歐前胡素增強多柔比星對HeLa細胞的抗腫瘤效應
鄭 穎,姜 凱(浙江省立同德醫院婦產科,檢驗科,浙江杭州300)
歐前胡素增強多柔比星對HeLa細胞的抗腫瘤效應
鄭穎1△,姜凱2
(浙江省立同德醫院1婦產科,2檢驗科,浙江杭州310012)
[摘要]目的:研究歐前胡素是否能提高宮頸癌HeLa細胞株對多柔比星的敏感性。方法: MTT法檢測HeLa細胞用歐前胡素和多柔比星處理后的活力。Western blot檢測HeLa細胞用歐前胡素和多柔比星處理后Bcl-2蛋白家族成員(Mcl-1、Bcl-2、Bcl-xL、Bad和Bax)的表達水平。流式細胞術檢測HeLa細胞用歐前胡素和多柔比星處理后的凋亡水平和線粒體膜電位的變化情況。構建Mcl-1真核表達載體,MTT法檢測Mcl-1表達載體轉染對歐前胡素聯合多柔比星治療宮頸癌效果的影響。結果:歐前胡素在體外可顯著提高多柔比星對宮頸癌細胞系HeLa的殺傷活性。歐前胡素可顯著降低HeLa細胞Mcl-1的表達,而多柔比星對Mcl-1的表達水平無影響。相比于歐前胡素或多柔比星單治療組,兩者聯合可顯著誘導HeLa細胞發生凋亡并降低其線粒體膜電位。體外轉染Mcl-1真核表達載體顯著降低多柔比星聯合歐前胡素對HeLa細胞的殺傷活性。結論:歐前胡素通過靶向于Mcl-1增強多柔比星對宮頸癌細胞的殺傷活性。
[關鍵詞]歐前胡素;多柔比星; Mcl-1;宮頸癌
1000-4718(2015) 09-1578-06
[修回日期]2015-06-05
宮頸癌是全球發病率第2位的婦科腫瘤,每年有超過50萬患者被診斷為宮頸癌,好發于40歲以上女性,手術和化療目前仍是治療宮頸癌的主要手段[1]。多柔比星是目前最主要的抗腫瘤藥物之一,能有效治療肺癌、宮頸癌、前列腺癌等[2-3]。以多柔比星為主的化療方案在腫瘤治療中越來越被重視,然而隨著多柔比星的反復使用,腫瘤細胞將逐漸對其產生耐藥,且藥物的心臟毒性也漸漸凸顯,因此目前亟待解決的問題就是如何選用最佳的輔助藥物以取得最好的療效并降低多柔比星的耐藥性[4]。歐前胡素是一種呋喃香豆素類化合物,是從中藥白芷中提取的主要活性成分[5]。現在臨床上主要用于抗炎癥、抗凝血、抑制心肌肥厚等[6-7]。最近有文獻報道歐前胡素還有一定的抗腫瘤作用,能抑制腫瘤細胞的增殖,阻礙其細胞周期,甚至可直接誘導腫瘤細胞發生凋亡[8-9]。然而歐前胡素單獨用藥的療效并不十分理想[10]。因此本文的目的在于研究中藥歐前胡素是否能提高宮頸癌細胞對多柔比星的敏感性。
材料和方法
1實驗材料
多柔比星、歐前胡素、MTT和Annexin V凋亡試劑盒購于Sigma; DMEM培養基、胎牛血清購于Gibco;細胞蛋白提取液購于江蘇碧云天;人Mcl-1、Bcl-2、Bcl-xL、Bad、Bax及β-actin多克隆抗體購于CST; TRIzol試劑、逆轉錄試劑盒、pcDNA3.1、Lipofectamine 2000購于Invitrogen; SYBR Green試劑購于日本TaKaRa; ECL試劑盒購于Pierce; 5,5’,6,6’-四氯-1,1’,3,3’-四乙基苯并咪唑羰花青碘化物(JC-1)購于Molecular Probes。各PCR引物由上海生工生物工程有限公司合成。
2主要方法
2.1細胞培養人宮頸癌細胞系HeLa購于ATCC。HeLa細胞系培養在含10%胎牛血清的DMEM培養基中,在37℃恒溫培養箱中培養,通入5% CO2。
2.2MTT法檢測HeLa的細胞活力及多柔比星對HeLa的半數抑制濃度(IC50)將HeLa細胞按每孔5×103接種在96孔板上孵育12 h。之后將歐前胡素和多柔比星加入培養體系中孵育48 h。加入20 mL MTT (5 g/L)培養4 h,移除孔內培養基,加入100 μL DMSO,振蕩后在570 nm波長下測定吸光度(A)。相對細胞活力用實驗組與對照組的A值的比值表示。IC50根據多柔比星濃度與相對細胞活力曲線確定。
2.3Real-time PCR檢測Mcl-1的表達宮頸癌細胞系HeLa總RNA用TRIzol試劑提取。cDNA用逆轉錄試劑盒按操作說明步驟由總RNA合成。Mcl-1的定量PCR擴增使用SYBR Green試劑,GAPDH作為內參照,Mcl-1的相對表達由2-ΔΔCt法計算[11]。Mcl-1的上游引物為5’-TGGCTAAACACTTGAAGACC-3’,下游引物為5’-GGAAGAACTCCACAAACCC-3’; GAPDH的上游引物為5’-CCACTCCTCCACCTTTG-3’,下游引物為5’-CACCACCCTGTTGCTGT-3’。
2.4Western blot實驗藥物處理后,收集HeLa細胞,用蛋白裂解液進行細胞裂解,提取總蛋白質。將蛋白提取液用12.5%的SDS-PAGE進行分離,將電泳分離膠通過電轉方法將蛋白質轉到PVDF膜上,用Mcl-1、Bcl-2、Bcl-xL、Bad、Bax或β-actin多克隆抗體孵育過夜,之后再用帶辣根過氧化物酶的II抗孵育2 h,蛋白條帶用ECL試劑盒顯色發光。
2.5HeLa細胞凋亡的檢測將HeLa細胞用歐前胡素和多柔比星處理24 h,收集細胞,按照凋亡檢測試劑盒說明書操作步驟將細胞用Annexin V和碘化丙啶(PI)室溫孵育15 min,用流式細胞術檢測細胞凋亡情況。
2.6線粒體膜電位(ΔΨm)的檢測將HeLa細胞接種在6孔板中孵育12 h。換新鮮培養基后加入歐前胡素和多柔比星繼續處理HeLa細胞24 h,收集細胞,加入5 μmol/L JC-1 37℃孵育20 min,用流式細胞術檢測細胞線粒體膜電位。正常細胞發生紅色熒光,膜電位越低,紅色熒光越弱[12]。
2.7質粒構建及轉染以HeLa細胞的cDNA為模板,將Mcl-1的開放閱讀框架以分子克隆的方法與pcDNA3.1連接后構建成pcDNA3.1-Mcl-1重組真核表達質粒[13]。待HeLa細胞生長到鋪滿培養瓶約80%密度后,將pcDNA3.1-Mcl-1質粒(2 mg/L)用Lipofectamine 2000試劑按照試劑說明書步驟轉染入HeLa細胞中。
3統計學處理
實驗數據用均數±標準差(mean±SD)表示,用SPSS 12.0統計分析軟件進行處理,實驗重復3次,采用非配對雙側t檢驗進行組間分析,以P<0.05為差異有統計學意義
結果
1歐前胡素增強多柔比星對HeLa細胞的殺傷活性
歐前胡素單獨作用對HeLa細胞的抑制作用不強,歐前胡素需超過80 μmol/L時才能顯著抑制He-La細胞的細胞活力(圖1)。選擇低濃度10 μmol/L歐前胡素與多柔比星一起聯合治療HeLa細胞,結果發現低濃度的歐前胡素可顯著提高多柔比星對He-La細胞的殺傷活性并顯著降低HeLa細胞對多柔比星的IC50(圖2)。
2歐前胡素抑制HeLa細胞Mcl-1的表達
歐前胡素(10 μmol/L)可顯著降低HeLa細胞Mcl-1的表達,但對其它Bcl-2家族蛋白(Bcl-2、BclxL、Bad和Bax)無影響,多柔比星(4 μmol/L)對He-La細胞Mcl-1的表達亦無影響(圖3)。
3歐前胡素增強多柔比星對HeLa細胞凋亡的誘導效應
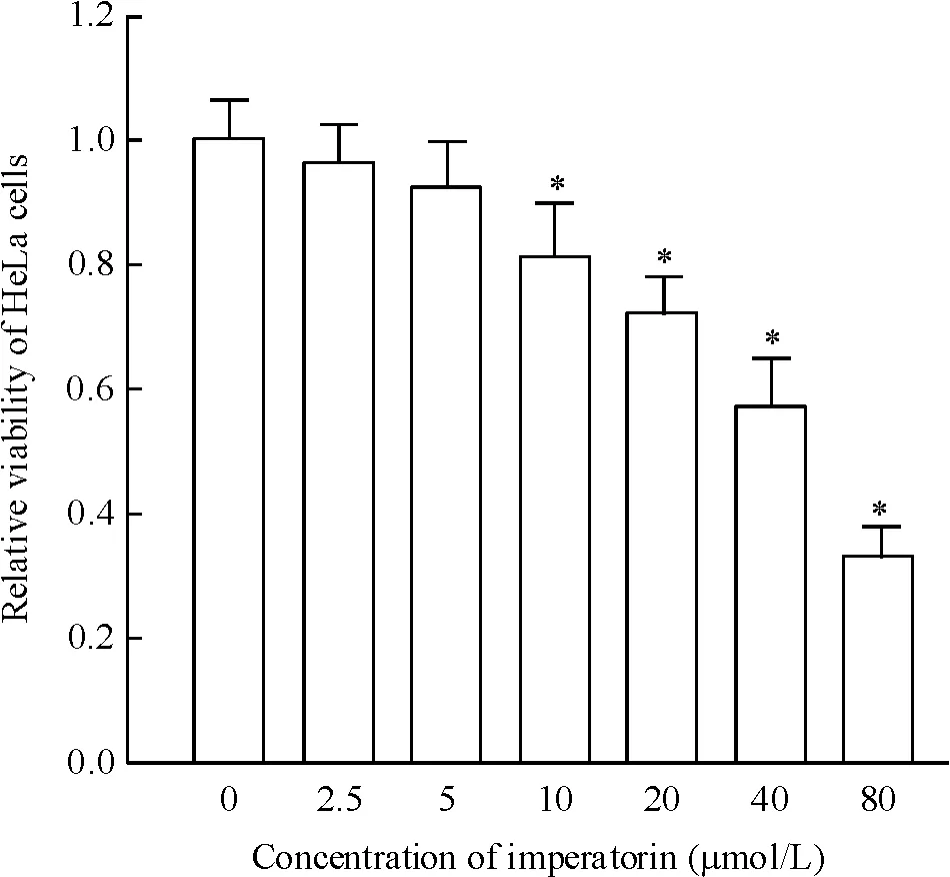
Figure 1.The relative viability of HeLa cells treated with various concentrations of imperatorin.Mean±SD.n=3.*P<0.05 vs 0 μmol/L group.圖1不同濃度歐前胡素對HeLa細胞活力的影響
低濃度的歐前胡素(10 μmol/L)和多柔比星(4 μmol/L)單治療組HeLa細胞的凋亡水平差異不顯著。然而將兩者聯合使用后,對HeLa細胞的凋亡誘導效應顯著提升(圖4),表明歐前胡素和多柔比星存在藥物協同效應。
4歐前胡素促進多柔比星的抗腫瘤效應是通過下調Mcl-1起作用
MTT實驗結果發現外源性Mcl-1的強制表達可顯著抑制歐前胡素(10 μmol/L)對多柔比星(4 μmol/L)的協同效應(圖5)。另外,相比于歐前胡素(10 μmol/L)和多柔比星(4 μmol/L)單治療組,兩者聯合能顯著誘導HeLa細胞線粒體膜電位的下降,而外源性Mcl-1可阻止ΔΨm的降低(圖6)。
討論
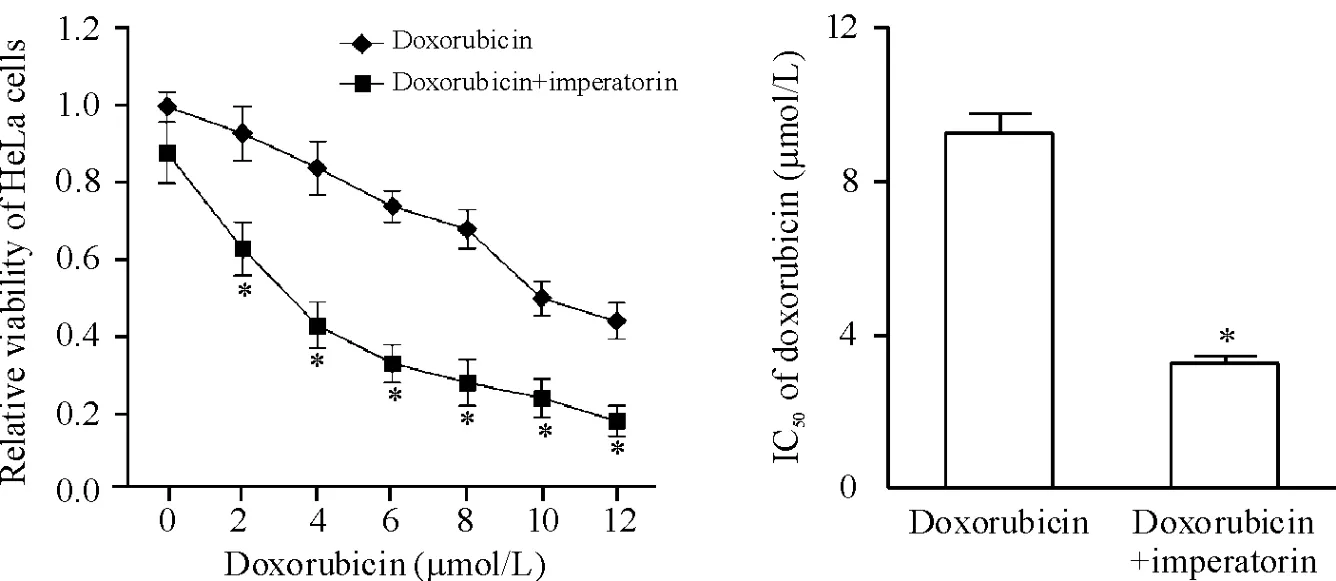
Figure 2.Imperatorin enhanced the cytotoxicity of doxorubicin to HeLa cells.Mean±SD.n=3.*P<0.05 vs doxorubicin group.圖2歐前胡素增強多柔比星對HeLa細胞的殺傷活性

Figure 3.Imperatorin down-regulated the expression of Mcl-1 in the HeLa cells.Mean±SD.n=3.*P<0.05 vs control group;#P<0.05 vs doxorubicin group.圖3歐前胡素下調HeLa細胞Mcl-1的表達水平
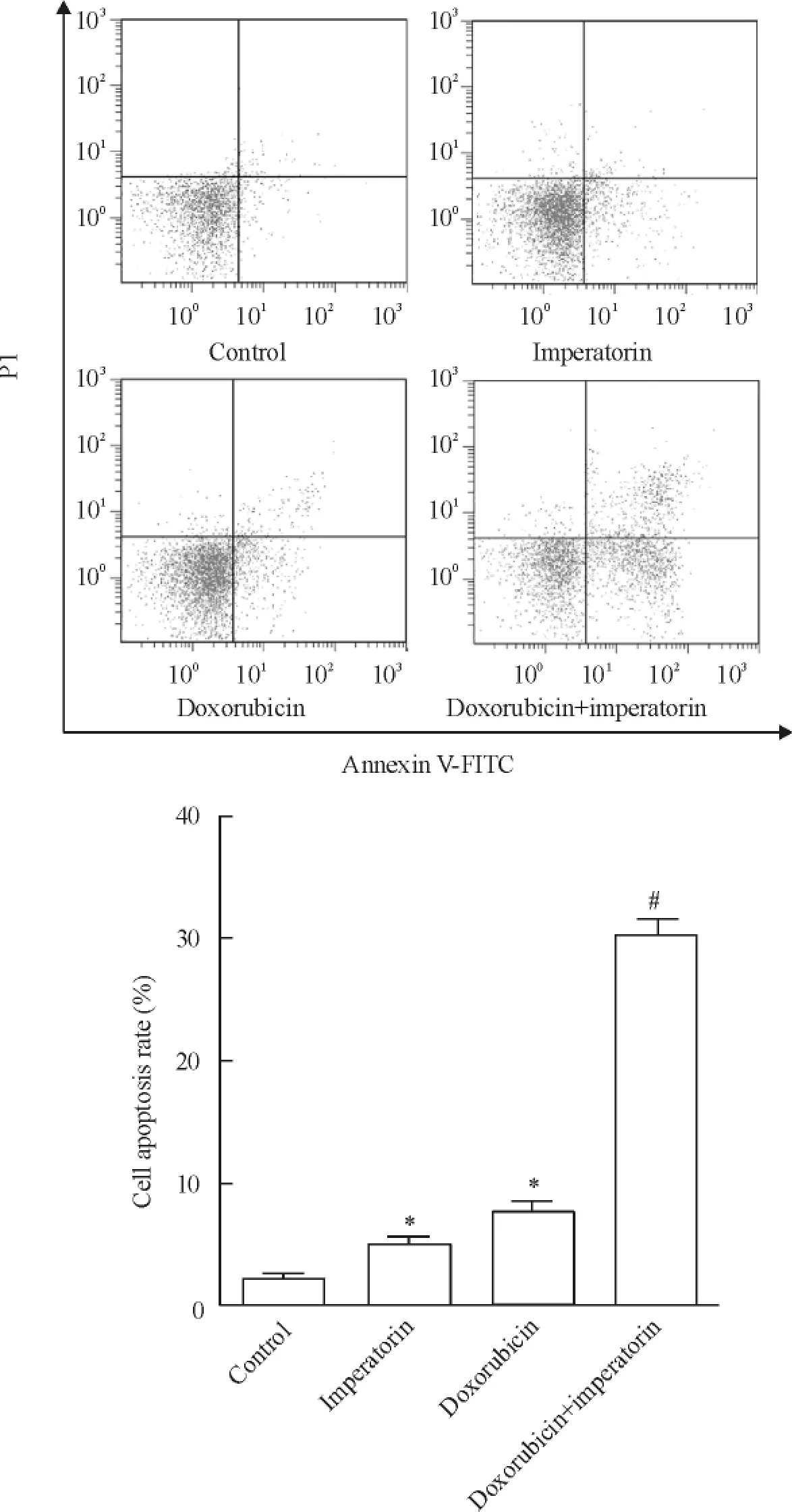
Figure 4.Imperatorin increased the apoptotic rate of HeLa cells induced by doxorubicin.Mean±SD.n=3.*P<0.05 vs control group;#P<0.05 vs doxorubicin group.圖4歐前胡素增強多柔比星對HeLa細胞的凋亡誘導效應
盡管多柔比星是目前治療腫瘤的一線化療藥物,然而在經過重復用藥后,腫瘤細胞往往通過減少多柔比星的轉運攝取、增加DNA修復能力、降低凋亡率等機制產生對多柔比星的耐藥性[14-15]。為了克服這一局限性,在進行多柔比星化療的基礎上,再聯合另外一種藥物以減弱腫瘤細胞對多柔比星的耐藥顯得十分重要。在本研究中,作者發現雖然低劑量歐前胡素的直接抗宮頸癌作用比較弱,但卻可顯著提升一線化療藥物多柔比星對HeLa的殺傷活性,表明中藥歐前胡素可與多柔比星發揮協同治療作用。
Bcl-2蛋白家族包括促凋亡成員和抗凋亡蛋白成員(如Mcl-1、Bcl-2、Bcl-xL、Bad和Bax等),這些蛋白的相對表達水平決定了細胞是否進入凋亡程序[16]。Mcl-1是Bcl-2蛋白家族中的抗凋亡蛋白成員,包含3個BH(Bcl-2 homology)位點。Mcl-1蛋白定位于細胞線粒體膜上,通過與促凋亡蛋白Noxa、Puma、Bim、Bid等結合,使它們失活從而發揮抗細胞凋亡作用[17]。因此,Mcl-1的過表達有助于保護細胞逃避凋亡信號,包括宮頸癌在內的多種腫瘤,其Mcl-1的表達水平均顯著上調[18]。Wei等[19]發現敲除Mcl-1基因后,胰腺癌細胞可發生自發性凋亡,且其對化療藥物吉西他濱的敏感性顯著增強,可見由于Mcl-1的抗凋亡作用,高表達的Mcl-1成為腫瘤細胞抵抗化療藥物殺傷活性的重要機制[20],Mcl-1基因已經成為腫瘤治療的新靶點。
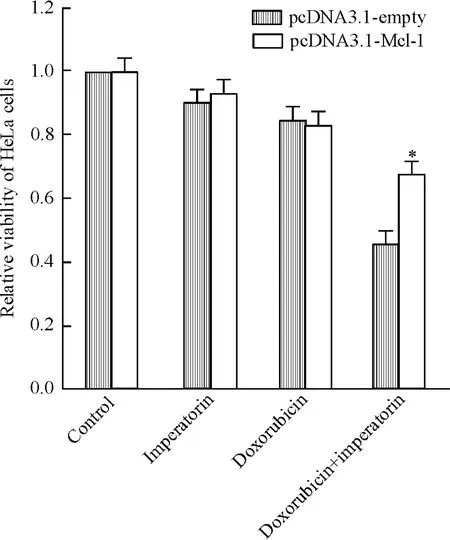
Figure 5.Imperatorin enhanced the cytotoxicity of doxorubicin to HeLa cells via down-regulating Mcl-1 expression,and this effect was inhibited by the transfection of pcDNA3.1-Mcl-1 plasmid.Mean±SD.n=3.*P<0.05 vs pc-DNA3.1-empty.圖5歐前胡素通過下調Mcl-1的表達增強多柔比星對He-La細胞的殺傷活性
在本研究中,我們發現歐前胡素能顯著降低He-La細胞Mcl-1的表達水平,而多柔比星對Mcl-1基因的表達無影響,因此我們推測歐前胡素增強多柔比星抗宮頸癌活性的機制可能和Mcl-1有關。為了驗證這一觀點,我們將外源性Mcl-1通過質粒在HeLa細胞中強制高表達,之后發現歐前胡素對多柔比星的協同抗腫瘤作用喪失,證實了歐前胡素增強多柔比星抗腫瘤活性的機制可能和下調Mcl-1表達水平有關。由于Mcl-1的表達水平和凋亡密切相關,而凋亡的誘導往往和線粒體的失能有關[21]。我們進一步發現歐前胡素聯合多柔比星能顯著降低HeLa細胞的線粒體膜電位,提示歐前胡素促進多柔比星引起的凋亡誘導效應的機制可能是通過下調宮頸癌細胞Mcl-1的表達,進而引起線粒體膜電位喪失,誘導細胞發生線粒體途徑的凋亡。綜上所述,歐前胡素-Mcl-1-線粒體途徑與多柔比星的抗宮頸癌活性密切相關,它可能成為腫瘤化療的一個新的靶點。
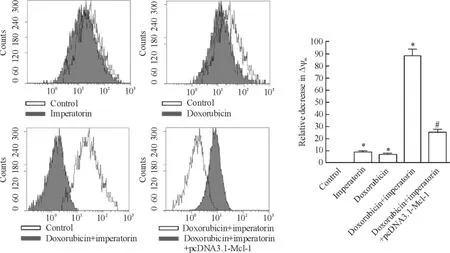
Figure 6.Doxorubicin plus imperatorin significantly decreased the ΔΨmin the HeLa cells,and this effect was inhibited by the transfection of pcDNA3.1-Mcl-1 plasmid.Mean±SD.n=3.*P<0.05 vs control group;#P<0.05 vs doxorubicin+ imperatorin group.圖6多柔比星聯合歐前胡素顯著降低HeLa細胞的線粒體膜電位
[參考文獻]
[1]Siegel R,Naishadham D,Jemal A.Cancer statistics,2013[J].CA Cancer J Clin,2013,63(1) : 11-30.
[2]Orzechowska EJ,Girstun A,Staron K,et al.Synergy of BID with doxorubicin in the killing of cancer cells[J].Oncol Rep,2015,33(5) : 2143-2150.
[3]曾小平,王紅梅,黃永紅,等.阿霉素聯合致敏樹突狀細胞對荷宮頸癌小鼠的治療作用[J].中國病理生理雜志,2013,29(4) : 734-738.
[4]Wang Z,Yang L,Xia Y,et al.Icariin enhances cytotoxicity of doxorubicin in human multidrug-resistant osteosarcoma cells by inhibition of ABCB1 and down-regulation of the PI3K/Akt pathway[J].Biol Pharm Bull,2015,38 (2) : 277-284.
[5]Appendino G,Bianchi F,Bader A,et al.Coumarins from Opopanax chironium.New dihydrofuranocoumarins and differential induction of apoptosis by imperatorin and heraclenin[J].J Nat Prod,2004,67(4) : 532-536.
[6]García-Argáez AN,Ramírez Apan TO,Parra Delgado H,et al.Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model[J].Planta Med,2000,66(3) : 279-281.
[7]Zhang Y,Cao Y,Zhang Y,et al.Furanocoumarins-imperatorin inhibits myocardial hypertrophy both in vitro and in vivo[J].Fitoterapia,2010,81(8) : 1188-1195.
[8]Choochuay K,Chunhacha P,Pongrakhananon V,et al.Imperatorin sensitizes anoikis and inhibits anchorage-independent growth of lung cancer cells[J].J Nat Med,2013,67(3) : 599-606.
[9]Luo KW,Sun JG,Chan JY,et al.Anticancer effects of imperatorin isolated from Angelica dahurica: induction of apoptosis in HepG2 cells through both death-receptor-and mitochondria-mediated pathways[J].Chemotherapy,2011,57(6) : 449-459.
[11]Livak KJ,Schmittgen TD.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCtmethod[J].Methods,2001,25(4) : 402-408.
[12]Prathapan A,Vineetha VP,Raghu KG.Protective effect of Boerhaavia diffusa L against mitochondrial dysfunction in angiotensin II induced hypertrophy in H9c2 cardiomyoblast cells[J].PLoS One,2014,9(4) : e96220.
[13]Sun JG,Xiang J,Zeng XL,et al.Clitocine induces apoptosis and enhances the lethality of ABT-737 in human colon cancer cells by disrupting the interaction of Mcl-1 and Bak[J].Cancer Lett,2014,355(2) : 253-263.
[14]Wang T,Huang B,Lei X,et al.A let-7b binding site SNP in the 3’-UTR of the Bcl-xL gene enhances resistance to 5-fluorouracil and doxorubicin in breast cancer cells [J].Oncol Lett,2015,9(4) : 1907-1911.
[15]Kang Q,Yan S.Piperlongumine reverses doxorubicin resistance through the PI3K/Akt signaling pathway in K562/A02 human leukemia cells[J].Exp Ther Med,2015,9 (4) : 1345-1350.
[16]Zhao J,Li X,Zou M,et al.miR-135a inhibition protects A549 cells from LPS-induced apoptosis by targeting Bcl-2 [J].Biochem Biophys Res Commun,2014,452 (4) : 951-957.
[17]Thomas LW,Lam C,Edwards SW.Mcl-1: the molecular regulation of protein function[J].FEBS Lett,2010,584 (14) : 2981-2989.
[18]Pignochino Y,Grignani G,Cavalloni G,et al.Sorafenib blocks tumour growth,angiogenesis and metastatic potential in preclinical models of cervical cancer through a mechanism potentially involving the inhibition of ERK1/2,MCL-1 and ezrin pathways[J].Mol Cancer,2009,8: 118.
[19]Wei SH,Dong K,Lin F,et al.Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell [J].Cancer Chemother Pharmacol,2008,62(6) : 1055-1064.
[20]Michels J,Obrist F,Vitale I,et al.MCL-1 dependency of cisplatin-resistant cancer cells[J].Biochem Pharmacol,2014,92(1) : 55-61.
[21]Zhang XJ,Mei WL,Tan GH,et al.Strophalloside induces apoptosis of SGC-7901 cells through the mitochondrion-dependent caspase-3 pathway[J].Molecules,2015,20(4) : 5714-5728.
(責任編輯:林白霜,羅森)
Antitumor effect of imperatorin enhances cytotoxicity of doxorubicin to HeLa cells
ZHENG Ying1,JIANG Kai2
(1Department of Obstetrics&Gynaecology,2Department of Laboratory Medicine,Tongde Hospital of Zhejiang Province,Hangzhou 310012,China.E-mail: tongdezhengying@163.com)
[ABSTRACT]AIM: To determine whether imperatorin would enhance the effect of doxorubicin therapy on cervical cancer in vitro.METHODS: The viability of HeLa cells treated with imperatorin and doxorubicin was determined by MTT assay in vitro.The expression of Bcl-2 protein family (Mcl-1,Bcl-2,Bcl-xL,Bad and Bax) in HeLa cells treated with imperatorin and doxorubicin was evaluated by Western blot analysis.The apoptosis and mitochondrial membrane potential (ΔΨm) in the HeLa cells treated with imperatorin and doxorubicin were analyzed by flow cytometry.A Mcl-1 expression vector was constructed,and its role in the cytotoxicity of imperatorin plus doxorubicin to HeLa cells was detected by MTT assay.RESULTS: Addition of imperatorin significantly enhanced the cytotoxicity of doxorubicin to HeLa cells in vitro.Mcl-1 expression was down-regulated by imperatorin but was not influenced by doxorubicin in the HeLa cells.A combination of imperatorin and doxorubicin induced apoptosis and ΔΨmcollapse more significantly compared with the treatment with imperatorin or doxorubicin alone.Furthermore,the imperatorin-induced sensitization for doxorubicin cytotoxicity to HeLa cells was abolished by the transfection with Mcl-1 expression plasmid.CONCLUSION: The combination of doxorubicin with imperatorin enhances the antitumor effect of doxorubicin on cervical cancer cells via targeting Mcl-1.
[KEY WORDS]Imperatorin; Doxorubicin; Mcl-1; Cervical cancer
通訊作者△Tel: 0571-89972339; E-mail: tongdezhengying@163.com
[收稿日期]2015-04-13
[中圖分類號]R730.23; R735.7
[文獻標志碼]A
doi:10.3969/j.issn.1000-4718.2015.09.008
[文章編號]1000-4718(2015)09-1578-06

