Adsorption Refrigeration Performance of Shaped MIL-101-Water Working Pair
RUI Zhengqiu (芮征球), LI Quanguo (李全國), CUI Qun (崔群)*, WANG Haiyan (王海燕), CHEN Haijun (陳海軍) and YAO Huqing (姚虎卿) College of Chemistry and Chemical Engineering, Nanjing Tech University, Nanjing 210009, China
Adsorption Refrigeration Performance of Shaped MIL-101-Water Working Pair
RUI Zhengqiu (芮征球), LI Quanguo (李全國), CUI Qun (崔群)*, WANG Haiyan (王海燕), CHEN Haijun (陳海軍) and YAO Huqing (姚虎卿) College of Chemistry and Chemical Engineering, Nanjing Tech University, Nanjing 210009, China
A new metal-organic framework of MIL-101 was synthesized by hydrothermal method and the powder prepared was pressed into a desired shape. The effects of molding on specific surface area and pore structure were investigated using a nitrogen adsorption method. The water adsorption isotherms were obtained by high vacuum gravimetric method, the desorption temperature of water on shaped MIL-101 was measured by thermo gravimetric analyzer, and the adsorption refrigeration performance of shaped MIL-101-water working pair was studied on the simulation device of adsorption refrigeration cycle system. The results indicate that an apparent hysteresis loop appears in the nitrogen adsorption/desorption isotherms when the forming pressure is 10 MPa. The equilibrium adsorption capacity of water is up to 0.95 kg·kg?1at the forming pressure of 3 MPa (MIL-101-3). The desorption peak temperature of water on MIL-101-3 is 82 °C, which is 7 °C lower than that of silica gel, and the desorption temperature is no more than 100 °C. At the evaporation temperature of 10 °C, the refrigeration capacity of MIL-101-3-water is 1059 kJ·kg?1, which is 2.24 times higher than that of silica gel-water working pair. Thus MIL-101-water working pair presents an excellent adsorption refrigeration performance.
adsorption refrigeration, MIL-101, forming, adsorption capacity, refrigeration capacity
1 INTRODUCTION
Adsorption refrigeration systems can be driven by low-grade thermal energy (industrial waste heat, solar energy, etc.), without the need for CFCs (refrigerant) and electricity, which are energy efficient and environmentally friendly [1-4]. The core of adsorption refrigeration systems is the working pair, which is the match of adsorbent and adsorbate (refrigerant). At the present stage, working pairs used in adsorption refrigeration study have disadvantages of small adsorption capacity, low refrigeration power and relatively high desorption temperature, restricting the industrialization process of this technology [5-7]. Thus, developing efficient working pairs, especially adsorbents with excellent adsorption performance, is the key to promote the performance and development of adsorption refrigeration systems.
In recent years, metal-organic frameworks (MOFs) have revealed promising applications in catalysis, adsorption and gas storage [8-11]. MIL-101, first reported by Ferey et al. [12], has two types of mesopores (2.9 and 3.4 nm), a tremendous Langmuir specific surface area (5900 m2·g?1), and better thermal stability compared with other MOFs. Moreover, there are lots of unsaturated metal active sites in the framework [13-15]. Henninger et al. [16, 17] reported that the water adsorption quantity of powdered MIL-101 was 1.01 kg·kg?1, and the structure remained stable after repetitive adsorption-desorption cycles. The results indicate that MIL-101 could serve as a potential adsorbent in adsorption refrigeration. The study on the adsorption refrigeration circulation performance of MIL-101 has not been reported yet.
In this paper, we select shaped MIL-101 and water as the working pair and study the performance of adsorption refrigeration circulation. We synthesize powdered MIL-101 by hydrothermal method and preform it with rotary tablet press. We investigate the effects of molding on structure and performance, water adsorption and desorption performance, as well as the adsorption refrigeration performance of shaped MIL-101-water working pair on a self-built simulation device of adsorption refrigeration cycle system, providing the basic parameters for MIL-101 used in adsorption refrigeration processes.
2 EXPERIMENTAL
2.1 Raw materials
Chromium nitrate [Cr(NO3)3·9H2O, AR, Xilong Chemical Co., Ltd., Guangdong, China, 99.0%], H2BDC [C6H4(CO2H)2, AR, Sinopharm Chemical Reagent Co., Ltd., China, 99.0%], and DMF (N,N-dimethylformamide, AR, Sinopharm Chemical Reagent Co., Ltd., China, 99.5%) were used without further purification. The deionized water was made in our laboratory.
2.2 Synthesis and molding
The preparation of MIL-101 was based on reference [18]. Cr(NO3)3·9H2O (4.00 g) was dissolved in deionized water (48 ml) and 1,4-benzene dicarboxylic acid (1.64 g) was added. The solution was stirred for 15 min. The complete solution was then transferred toa Tef l on-lined stainless steel reactor and sealed. The reactor was placed inside a hot air oven at 220 °C and held for 8 h. After the reaction time, the reactor was cooled to room temperature. A fine green colored powder was obtained as major product, with significant amount of H2BDC present in the form of needle-shaped colorless crystals along with the product. To remove this impurity, contents were completely transferred into a conical flask, and DMF was added incrementally with continuous shaking to dissolve H2BDC. Then the solution was filtered and washed with water. The resulting product was dried at 150 °C overnight. The powder was pressed into a desired shape by powder pressing machine at different pressures. Then the flaky MIL-101 was smashed into particles ranging from 0.42-0.85 mm (20-40 mesh).
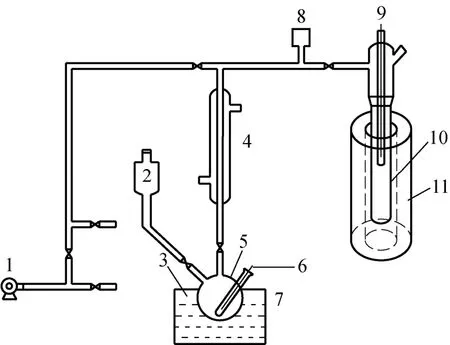
Figure 1 Simulation device of adsorption refrigeration cycle system 1—vacuum system; 2—liquid storage; 3,6,9—thermocouple; 4—condenser; 5—evaporator; 7—refrigerator; 8—vacuum gauge; 10—adsorption bed; 11—heat exchanger
2.3 Characterization
The crystal structure was examined on D8 ADVANCE X-ray diffractometer. The scan range was from 2° to 20° (2θ) at 5 (°)·min?1. Desorption temperature of water on shaped MIL-101 was measured by thermo gravimetric analyzer (TGA), in atmosphere at a heating rate of 5 °C·min?1. Nitrogen adsorption/ desorption isotherms were obtained on a Tristar II 3020 automatic specific surface area and pore analyzer at 77 K. The sample was first activated at 170 °C for 10 h.
2.4 Adsorption isotherms of water on shaped MIL-101
Adsorption isotherms of water on shaped MIL-101 were investigated by a high vacuum gravimetric method, and the detailed operating procedures can be found in reference [19]. The samples were evacuated at 200 °C for 2 h in advance.
2.5 Simulated experiment of adsorption refrigeration
Adsorption refrigeration performance of shaped MIL-101-water working pair was examined on a simulation device of adsorption refrigeration, as shown in Fig. 1. The experimental system consists of adsorption bed, evaporator, condenser, refrigerator, heat exchanger and vacuum system. The experimental conditions are as follows, adsorbent: 10-50 g; refrigerant volume: 50 ml; water volume in refrigerator: 500 ml. The adsorption refrigeration experiment includes two processes, adsorption refrigeration process and heating desorption process. The detail experimental method and procedure refer to reference [20].
3 RESULTS AND DISCUSSION
3.1 XRD analysis
The XRD pattern of synthesized sample is shown in Fig. 2. The diffraction peak positions are the same as the standard data for MIL-101 [12], so the sample is MIL-101 powder.
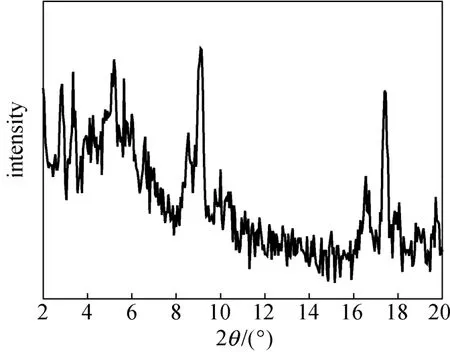
Figure 2 XRD pattern of MIL-101
3.2 Effects of molding on pore structure of MIL-101
In this study, the powdered MIL-101 was pressed at the forming pressures of 3, 5, and 10 MPa and the shaped MIL-101 was named MIL-101-3, MIL-101-5 and MIL-101-10, respectively. The nitrogen adsorption/desorption isotherms of MIL-101 are shown in Fig. 3. The specific surface area and pore parameters calculated according to the nitrogen adsorption/ desorption isotherms are listed in Table 1, and the pore distribution is shown in Fig. 4. Fig. 3 shows that the nitrogen adsorption/desorption isotherms of MIL-101-3, MIL-101-5 and powdered MIL-101 are similar, expect for the adsorption capacity. When the molding pressure extends to 10 MPa, an apparent hysteresis loop appears in the nitrogen adsorption/desorption isotherm, and different pore distribution is found in the framework of MIL-101-10 [Fig.4 (b)]. This phenomenon may be due to the appearance of intergranular pores in the process of molding. Table 1 shows that, with the increase of molding pressure, the specific surface areaand pore volume decreases, caused by the extrusion of surface and inner pores [Fig. 4 (a)]. The variation of pore size may be related to the appearance of intergranular pores. From Fig. 3 and Table 1, we can conclude that the decrease of specific surface area and pore volume leads to the attenuation of the adsorption performance.
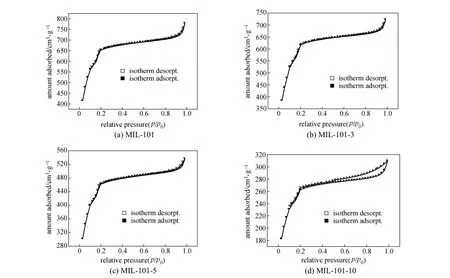
Figure 3 Nitrogen adsorption/desorption isotherms at 77 K for powdered MIL-101 and shaped MIL-101
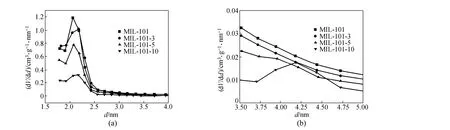
Figure 4 Pore distribution of powdered MIL-101 and shaped MIL-101

Table 1 Specific surface area and pore parameters of powdered MIL-101 and shaped MIL-101
3.3 Water adsorption isotherms of shaped MIL-101
Water adsorption isotherms of shaped MIL-101 are shown in Fig. 5, which are of an S-shaped characteristic. Water uptake is small at low partial pressure, and the adsorption mainly takes place within a relative pressure of 0.35 and 0.65. There are two obvious steps in the isotherm, attributing to the two types of mesoporous in the framework. The equilibrium adsorption capacity of water on MIL-101-3 can reach0.95 kg·kg?1, and the adsorption quantity decays with the increase of molding pressure. The equilibrium adsorption capacities on MIL-101-5 and MIL-101-10 are 0.83 and 0.66 kg·kg?1, respectively, which is 13% and 30% lower than that on MIL-101-3. The variation tendency of water adsorption quantity on different shaped MIL-101 is similar with the relationship between nitrogen adsorption quantity and the forming pressure (Fig. 3), mainly caused by the decrease of specific surface area and pore volume after the powdered MIL-101 is pressed at different pressures.
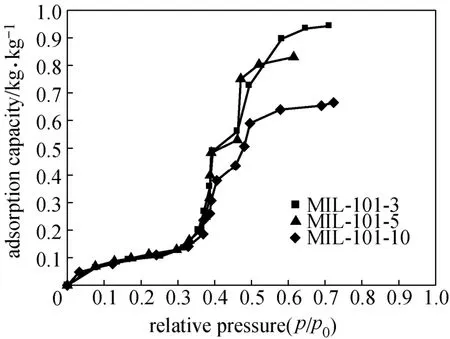
Figure 5 Water adsorption isotherms of shaped MIL-101 at 25 °C
3.4 Desorption temperature of water on shaped MIL-101
For the sake of contrastive analysis, the desorption temperature of water on shaped MIL-101 and silica gel were examined. Fig. 6 shows the differential thermal analysis (DTA) curves of MIL-101-3 and silica gel. The desorption peak temperature of water on MIL-101-3 is 82 °C, which is 7 °C lower than that of silica gel, and the complete desorption temperature is approximately 100 °C. The excellent desorption performance of shaped MIL-101 indicates that it can be regenerated at a relatively low temperature, which is appropriate for adsorption refrigeration using low-grade thermal energy.
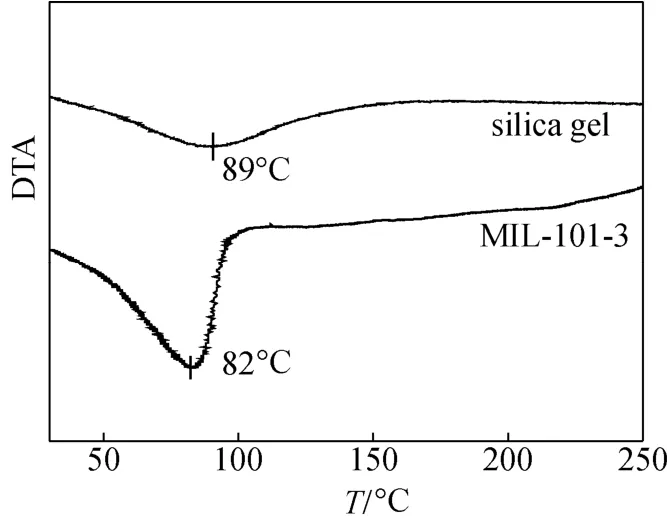
Figure 6 DTA analysis of MIL-101-3 and silica gel
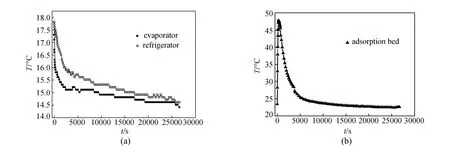
Figure 7 Characteristic curves of adsorption refrigeration for MIL-101-3-water
3.5 Adsorption refrigeration performance of shaped MIL-101-water working pair
3.5.1 Characteristic curves of adsorption refrigeration for shaped MIL-101-water working pair
In the adsorption refrigeration process (Fig. 1), the temperature of evaporator, refrigerant water in refrigerator and adsorption bed are main characteristic parameters to evaluate the adsorption refrigeration performance. The characteristic curves (the variation of characteristic temperature with adsorption time) can reflect the dynamic characteristics of the whole process. Fig. 7 shows the characteristic curves of adsorption refrigeration for MIL-101-3-water working pair obtained on the simulation device of adsorption refrigeration. As Fig. 7 (a) shows, when adsorption process begins, the temperature of evaporator and refrigerator declines quickly, then the drop of temperature becomes slower, finally the temperatures of the two units approach a same value. This phenomenon is due to the fast water adsorption rate in the initial stage of adsorption. A large amount of water in the evaporator is adsorbed, and the process of liquid water changing into vapor needs to absorb heat from ambient environment, leading to a sharply decline of the temperature of evaporator. Because of the heat exchange between evaporator and refrigerator, the temperature difference becomes small gradually. The temperature of refrigerator is controlled by that of evaporator, so the temperature drop range is relatively stable. In Fig. 7 (b), the temperature of adsorption bed risesrapidly in the initial stage, since the adsorption process releases enormous adsorption heat. The temperature reaches its peak and then falls quickly, at last achieves room temperature. In the adsorption refrigeration process, the temperature of evaporator declines from 17.3 to 14.4 °C, the temperature of refrigerator drops from 17.8 to 14.6 °C, and that of the adsorption bed rises from 23.4 to 47.8 °C. We can calculate the refrigeration capacity according to the temperature drop of refrigerator and the amount of the refrigerant water. 3.5.2 Adsorption refrigeration capacity of shaped MIL-101-water working pair
For the adsorption refrigeration process, we use the refrigeration capacity produced by unit mass adsorbent to evaluate the performance of the working pair. The calculation of refrigeration capacity according to characteristic temperature is related to the heat loss in the heat exchange process, which is considerably affected by the environmental factor. Consequently, we usually use the amount of desorption to calculate refrigeration capacity, and the variation of sensible heat is ignored. We regard the water volume of desorption as effective adsorption quantity. The calculation formula of refrigeration capacity is
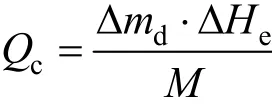
where Δmdis the amount of desorption, kg; ΔHeis the latent heat of vaporization of refrigerant at the evaporation temperature, kJ·kg?1; and M is the adsorbent load, kg.
The circulation adsorption capacity and refrigeration capacity of MIL-101-3-water and silica gel-water were measured on the simulation device of adsorption refrigeration at the evaporation temperatures of 10 and 15 °C (typical air conditioning conditions). The results are listed in Table 2.
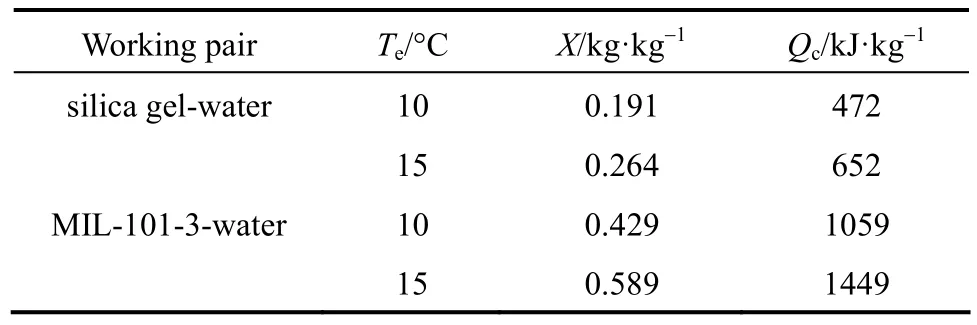
Table 2 Refrigerating capacity of MIL-101-3-water and silica gel-water
At the evaporation temperature of 10 °C, the circulation adsorption capacity of MIL-101-3-water working pair is 0.429 kg·kg?1and the refrigeration capacity is 1059 kJ·kg?1, which is 2.24 times higher than that of silica gel-water. At 15 °C, the circulation adsorption capacity of MIL-101-3-water working pair is 0.589 kg·kg?1and the refrigeration capacity is up to 1449 kJ·kg?1, which is 2.22 times higher than that of silica gel-water. At the evaporation temperature rises from 10 to 15 °C, the refrigeration capacity increases by 37%. Thus the MIL-101-3-water working pair presents excellent refrigeration performance, especially in relatively high evaporation temperature, which is related with the shape of water adsorption isotherms of MIL-101.
4 CONCLUSIONS
(1) Forming pressure has a large influence on pore structure of shaped MIL-101, and an apparent hysteresis loop appears in the nitrogen adsorption/ desorption isotherms of MIL-101-10. As the forming pressure increases from 3 to 5 MPa, the specific surface area of shaped MIL-101 reduces from 2047 to 1494 m2·g?1.
(2) The water adsorption isotherms are of an S-shaped characteristic and the equilibrium adsorption capacity of water on MIL-101-3 reaches 0.95 kg·kg?1.
(3) The desorption peak temperature of water on MIL-101-3 is 82 °C, and the complete desorption temperature is approximately 100 °C, which is apparently lower than that of silica gel. Shaped MIL-101 can be applied to adsorption refrigeration systems driven by low-grade thermal energy.
(4) At the evaporation temperature of 10 °C, the refrigeration capacity of MIL-101-3-water working pair is 1059 kJ·kg?1, which is 2.24 times higher than that of silica gel-water. As the evaporation temperature extends from 10 to 15 °C, the refrigeration capacity increases by 37%, reaching 1449 kJ·kg?1. For high evaporation temperature conditions, MIL-101-3-water working pair has excellent refrigeration performance.
(5) Compared with silica gel, shaped MIL-101 has advantages of large adsorption capacity and low desorption temperature in the adsorption refrigeration process using water as adsorbate. MIL-101 has promising application as an efficient adsorbent in adsorption refrigeration.
NOMENCLATURE

REFERENCES
1 Wang, R.Z., Oliveira, R.G., “Adsorption refrigeration-An efficient way to make good use of waste heat and solar Energy”, Prog. Energy Combust. Sci., 32 (24), 424-458 (2006).
2 Saha, B.B., Koyama, S., Lee, J.B., Kuwahara, K., Alam, K.C.A.,“Performance evaluation of a low-temperature waste heat driven multi-bed adsorption chiller”, Int. J. Multiphase Flow, 29 (8), 1249-1263 (2003).
3 Saha, B.B., Koyama, S., Kashiwagi, T., Akisawa, A., Ng, K.C., Chua, H.T., “Waste heat driven dual-mode, multi-stage, multi-bed regenerative adsorption system”, Int. J. Refriger., 26 (7), 749-757 (2003).
4 Boubakri, A., Guillemiont, J.J., Meunier, F., “Adsorptive solar powered ice-maker: Experiments and model”, Solar Energ, 69 (3),249-263 (2000).
5 Henninger, S.K., Schicktanz, M., Hugenell, P.P.C., Sievers, H., Henning, H.M., “Evaluation of methanol adsorption on activated carbons for thermally driven chillers part I: Thermophysical characterization”, Int. J. Refriger., 35 (3), 543-553 (2012).
6 Henninger, S.K., Schmidt, F.P., Henning, H.M., “Water adsorption characteristics of novel materials for heat transformation applications”, Appl. Thermal Eng., 30 (13), 1692-1702 (2010).
7 Zhong, Y., Critoph, R.E., Thorpe, R., “Evaluation of the performance of solid sorption refrigeration systems using carbon dioxide as refrigerant”, Appl. Thermal Eng., 26 (16), 1807-1811 (2006).
8 Ferey, G., “Hybrid porous solids: past, present, future”, Chemical Society Reviews, 37 (1), 191-214 (2008).
9 Chae, H.K., Siberio-Perez, D.Y., Jaheon, K., Go, Y.B., Eddaoudi, M., Matzger, A.J., Okeeffe, M., Yaghi, O.M., “A route to high surface area, porosity and inclusion of large molecules in crystals”, Nature, 427 (6974), 523-527 (2004).
10 Zhao, X.B., Xiao, B., Fletcher, A.J., Thomas, K.M., Bradshaw, D., Rosseinsky, M.J., “Hysteretic adsorption and desorption of hydrogen by nanoporous metal-organic frameworks”, Science, 306 (5698), 1012-1015 (2004).
11 Kitagawa, S., Kitaura, R., Noro, S., “Functional porous coordination polymers”, Angewandet Chemie International Edition, 43 (18), 2234-2375 (2004).
12 Ferey, G., Mellot-Draznieks, C., Serre, C., Millange, F., Dutour, J., Surble, S., Margiolaki, I., “Achromium terephthalate-based solid with unusually large pore volumes and surface area”, Science, 309 (5743), 2040-2043 (2005).
13 Latroche, M., Surble, S., Serre, C., Mellot-Draznieks, C., Llewellyn, P.L., Lee, J., Chang, J., Jhung, S.H., Ferey, G., “Hydrogen storage in the giant-pore metal-organic frameworks MIL-100 and MIL-101”, Angewandet Chemie International Edition, 45 (48), 8227-8231 (2006).
14 Khan, N.A., Kang, I.J., Seok, H.Y., Jhung, S.H., “Facile synthesis of nano-sized metal-organic frameworks, chromium-benzenedicarboxylate, MIL-101”, Chem. Eng. J., 166 (3), 1152-1157 (2011).
15 Jhung, S.H., Lee, J.H., Yoon, J.W., Serre, C., Ferey, G., Chang, J.S.,“Microwave synthesis of chromium terephthalate MIL-101 and its benzene sorption bbility”, Adv. Mater., 19 (1), 121-124 (2007).
16 Henninger, S.K., Hesham, A.H., Christoph, J., “MOFs as adsorbents for low temperature heating and cooling applications”, JACS, 131 (8), 2776-2777 (2009).
17 Ehrenmann, J., Henninger, S.K., Janiak, C., “Water adsorption characteristics of MIL-101 for heat transformation applications of MOFs”, Eur. J. Inorg. Chem., 2011 (4), 471-474 (2011).
18 Pradip, C., Chaitanya, B., Sasidhar, G., “Gas adsorption properties of the chromium-based metal organic framework MIL-101”, American Chemical Society, 113 (16), 6616-6621 (2009).
19 Chen, H.J., Cui, Q., Tang, Y., Chen, X.J., Yao, H.Q., “Attapulgite based LiCl composite adsorbents for cooling and air conditioning applications”, Appl. Thermal Eng., 28 (17/18), 2187-2193 (2008).
20 Cui, Q., Tao, G., Chen, H.J., Guo, X.Y., Yao, H.Q., “Environmentally benign working pairs for adsorption refrigeration”, Energy, 30 (2-4), 261-271 (2005).
2012-09-07, accepted 2012-10-14.
* To whom correspondence should be addressed. E-mail: cuiqun@njtech.edu.cn
 Chinese Journal of Chemical Engineering2014年5期
Chinese Journal of Chemical Engineering2014年5期
- Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor Model Derived from Wiener Model Structure: Modeling and Identification*
- Kinetics of Forward Extraction of Boric Acid from Salt Lake Brine by 2-Ethyl-1,3-hexanediol in Toluene Using Single Drop Technique*
- Influence of Solvent on Reaction Path to Synthesis of Methyl N-Phenyl Carbamate from Aniline, CO2and Methanol*
- Effect of Adsorbent Diameter on the Performance of Adsorption Refrigeration*
- High-Thermal Conductive Coating Used on Metal Heat Exchanger*
- A Facile Route for Synthesis of LiFePO4/C Cathode Material with Nano-sized Primary Particles*
