Effect of PSA tin plating process on trace lead in tin coating☆
Binghu Li,Kuaikuai Guo ,Changsheng Liu ,*
1 Key Laboratory for Anisotropy and Texture of Materials of Ministry of Education,Northeastern University,Shenyang 110819,China
2 Baoshan Iron&Steel Group,Shanghai 201900,China
Keywords:Optimal design Process control Safety Tin plating Trace Pb Current density Bath temperature
ABSTRACT In this paper,effects of conditions in phenol sulfonic acid(PSA)plating for tin coating of MR low carbon aluminum killed steel on trace Pb were examined.Trace Pb was measured by atomic absorption spectrometry(AAS)and glow discharge spectrometry,and coating morphology was observed by scanning electronic microscopy(SEM).Corrosion resistance of the tin coating was analyzed by electrochemical methods.The results indicated that Pb content in the tin coating reduced as bath temperature increased.When the temperature exceeded 40°C,the grains in the coating were coarse and loose,reducing the corrosion resistance.As current density increased,Pb contentincreased rapidly,while low currentdensity plating could lead to drain regions.The plating speed had no obvious effect on trace Pb in tin coating.In the tin plating layer,Pb was enriched at the surface and gradually reduced to zero along the depth.At bath temperature of 40 °C and current density of 20 A·dm-2,the amount of Pb could be less than 100 mg·kg-1 with excellent corrosion resistance.
1.Introduction
Tinplate is widely used in food and beverage packaging due to its non-toxicity,beautifulappearance,corrosion resistance,and other characteristics[1-4].For example,the consumption of tinned steel in above industry is up to 1300 ton·a-1in recentyears.With the developmentof living standards ofpeople,highersafety in food packaging is strongly required.In 2005,EU issued the law of“Steel for packaging — Flat steel products intended for use in contactwith food stuffs,products or beverages for human and animal consumption — Tin coated steel”[5],which demands that trace Pb in tin coating should be less than 0.01%.A new standard is ten times lower than the original standard so that the old tin plating process has to be modi fied as soon as possible.It is known that the tin used in the tinplate usually contains trace Pb,and the standard potential of Sn/Sn2+and Pb/Pb2+is-0.136 V and-0.126 V vs.normal hydrogen electrode,respectively,leading to codeposition easily in electroplating process[6-11].The trace Pb in tin coating often exceeds 100 mg·kg-1,which is unable to meet new environmental requirement.
In tin plating industry,high rate phenol sulfonic acid(PSA)plating[12]is widely applied due to its high ef ficiency,low cost,and highperformance products.Researches have focused on the process of lead-tin alloy plating and effects of parameters and additives on coating quality[13-15],while the trace Pb in tin coating is rarely reported.Therefore,it is urgent to examine the effect of operation parameters in PSA bath plating process on trace Pb.
In this paper,the in fluence of bath temperature,current density and plating speed on trace Pb in tin coating is studied.A briefanalysis on the deposition mechanism of tin plating process and appropriate methods to reduce the Pb content and maintain coating quality is given.
2.Experimental
2.1.Preparation for test
Test substrate is MR low carbon aluminum killed steel.Its ingredients are as follows(mass fraction,%):C 0.12,Mn 0.60,P 0.02,S 0.05,Si 0.02,Cu 0.20.Specimen was cut into rectangular piece(100 mm×60 mm×0.20 mm),with the pretreatment as follows:alkali cleaning(NaOH 15-25 g·L-1;SNN41 2-4 g·L-1;1 min;45 °C)→electrochemical alkali cleaning(NaOH 15-25 g·L-1;SNN41 2-4 g·L-1;5 A·dm-2;2 min;50 °C) → water cleaning(pH ≤ 11;1 min;40 °C) → electrolytic picking(H2SO460-80 g·L-1;3 A·dm-2;2 min;30°C)→ water cleaning(pH ≥3;1 min;35°C).
2.2.Electroplating process conditions
Tin plating was performed in a self-made rotating-cathode plating apparatus.The plating solution is PSA system,with bath condition as follows:Sn2+27 g·L-1,free acid H+16 g·L-1,ENSA 2.8 g·L-1,EN 4.6 g·L-1,Pb 17 mg·L-1,pH 1.0.Bath temperature was set at 35,40,45,and 50 °C;current density was 10,15,20,25,and 30 A·dm-2;plating speed was 80,100,120 and 140 m·min-1.To ensure singleside tin coating weight2.8 g·m-2atleast,the plating time was adjusted appropriately between 2 s and 20 s.
2.3.Characterization tests
Trace Pb in tin coating was detected by atomic absorption spectrometry.The tin coating was firststripped by hydrochloric acid;then,the tin in the solution was removed by hydrobromic acid;graphite furnace atomic absorption(HITACHI,Z2700,Japan)was used to detect trace Pb of stripping solution.The surface morphology of the coating under different processing conditions was observed by scanning electron microscope(JEOL,JSM-6510A,Japan).
In order to examine corrosion behavior of the tinplates,Tafel measurements[16,17]were performed by electrochemical workstation(CorrTest,CS350,China).A three-electrode arrangement was adopted,with the working electrode(1 cm2of exposed area),saturated calomel electrode as the reference electrode,and platinum mesh as the counter electrode.The test solution was 3.5%sodium chloride with scanning rate of 10 mV·s-1and scanning range of-0.4 to-1.2 V.
The distribution of elements Pb,Sn,Fe and O along coating depth in tin coating was determined by glow discharge spectrometer(SPECTRO,GDA750,Germany).
3.Results and Discussion
3.1.Effect of bath temperature on trace Pb in tin coating
Fig.1 illustrates the in fluence of bath temperature on Pb content at current density of 20 A·dm-2and plating speed of 120 m·min-1.Pb content in the tin coating reduces quickly as bath temperature increases.At temperatures higher than 40°C,Pb content is lower than 100 mg·kg-1.
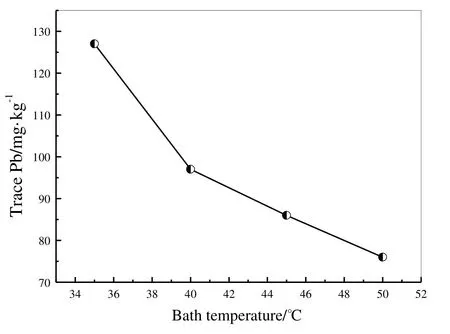
Fig.1.Effect of bath temperature on Pb content in tin coating.
Higher bath temperature is bene ficial to lowering Pb content in tin coating,but introduces adverse effect on corrosion resistance.At high bath temperature,the microstructure of tin coating is loose,as shown in Fig.2.Tin grains are larger.At 40°C,the density of uniform grains of coating is the lowest,suggesting best corrosion resistance.Considering both coating quality and Pb content,the optimal temperature is 40°C.
Fig.3 shows polarization curves of tin coating prepared at different bath temperatures.Fitted corrosion current and potential of each tin coating are list in Table 1.As bath temperature increases from 35°C to 50°C,corrosion current of tin coating reduces first and then increases,while the potential is opposite.It means that the tinplate shows the best corrosion resistance at bath temperature 40°C,consistent with the smallest coating grains in Fig.2.
3.2.Effect of current density on trace Pb
Fig.4 illustrates the in fluence of current density on Pb content in tin coating at bath temperature 20 °C and plating rare 120 m·min-1.Pb content in tin coating increases gradually as current density increases.When the current density is over 25 A·dm-2,Pb content is higher than 100 mg·kg-1,which cannot meet the new standard.
Current density has signi ficant in fluence on the polarization and Pb content in tinplate coating.According to the electrochemical polarization potential and current reaction Butler-Volmer equation[18],
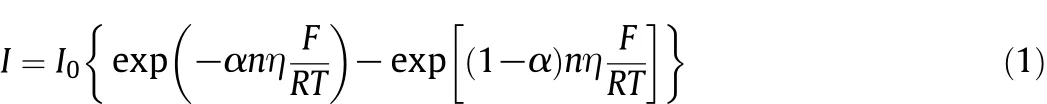
where I is the current density,I0is the exchange current,α is the electron transfer coef ficient,n is the number of electron transferred reaction,F is the Faraday constant,R is the molar gas constant,and T is the bath temperature.In a high-speed tin plating process,bath and cathode plate move relatively and quickly so that exchanged ions can be supplied suf ficiently in time.
Eq.(1)leads to

where η is the electrochemical polarization potential,a and b are the Tafel constants.
According to Eq.(2),in the electrochemical polarization,a linear relation exists between electrode potential and logarithm of current density.Tin plating is essentially an electrocrystallization of metal,including the formation and growth of crystal nucleus[19].The two processes occur simultaneously and their relative rate determines the size of tin grains.When the formation rate of crystal nucleus is higher than the growth rate, fine crystals and dense deposition can be obtained in the plating process.On the contrary,the crystallization becomes coarse.When the cathode current density is small,low cathode polarization formats less crystal nucleus at low rate[20],resulting in larger grains.As the current density increases,the electrochemical overpotential increases.As a result,the number of crystal nuclei increases and the grain size decreases.Nucleation rate is derived by Volmer and Weber[21]:

where v1is the nucleation rate,v2is the crystal growth rate,η is the electrochemical polarization overpotential,and K1and K2are the constants.
Eqs.(3)and(4)show thatthe electrochemicalpolarization increases with the current density.Nucleation rate is much higher than the growth rate,which re fine grains.
Fig.5 shows the surface morphologies of the coating at different current densities.Some leakage areas appear at low cathode current density(Fig.5(a)).As current density increases,the nucleation rate is greatly improved,which lead to grain re finement of electrodeposited tin layer.
Fig.6 shows polarization curves at different current densities.Fitted corrosion currents and potentials are list in Table 2.With the increase of current density,corrosion current of tin coating decreases,the crystallization of tin plating layer tends to be uniform and fine.The corrosion current is gradually reduced and the corrosion resistance is better.Considering both Pb content in coating and corrosion resistance,the current density in PSA should be less than 20 A·dm-2.
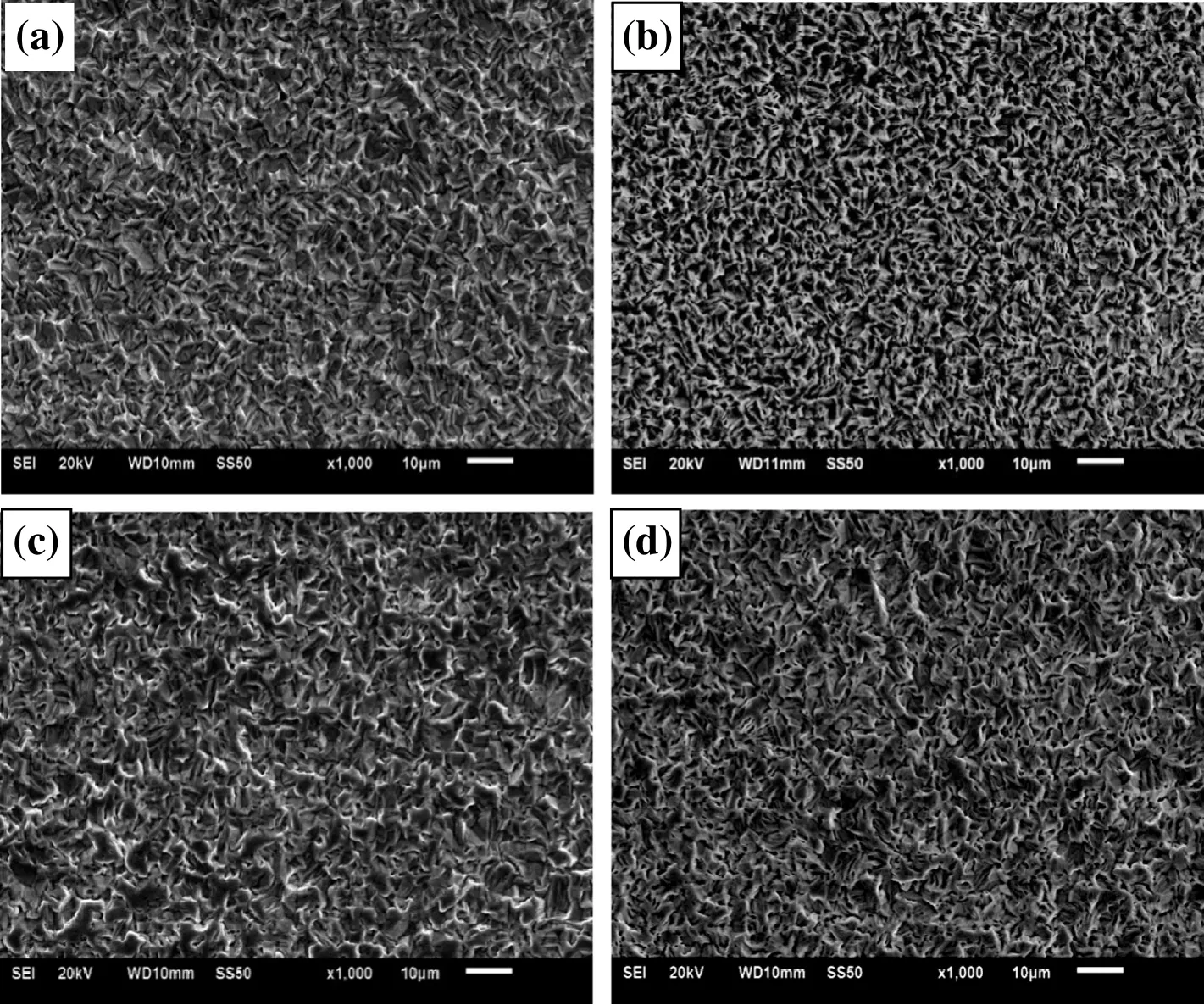
Fig.2.Morphology of tin coatings at different bath temperatures.(a)35 °C;(b)40 °C;(c)45 °C;(d)50 °C.
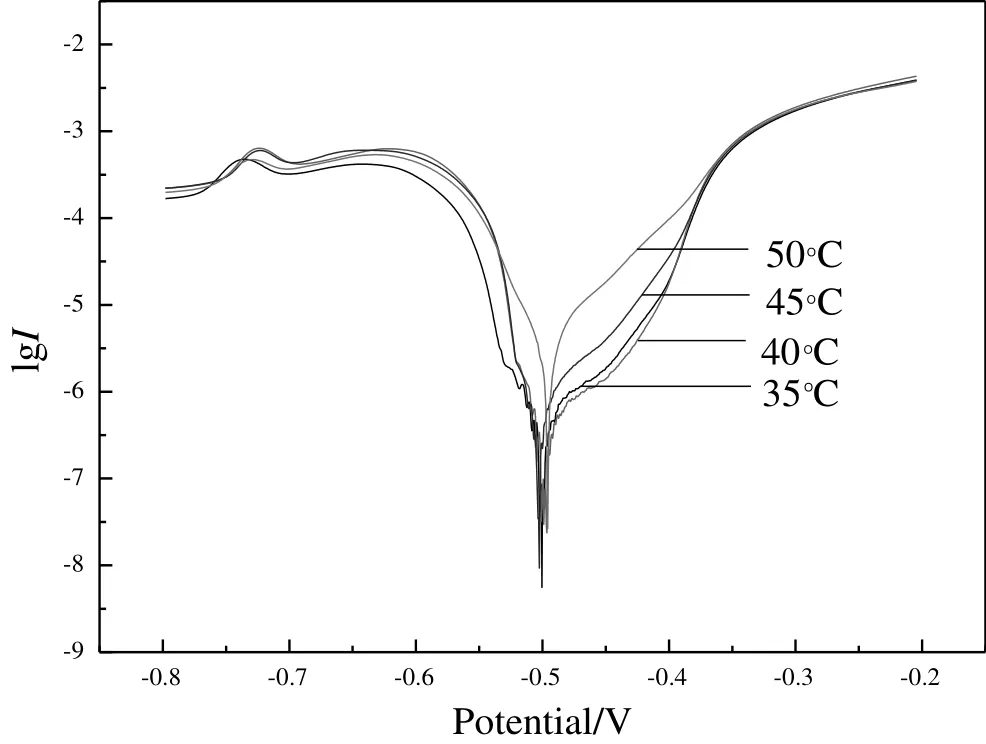
Fig.3.Tafel curves of tin plating at different bath temperatures.
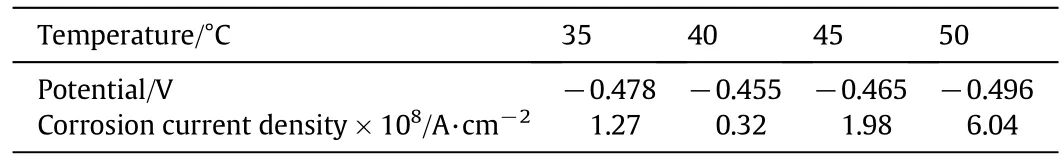
Table 1 Electrochemical corrosion data of tin plating prepared at different bath temperatures
3.3.Effect of plating speed on trace Pb
Fig.7 illustrates the in fluence of plating speed on Pb content at current density 20 A·cm-2and bath temperature 40 °C.The plating speed has little in fluence on trace Pb in tin coating.Itcould be explained as follows:the plating speed is much higher than ion exchange rate,so thatspecimen is always surrounded by fresh bath even atthe minimum speed.Therefore,it is not needed to consider the effect of plating speed in production.
3.4.Distribution of Pb along coating depth in coating
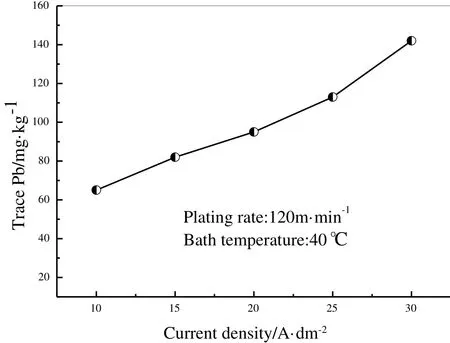
Fig.4.Effect of current density on trace Pb in tin coating.
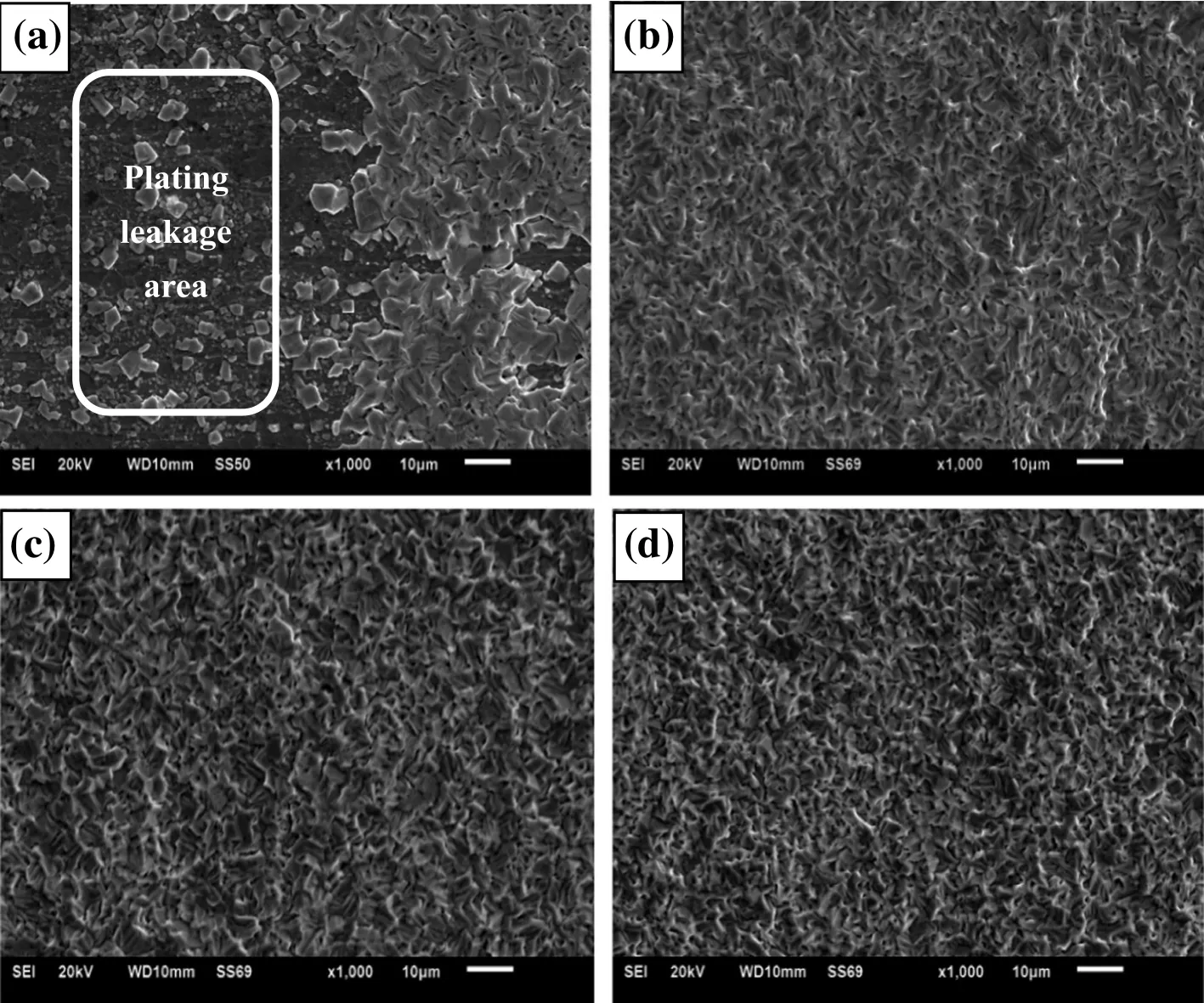
Fig.5.Scanning electronic microscopy of tin coatings at different current densities.(a)10 A·dm-2;(b)20 A·dm-2;(c)25 A·dm-2;(d)30 A·dm-2.
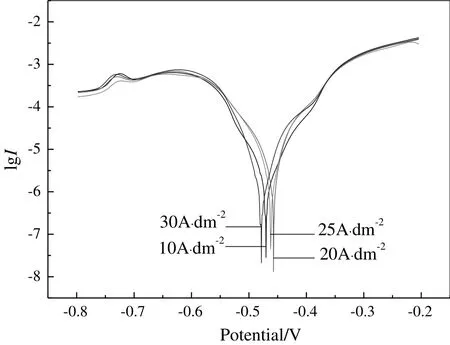
Fig.6.Tafel curves of tin plating at different current densities.
Fig.8 shows the result of glow discharge spectrometry(GDS)at current density of 20 A·dm-2,bath temperature of 40 °C,and plating speed of 120 m·min-1,with the distribution of Pb,Sn,Fe,and O contents in coating along the depth.The Pb content is relatively high at the coating surface and gradually reduces along the coating depth.Fig.8 could be divided into four parts according to the content of elements Sn and Fe.Parts I,II,III and IV are coating surface layer,pure tin layer,Sn-Fe alloy interlayer,and Fe substrate,respectively.Part I is mainly tin and tin oxide with the thickness of 0.03 μm,enrichment ofPb element.Part II is about 0.23 μm in-depth with a gradual decline of Pb content.In part III,Pb has a peak and then declines,while oxygen has a weak peak.One of possible explanations is that tin reacts with increasing oxygen and tin oxide reduces cathode polarization voltage,reducing Pb deposition in Part III.
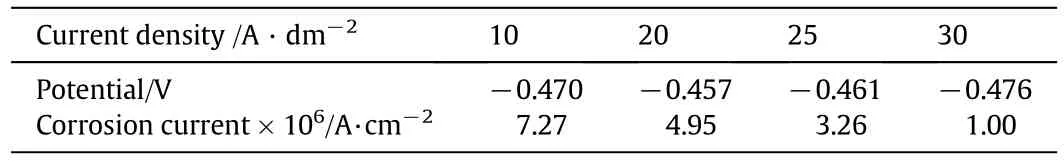
Table 2 Electrochemical corrosion data of tin plating at different current densities
4.Conclusions
As bath temperature increases,trace Pb in tin coating reduces quickly;when bath temperature exceeds 40°C,tin grains in coating are coarse and loose,and the corrosion resistance of coating decreases.As current density increases,Pb content increases rapidly,but low current density in plating could lead to drain regions.In high speed plating process in PSA bath,the plating speed has no obvious effect on trace Pb in tin coating.Trace Pb is associated with the tin plating layer,enriched near the surface and gradually reduced to zero along the depth in coating.At bath temperature of 40°C and current density of 20 A·dm-2,Pb content maintains less than 100 mg·kg-1in tinplated coating with excellent corrosion resistance.
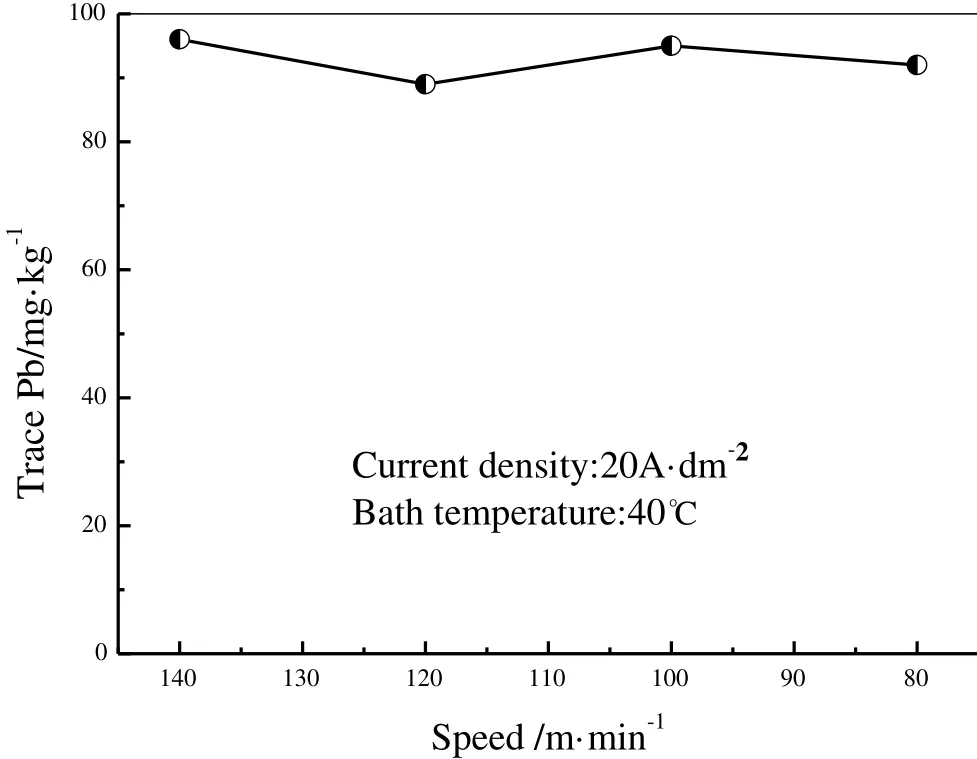
Fig.7.Effect of plating speed on trace Pb in tin coating.
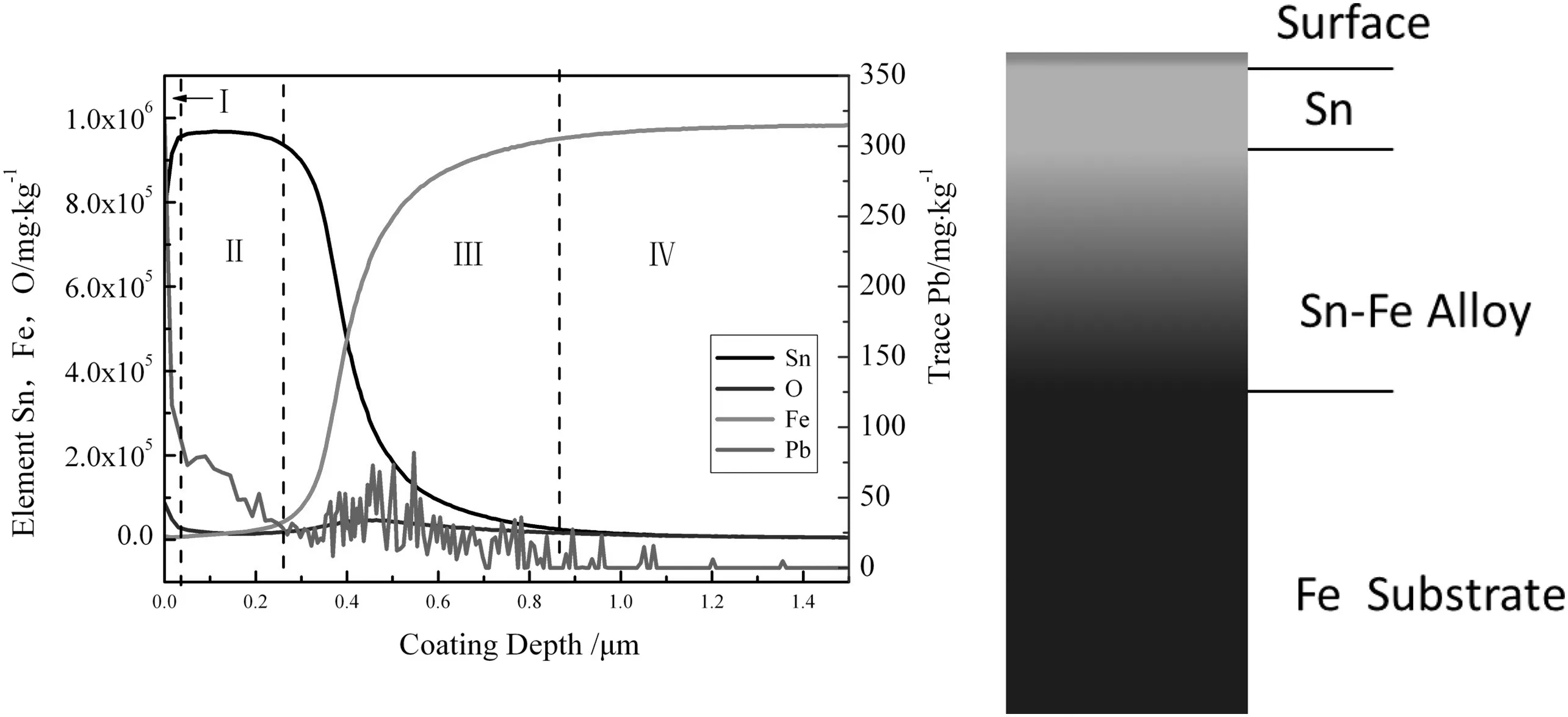
Fig.8.Distribution of elements Sn,Fe,O and Pb along tin coating depth by GDS.
 Chinese Journal of Chemical Engineering2015年10期
Chinese Journal of Chemical Engineering2015年10期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis and characterization of switchable ionic compound based on DBU,CH3OH,and CO2☆
- Preparation and simulation of a taro flavor☆
- Preparation and characterization of monodisperse zirconia spherical nanometer powder via lamellar liquid crystal template method
- Biological treatment of high wastewater using an ammonia-tolerant photosynthetic bacteria strain(ISASWR2014)☆
- Adsorption and degradation of nor floxacin by a novel molecular imprinting magnetic Fenton-like catalyst☆
- TG-FTIR analysis of pyrolusite reduction by major biomass components☆
