以對苯二氧基二乙酸和咪唑基化合物為配體的鎳、鎘配合物的合成和晶體結構
陳 宏 喻 敏 劉光祥*,
(1巢湖學院化學化工與生命科學學院,巢湖 238000) (2南京曉莊學院環境科學學院,南京 211171)
以對苯二氧基二乙酸和咪唑基化合物為配體的鎳、鎘配合物的合成和晶體結構
陳宏1喻敏2劉光祥*,2
(1巢湖學院化學化工與生命科學學院,巢湖238000) (2南京曉莊學院環境科學學院,南京211171)
以對苯二氧基二乙酸(H2BDOA)和4,4′-二咪唑基二苯醚(BIDPE)為原料,分別與氯化鎳、氯化鎘在水熱條件下反應,得到2個結構不同的配位聚合物[Ni(BDOA)(BIDPE)(H2O)]n(1)和[Cd(BDOA)0.5(BIDPE)Cl]n(2)。對它們進行了元素分析、紅外光譜分析,并利用X-射線衍射測定了它們的單晶結構。配合物1屬于三斜晶系,P1空間群,a=0.934 97(8)nm,b=1.032 31(9)nm,c=1.352 91 (12)nm,α=96.398 0(10)°,β=90.729 0(10)°,γ=102.027 0(10)°,V=1.268 31(19)nm3,Z=2,Mw=603.22,Dc=1.580 g·cm-3,μ=0.827,F (000)=624,R1=0.039 5,wR2=0.089 5(I>2σ(I))。配合物2也屬于三斜晶系,P1空間群,a=1.017 51(7)nm,b=1.042 99(7)nm,c=1.173 38(8)nm,α=68.723 0(10)°,β=71.826 0(10)°,γ=76.091 0(10)°,V=1.091 05(13)nm3,Z=2,Mw=562.26,Dc=1.711 g·cm-3,μ=1.163,F (000)=562,R1=0.021 0,wR2=0.053 7(I>2σ(I))。配合物1中鎳離子通過2種配體橋聯成二維波浪形層狀結構,而配合物2中鎘離子通過2個Cl離子連接成一個雙核Cd2單元,雙核單元再通過2種配體連接成一維鏈狀結構。結果說明了金屬離子在配合物組裝過程中起著非常重要的作用。此外,研究了它們的熒光性質。
配位聚合物;雙咪唑配體;芳香羧酸配體;晶體結構
0 Introduction
Construction of coordination polymers(CPs)is experiencing great growth in crystal engineering due to their fascinating structural topologies and potential applications as functional materials in the fields of catalysis,gas absorption,magnetism,nonlinear optics, and luminescence[1-9].However,it would still be a challenge for a long time to design and construct coordination compounds with the desired topologies and properties,because the molecular architectures of coordination compounds are affected by many factors, such as organic ligands,metal ions,metalligand ratio, solvents,counter ions,pH value,and temperature[10-17]. Of all these influential factors,the intrinsic geometric preferences of central metal atom and various coordination sites of bridging ligands are the pivotal factors in determining the supramolecular architectures[18-21]. Therefore,through the choice of organic ligands and metal ions,it is possible to develop a targeted architecture.Amongvariousorganicligands,aromatic polycarboxylateligandshavebeenextensively employed in the preparation of such metal-organic complexeswithmultidimensionalnetworksand interestingproperties[22-28].Hence,thereported coordination polymers are mostly constructed by rigid polycarboxylate ligands[22-24].Recently,more and more attention has been paid to the flexible polycarboxylate ligands[25-28],because the flexible ligands have different conformations when they react with metal salts,and thus can form metal complexes with great structural diversity.
Recently,alargenumberofmixed-ligand coordination compounds have been reported[29-31],most of which contain N-containing ligands introduced into metal-polycarboxylate systems.The combination of different ligands may result in greater tunability of structural frameworks than that present with single ligands.Hence,a mixed-ligand is undoubtedly a good choice for the construction of new polymeric structures. Imidazole ligands have been used in the synthesis of unique CPs which possess excellent coordination ability and allow free rotation of the imidazole ring to meet the requirement of coordination geometries of metal ions. Until now,a great number of ingenious CPs have been synthesized based on imidazole ligands[32-33].Syntheses of new imidazole ligands are still a long-standing fascination of chemists,and we have designed and synthesized a new V-shape imidazole ligand,4,4′-bis (imidazol-1-yl)diphenyl ether(BIDPE),which may be regarded as a semi-rigid ligand.To test the ability of this ligand to give new architectures and topologies,we have selected a new ligand,H2BDOA ligand(H2BDOA= benzene-1,4-dioxydiacetic acid),and different bivalent metal salts to prepare two coordination polymers with intriguing structures,namely,[Ni(BDOA)(BIDPE) (H2O)]n(1)and[Cd(BDOA)0.5(BIDPE)Cl]n(2).Herein,we reporttheirsyntheses,crystalstructuresand photochemical properties.
1 Experimental
1.1Materials and general methods
All reagents for syntheses and analyses were purchased from commercial sources and used as receivedwithoutfurtherpurification.TheBIDPE ligand was synthesized according to the reported method[34].Elemental analyses(C,H and N)were performed on a Vario ELⅢelemental analyzer. InfraredspectrawereperformedonaNicolet AVATAR-360 spectrophotometer with KBr pellets in the 4 000~400 cm-1region.Solid-state UV-Vis diffuse reflectance spectra were obtained at room temperature using a Shimadzu UV-3600 double monochromator spectrophotometer,and BaSO4was used as a 100%reflectance standard for all materials.The luminescent spectra for the powdered solid samples were measured at room temperature on a Horiba FluoroMax-4PTCSPC fluorescence spectrophotometer with a xenon arc lamp as the light source.In the measurements of emission and excitation spectra the pass width is 5 nm.All the measurements were carried out under the same experimental conditions.
1.2Synthesis of[Ni(BDOA)(BIDPE)(H2O)]n(1)
A mixture containing NiCl2·6H2O(23.7 mg,0.1 mmol),H2BDOA(22.6 mg,0.1 mmol),BIDPE(30.2 mg,0.1 mmol)and LiOH·H2O(8.4 mg,0.2 mmol)in 15 mL of deionized water was sealed in a 25 mL Teflon lined stainless steel container and heated at 140℃ for 3 days.Green block crystals of 1 were collected by filtration and washed with water and ethanol several times with a yield of 59%based on BIDPE ligand.Anal.Calcd.for C28H24N4O8Ni(%):C, 55.75;H,4.01;N,9.29.Found(%):C,55.73;H,4.03; N,9.31.IR spectrum(cm-1):3 445(s),3 138(m),2 927 (w),1 609(s),1 559(s),1 519(m),1 422(m),1 371(s), 1 289(s),1 241(m),1 172(w),1 072(s),1 032(m),973 (w),832(w),788(w),689(w),572(w)and 537(w).
1.3Synthesis of[Cd(BDOA)0.5(BIDPE)Cl]n(2)
A mixture containing CdCl2·2.5H2O(45.6 mg, 0.2 mmol),H2BDOA(22.6 mg,0.1 mmol),BIDPE (60.4 mg,0.2 mmol)and LiOH·H2O(8.4 mg,0.2 mmol)in 15 mL of deionized water was sealed in a 25 mL Teflon lined stainless steel container and heated at 140℃ for 3 days.Colorless block crystals of 2 were collected by filtration and washed with water and ethanol several times with a yield of 61%based on BIDPE ligand.Anal.Calcd.for C23H18N4O4ClCd(%):C, 49.13;H,3.23;N,9.64.Found(%):C,49.11;H,3.25; N,9.62.IR spectrum(cm-1):3427(s),3166(m),2 827 (w),1 595(s),1 519(s),1 422(m),1 362(s),1 249(m), 1 126(m),1 089(s),1 012(w),965(w),929(w),857(w), 822(w),755(w),657(w)and 562(w).
1.4X-ray crystallography
Two block single crystals with dimensions of 0.22 mm×0.16 mm×0.14 mm for 1 and 0.18 mm×0.16 mm× 0.12 mm for 2 were mounted on glass fibers for measurement,respectively.X-ray diffraction intensity data were collected on a Bruker APEXⅡ CCD diffractometerequippedwithagraphitemonochromatic Mo-Kα radiation(λ=0.071 073 nm) using the φ-ω scan mode at 293(2)K.Data reduction and empirical absorption correction were performed using the SAINT and SADABS program[35],respectively.The structures were solved by the direct method using SHELXS-97[36]and refined by full-matrix least squares on F2using SHELXL-97[37].All of the nonhydrogen atomswererefinedanisotropically.The details of the crystal parameters,data collection and refinement for 1 and 2 are summarized in Table1, and selected bond lengths and angles with their estimated standard deviations are listed in Table2.
CCDC:1018030,1;1018031,2.
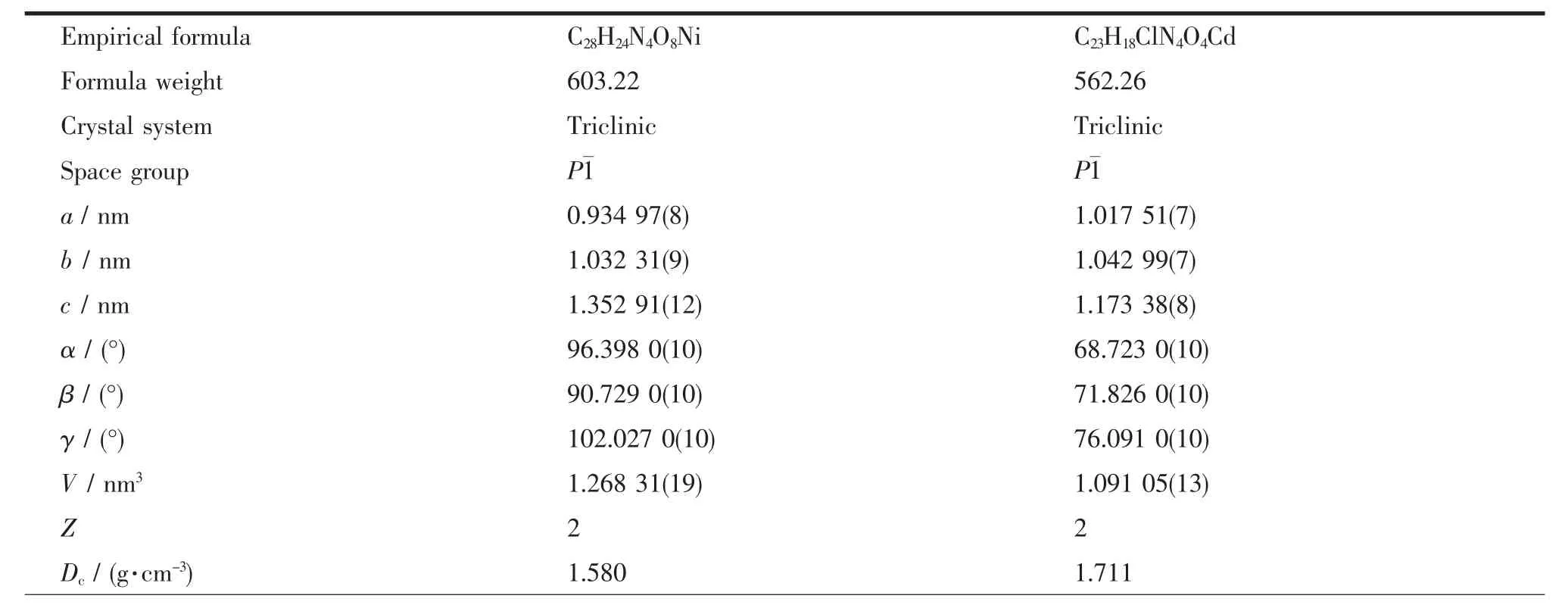
Table1 Crystal data and structure refinement for 1 and 2
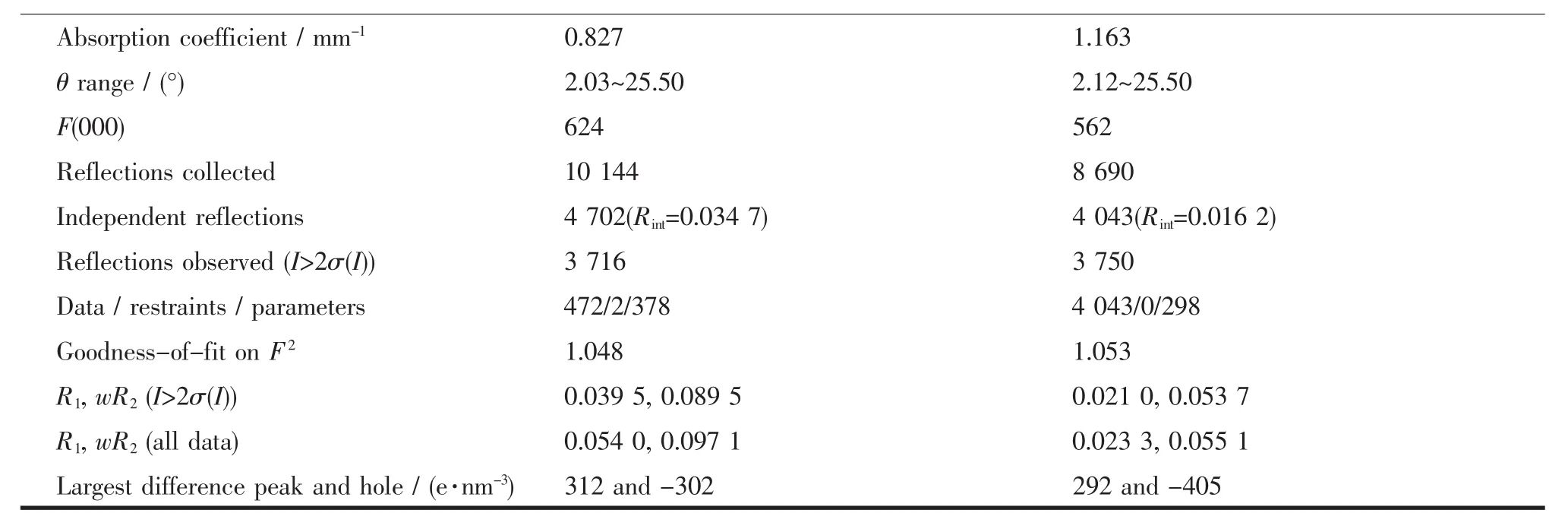
Continued Table1
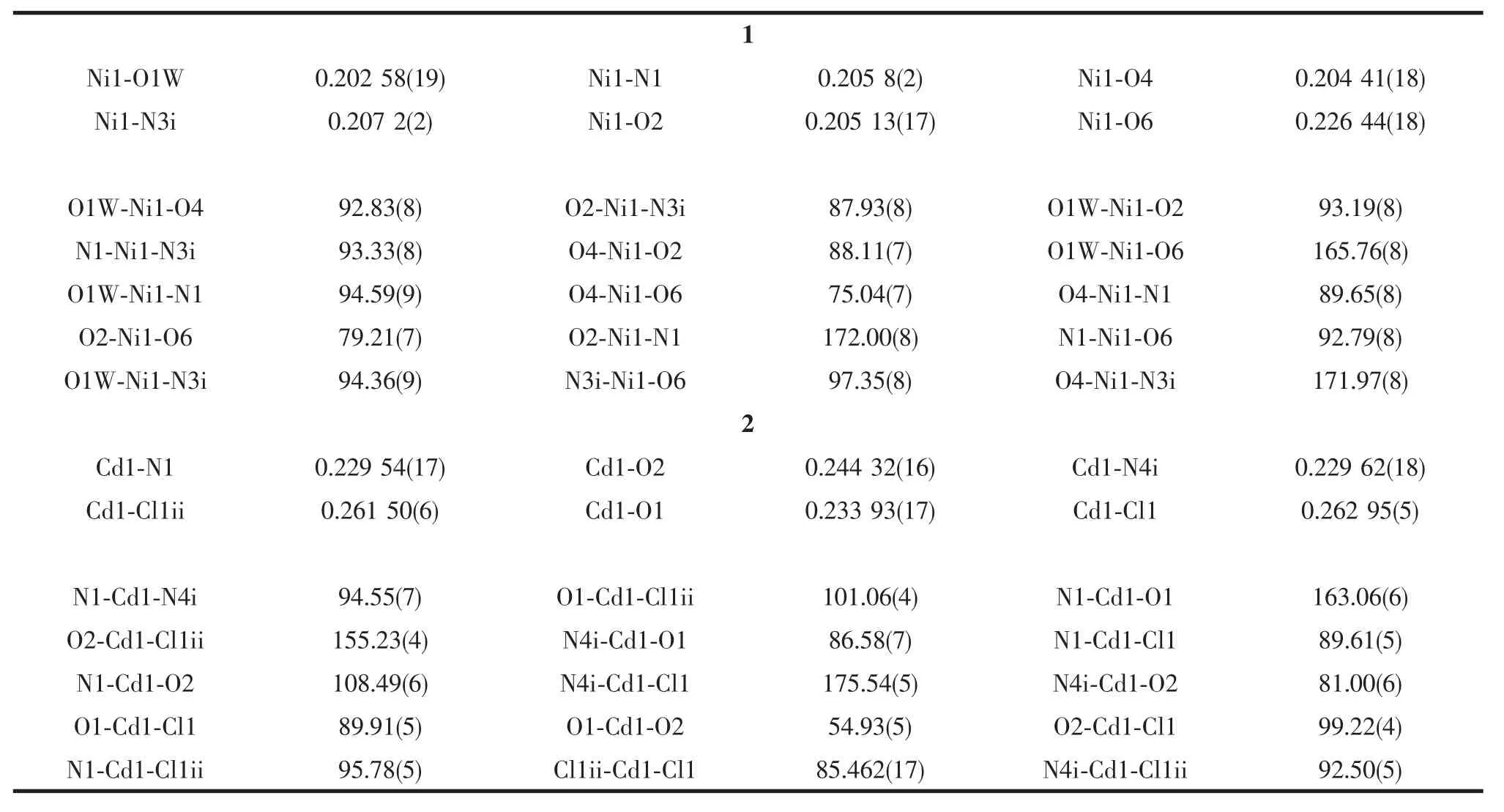
Table2 Selected bond lengths(nm)and angles(°)for 1 and 2
2 Results and discussion
2.1Crystal structure
The crystallographic analysis reveals that 1 is a two-dimensional corrugated layer structure.As shown in Fig.1,the asymmetric unit contains one Niion, oneBDOAanion,oneBIDPEligandandone coordinated water molecule.Each Niion is sixcoordinated by three oxygen atoms from two different BDOA2-anions,two nitrogen atoms from two BIDPE ligands and one coordinated water molecule to form a distorted octahedralgeometry.Itsbasalplaneis occupied by three oxygen atoms,O4,O6 and O1W, and one nitrogen atom,N3i,while the apical position is occupied by the other nitrogen atom(N1)and one oxygen atom(O2).The Ni-O bond lengths are in the range of 0.202 58(19)~0.226 44(18)nm.The Ni-N bond lengths are in the range of 0.205 8(2)~0.207 2(2) nm and the coordination angles around Ni ion are in the range of 75.04(7)°~172.00(8)°.
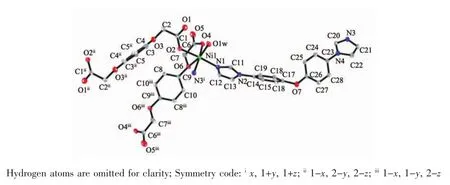
Fig.1 Coordination environment of Niin complex 1 with thermal ellipsoids at 30%probability
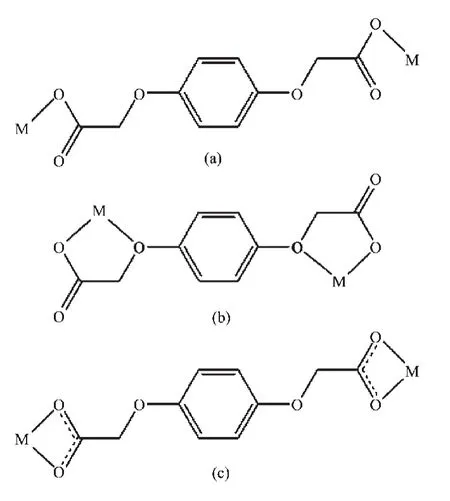
Scheme 1 Coordination modes of the BDOA2-ligand
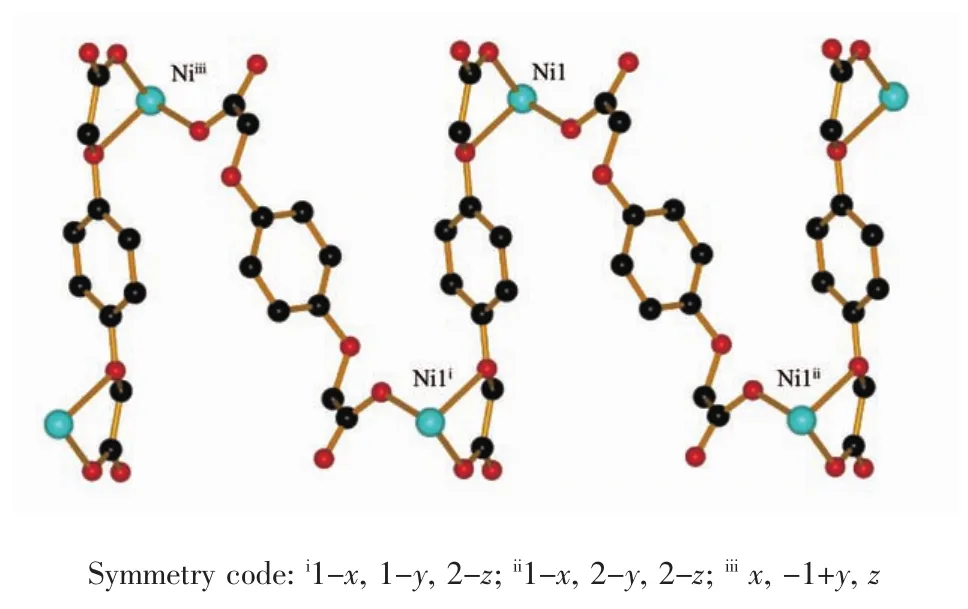
Fig.2 View of 1D infinite[Ni(BDOA)]nchain in 1
Fig.3 Corrugated 2D(4,4)layer bridged the BDOA and BIDPE ligands in 1
WhenCdCl2wasreactedwithBIDPEand H2BDOA using a preparation procedure similar to that of 1,complex 2 was isolated.As shown in Fig.5,the asymmetric unit consists of one crystallographically unique Cdions,one individual BIDPE ligand,half of BDOA anion and one chlorine ion.Each Cdion issix-coordinatedbytwooxygenatomsfroma carboxylate group of the BDOA2-anion,two nitrogen atoms from two different BIDPE ligands and two chlorine ions to form a distorted octahedral geometry. Its basal plane is occupied by one oxygen atom(O2), one nitrogen atom(N4ii)and two chlorine ions(Cl1, Cl1i),while the apical position is occupied by the other nitrogen atom(N1)and oxygen atom(O1).The Cd-O bond lengths are in the range of 0.233 93(17)~0.244 32(16)nm,and the Cd-N bond lengths are in the range of 0.229 54(17)~0.229 62(18)nm and the coordination angles around Cd ion are in the range of 54.93(5)°~163.06(6)°.Two Cdions are double bridged by two chlorine ions with Cd…Cd separations of 0.358 2 nm to from a binuclear Cd2unit,which is further linked by the BIDPE and BDOA2-ligands togenerate a one-dimensional chain structure(Fig.6).The fully deprotonated BDOA2-anion coordinates to two Cd ions with two carboxylate groups adopting monodentate mode(Scheme 1c).In the crystal,adjacent 1D chains are stacked into a three-dimensional supramolecular network through intermolecular C-H…Cl hydrogen bonding and weak π…π stacking interactions(the centroid-to-centroid distance between the two adjacent benzene rings of BIDPE ligands is 0.412 3 nm,and the dihedral angle is 3.785(2)°).
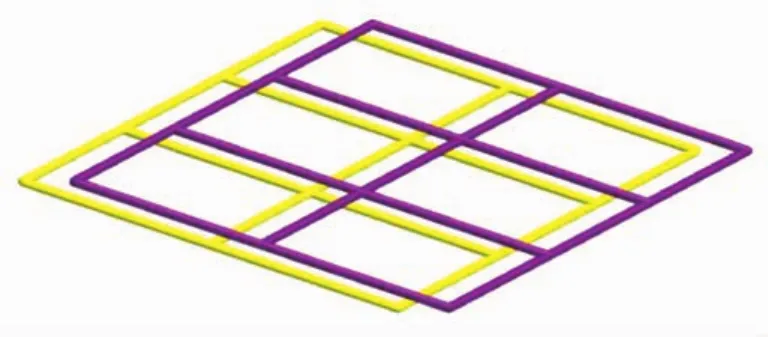
Fig.4 Schematic description of stacking sequence of the layers in 1
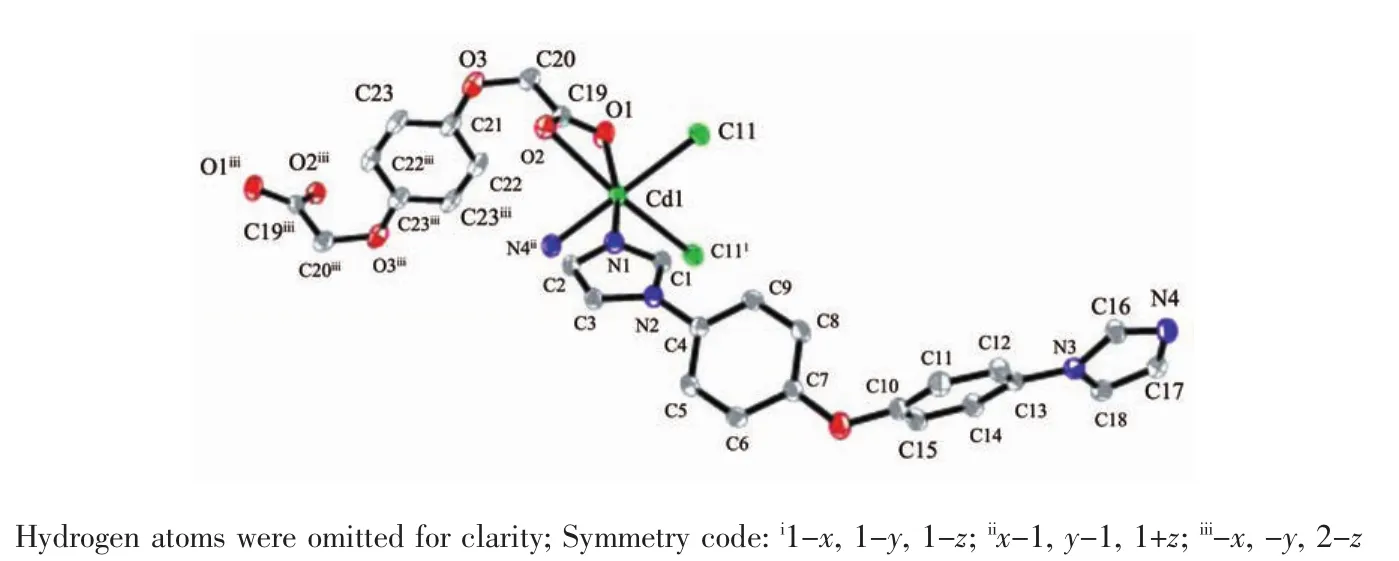
Fig.5 Coordination environments of the Cdatoms in 2 with the ellipsoids drawn at the 30%probability level
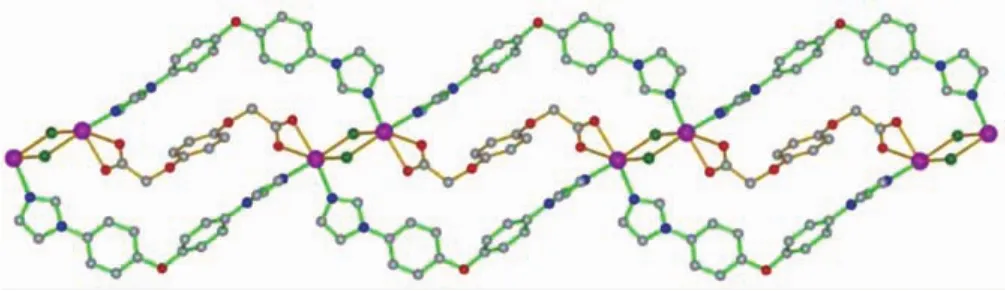
Fig.6 View of the 1D infinite chain formed by the BIDPE ligands and BDOA2-anions
As to the synthesis of 1 and 2,the reaction conditions are the same except for the difference of the metal ions.As a result,complex 1 is a 2D supramolecular structure,while complex 2 is a 1D infinite chain.The differences between 1 and 2 reveal that metal ions play an extreme role in the generation of crystal structure,due to the size of metal ions and the versatility of the metal coordination geometry.In other words,it is found that suitable metal ions may be a good candidate forthe targetcoordination polymeric frameworks.
2.2FTIR spectra
FTIR spectra revealed valuable information about the coordination modes of H2BDOA.The absence of the characteristic bands at around 1 700 cm-1attributed to the protonated carboxylate group indicates that the complete deprotonation of H2BDOA upon reaction with metal ion.The difference between asymmetric and symmetric carbonyl stretching frequencies(Δν=νasymνsym)was used to fetch information on the metalcarboxylate binding modes.Complex 1 showed two pairs of νasymand νsymfrequencies at 1 609,1 422 cm-1(Δν=187 cm-1)and 1 559,1 371 cm-1(Δν=188 cm-1) for the carbonyl functionality indicating two coordination modes as observed in the crystal structure. Complex 2 showed a pairs of νasymand νsymfrequencies at 1 695,1 362 cm-1(Δν=233 cm-1)corresponding to the carbonylfunctionalityofdicarboxylateligand indicatingasymmetricmonodentatecoordination mode.OH stretching broad bands at 3 445 cm-1for 1 are attributable to the coordinated lattice water.The bands in the region of 640~1 250 cm-1are attributed to the-CH-in-plane or out-of-plane bend,ring breathing,andringdeformationabsorptionsof benzene ring.The IR spectra exhibit the characteristic peaks of imidazole groups at ca.1 520 cm-1[39].
2.3Photoluminescence study
Coordination polymers and conjugated organic linkersarepromisingcandidatesforphotoactive materials with potential applications such as chemical sensors and photochemistry[40-41].Solid state photoluminescence behavior of 1 and 2 at ambient temperature was examined.Absorption maxima for ligands BIDPE and H2BDOA were observed at 272 and 287 nm respectively,however complex 1 and 2 showed major absorption bands at ca.369 and 291 nm,respectively. As illustrated in Fig.7,the intense emission band at 417 nm(λex=369 nm)for 1 is observed.Upon excitation with 291 nm,strong emission at 454 nm with an overall luminescence quantum yield(Φoverall)of 5.02%for 2 was recorded at room temperature.The red shift in the emission bands of 2 could be ascribed to the metal-perturbed intraligand emission and ligand field transitions.The good photoluminescence of 2 may also be attributed to the closed shell metal configuration of Cd.Furthermore,the higher quantum yield of 2 may also be attributed to the triple helical motif as such rigid structural features substantially cease the radiationless vibrational energy loss[42-44].
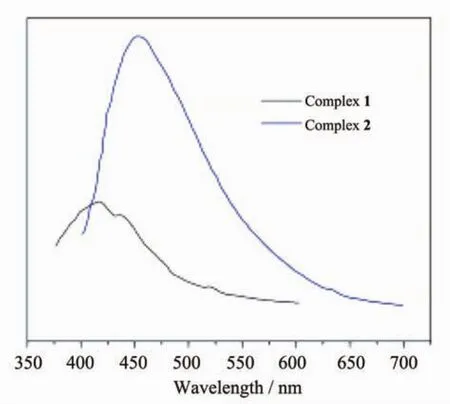
Fig.7 Solid-state photoluminescent spectra of complexes 1 and 2
[1]Horike S,Inubushi Y,Hori T,et al.Chem.Sci.,2012,3:116 -120
[2]Liu Y L,Eubank J F,Cairns A J,et al.Angew.Chem.Int. Ed.,2007,46:3278-3283
[3]Rowsell J L C,Millward A R,Park K S,et al.J.Am.Chem. Soc.,2004,126:5666-5667
[4]Zhang Y B,Zhang W X,Feng F Y,et al.Angew.Chem.Int. Ed.,2009,48:5287-5390
[5]Li J R,Kuppler R J,Zhou H C.Chem.Soc.Rev.,2009,38: 1477-1504
[6]Qin L,Hu J S,Huang L F,et al.Cryst.Growth Des.,2010, 10:4176-4183
[7]Ma Y S,Li H,Wang J J,et al.Chem.Eur.J.,2007,13: 4759-4769
[8]Nouar F,Eckert J,Eubank J F,et al.J.Am.Chem.Soc., 2009,131:2864-2870
[9]Xamena F X L I,Abad A,Corma A,et al.J.Catal., 2007,250:294-298
[10]Reger D L,Watson R P,Smith M D.Inorg.Chem.2006,45: 10077-10087
[11]Tian Y Q,Zhao Y M,Chen Z X,et al.Chem.Eur.J., 2007,13:4146-4154
[12]Sarma R,Kalita D,Baruah J B.Dalton Trans.,2009:7428 -7436
[13]Cao R,Sun D F,Liang Y C,et al.Inorg.Chem.,2002,41: 2087-2094
[14]Mahata P,Natarajan S.Inorg.Chem.,2007,46:1250-1258
[15]Zhang L X,Fan C J,Liu P,et al.Inorg.Chem.Commun., 2010,13:914-918
[16]Zheng B,Dong H,Bai J F,et al.J.Am.Chem.Soc., 2008,130:7778-7779
[17]Zhao L M,Zhai B,Gao D L,et al.Inorg.Chem.Commun., 2010,13:1014-1017
[18]Li Z,Li M,Zhou X P,et al.Cryst.Growth Des.,2007,7: 1992-1998
[19]Bai Z S,Qi Z P,Lu Y,et al.Cryst.Growth Des.,2008,8: 1924-1931
[20]He F,Tong M L,Yu X L,et al.Inorg.Chem.,2005,44:559 -565
[21]Yang J,Wu B,Zhu F Y,et al.Cryst.Growth Des.,2010, 10:2331-2341
[22]Schaate A,Klingelhofer S,Behrens P,et al.Cryst.Growth Des.,2008,8:3200-3205
[23]Lu Y L,Zhao W J,Liu Y,et al.J.Solid State Chem., 2012,192:144-152
[24]Jing X H,Yi X C,Gao E Q,et al.Dalton Trans.,2012,47: 14316-14328
[25]Liu G X,Huang Y Q,Chu Q,et al.Cryst.Growth Des., 2008,8:3233-3245
[26]Wang F,Ke X H,Zhao J,et al.Dalton Trans.,2011,40: 11856-11865
[27]Fan J,Hanson B E.Inorg.Chem.,2005,44:26998-27008
[28]Wang X Y,Wang Z M,Gao S.Sci.China Ser.B,2012,55: 1055-1063
[29]Sun D,Yan Z H,Blatov V A,et al.Cryst.Growth Des., 2013,13:1277-1289
[30]Bisht K K,Suresh E.Inorg.Chem.,2012,51:9577-9579
[31]Wang C J,Wang T,Li L,et al.Dalton Trans.,2013,42: 1715-1725
[32]Yang W,Wang C M,Ma Q,et al.CrystEngComm,2014,16: 4554-4561
[33]Yan Z H,Wang W,Zhang L L,et al.RSC Adv.,2015,5: 16190-16198
[34]Hu J S,Shang Y J,Yao X Q,et al.Cryst.Growth Des., 2010,10:4135-4142
[35]Sheldrick G M.SADABS,Program for Empirical Absorption Correction of Area Detector Data,University of G?ttingen, Germany,1996.
[36]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,University of G?ttingen,Germany,1997.
[37]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structure,University of G?ttingen,Germany,1997.
[38]Xu H B,Su Z M,Shao K Z,et al.Inorg.Chem.Commun., 2004,7:260-263
[39]Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordinated Compounds.5th Ed.New York:Wiley&Sons, 1997.
[40]Allendorf M D,Bauer C A,Bhakta R K,et al.Chem.Soc. Rev.,2009,38:1330-1352
[41]Braverman M A,LaDuca R L.Cryst.Growth Des.,2007,7: 2343-2351
[42]Bisht K K,Suresh E.Inorg.Chem.,2012,51:9577-9579
[43]Cui Y,Yue Y,Qian G,et al.Chem.Rev.,2012,112:1126 -1162
[44]Perry J J,Feng P L,Meek S T,et al.J.Mater.Chem.,2012, 22:10235-10248
Syntheses and Crystal Structures of Nickeland CadmiumCoordination Polymers Constructed by Benzene-1,4-dioxydiacetate and 4,4-Bis(imidazole-l-yl)diphenyl Ether
CHEN Hong1YU Min2LIU Guang-Xiang*,2
(1School of Chemical Engineering and Life Science,Chaohu University,Chaohu,Anhui 238000,China) (2School of Environmental Science,Nanjing Xiaozhuang University,Nanjing 211171,China)
Two coordination polymers,[Ni(BDOA)(BIDPE)(H2O)]n(1)and[Cd(BDOA)0.5(BIDPE)Cl]n(2),(H2BDOA= benzene-1,4-dioxydiacetic acid and BIDPE=4,4′-bis(imidazole-l-yl)diphenyl ether),have been synthesized and characterized by IR spectroscopy,elemental analysis and single-crystal X-ray diffraction.Complex 1 crystallizes in triclinic,space group P1 with a=0.934 97(8)nm,b=1.032 31(9)nm,c=1.352 91(12)nm,α=96.398 0(10)°,β= 90.729 0(10)°,γ=102.027 0(10)°,V=1.268 31(19)nm3,Z=2,Mw=603.22,Dc=1.580 g·cm-3,μ=0.827,F(000)=624, R1=0.039 5,wR2=0.089 5(I>2σ(I)).Complex 2 belongs to triclinic,space group P1 with a=1.017 51(7)nm,b= 1.042 99(7)nm,c=1.173 38(8)nm,α=68.723 0(10)°,β=71.826 0(10)°,γ=76.091 0(10)°,V=1.091 05(13)nm3,Z= 2,Mw=562.26,Dc=1.711 g·cm-3,μ=1.163,F(000)=562,R1=0.021 0,wR2=0.053 7(I>2σ(I)).Structural analyses reveal that complex 1 exhibits a two-dimensional(2D)corrugated layer structure,whereas complex 2 possesses a one-dimensional(1D)chain structure which is generated by joining binuclear Cd2units by BIDPE and BDOA ligands.The results show that the nature of metal ions plays an important role in governing the molecularframeworks.The photochemical properties of two complexes have also been studied.CCDC:1018030,1; 1018031,2.
coordination polymer;bis(imidazole)ligands;polycarboxylate;crystal structure
O614.81+3;O614.24+2
A
1001-4861(2015)06-1261-08
10.11862/CJIC.2015.170
2015-03-01。收修改稿日期:2015-03-31。
國家自然科學基金(No.21271106)和教育部科學技術重點項目(No.210102)資助。
*通訊聯系人。E-mail:njuliugx@126.com