A, Hwang YS (2012) Osteoblastic/cementoblastic and neural differentiation of dental stem cells and their applications to tissue engineering and regenerative medicine. Tissue Eng Part B 18:235-244.
Kiraly M, Kadar K, Horvathy DB, Nardai P, Racz GZ, Lacza Z, Varga G, Gerber G (2011) Integration of neuronal pre differentiated human dental pulp stem cells into rat brain in vivo. Neurochem Int 59:371-381.
Langer R, Vacanti JP (1993) Tissue Engineering. Science 260:920-926.
Martens W, Bronckaers A, Politis C, Jacobs R (2013) Dental stem cells and their promising role in neural regeneration: an update. Clin Oral Invest 17:1969-1983.
Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA (2001) Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci 19:2388-2398.
Park YJ, Cha S, Park YS (2016) Regenerative applications using tooth derived stem cells in other than tooth regeneration: a literature review. Stem Cells Int doi:10.1155/2016/9305986.
Ramon y Cajal S (1928) Degeneration and regeneration of the nervous system. Volume 2. Haffner Publishing Co. New York, NY, USA.
Robbins SL, Cotran RS, Kumar V, Eusebi V, et al. (2010) Le basi patologiche delle malattie. Milan. Elsevier Masson.
Sasaki R, Aoki S, Yamato M, Uchiyama H, Wada K, Ogiuchi H, Okano T, Ando T (2011) PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med 5:823-830.
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endodontics 34:166-171.
Takeyasu M, Nazaki I, Daito M (2006) Differentitation of dental pulp stem cells into a neural lineage. Pediatric Dent J 16:154-162. Tucker A, Sharpe P (2004) The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev 5:499-508.
Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S (2010) Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev 19:1375-1383.
Widera D, Grimm WD, Moebius JM, Mikenberg I, Piechaczek C, Gassman G, Wolff NA, Thevenod F, Kaltschmidt C, Kaltschmidt B (2007) Highly efficient neural differentiation of human somatic stem cells, isolated by minimally invasive periodontal surgery. Stem Cells Dev 16:447-460.
*Correspondence to: Ludovica Parisi, Ph.D., ludovica.paris@gmail.com.
orcid: 0000-0002-4673-3176 (Ludovica Parisi)
10.4103/1673-5374.194705
Accepted: 2016-10-27
- 中國神經再生研究(英文版)的其它文章
- Cortical spreading depression-induced preconditioning in the brain
- Nerve growth factor protects against palmitic acidinduced injury in retinal ganglion cells
- Tissue-engineered rhesus monkey nerve grafts for the repair of long ulnar nerve defects: similar outcomes to autologous nerve grafts
- HLA class II alleles and risk for peripheral neuropathy in type 2 diabetes patients
- Rab27a/Slp2-a complex is involved in Schwann cell myelination
- Key genes expressed in different stages of spinal cord ischemia/reperfusion injury