基于蒽/芘分子封端的芴-芳胺衍生物的可溶液加工的藍光材料的合成與光電性質
歐陽密 吳啟超 余振偉 李洪飛 張 誠
(浙江工業大學化工學院,杭州310014)
1 Introduction
Electroluminescent materials have made significant progress since the pioneering work of Tang and VanSlyke.1Although electroluminescent devices have recently been developed and commercialized for application in displays and solid state lightings,2-4there remain several obstacles to be overcome.A crucial one is the development of solution processed blue fluorescent materials for full color display and lighting.5-7Since solution processing affords simple preparation and the possibility of low cost large area devices,8and the performance of blue fluorescent emitters is still unsatisfactory compared with green and red fluorescent emitters.9-12
During the research of efficient blue fluorescent emitters,tremendous efforts focus on several compounds and their derivatives such as fluorene,13,14di(styryl)arylene,15pyrene,16,17and anthracene18,19derivatives.Among them,fluorene derivatives possessing excellent photoluminescence and electroluminescence properties have been studied intensively to develop efficient blue light emitting devices.For example,Kumaret al.20synthesized and characterized a series of fluorene derivatives as blue emitters suitable for application in light emitting devices,and proved that anthracene and pyrene containing derivatives exhibited blue photoluminescence with high quantum yield and thermal stability.Unfortunately,all these derivatives hold the highest occupied molecular orbital(HOMO)energy lower than-5.5 eV,with poor hole-injection ability,resulting in only decent device parameters.In fact,almost all fluorene derivatives as blue fluorescent emitters possessed lower HOMO energy level due to the intrinsically wide band-gap of blue-emitters.21
To obtain solution processed blue fluorescent emitter with high HOMO energy level,two novel fluorene-diphenylamine cored derivatives end-capped with anthracene and pyrene were designed and synthesized in this paper.We anticipate that the noncoplanar diphenylamine structure inserted between the two fluorenes could effectively restrain the intermolecular close packing in the solid state and increase the HOMO energy level for better hole-injection.22Moreover,the diphenylamine unit made alkyl substitution of the N-position easily,which ensures the solubility in common solvents for solution processed of devices.23Also,the polyaromatic anthracene and pyrene were attached to the core to improve photoluminescence performance of quantum yield and thermal stability.In consequence,the photophysical and electrochemical properties were tuned to develop good solution processed blue electroluminescence performance.
2 Experimental
2.1 General information
Commercially available starting chemicals,10-phenyl-9-anthraceneboronic acid(98%)and 1-pyrenylboronic acid(95%)were purchased from Aladdin and used as received without further purification.The catalyst,triflic acid(99%)and tetrakis(triphenylphosphine)palladium(99%)were purchased from Energy Chemical.Toluene,tetrahydrofuran(THF),and 1,4-dioxane were distilled from sodium under argon.1H and13C nuclear magnetic resonance(NMR)spectra were recorded on a Avance III 500 MHz spectrometer(Bruker,Switzerland)at 500 and 125 MHz,respectively,using tetramethylsilane(TMS)as the internal standard.Autoflex MALDI-TOF-MS spectrometer(Bruker,Switzerland)was used to obtain high-resolution mass spectrometric.Elemental analyses were performed on a Thermo-Finnigan Flash EA-1112(CE,Italy)instrument.UV-1800 spectrophotoscopy(HRMS)(Shimadzu,Japan)was used to measure the UV-Vis spectra.Fluorescence spectra were carried out using a Fluorolog-3 modular spectrofluorometer(HORIBA Jobin Yvon,Japan).Thermal analysis was characterized by a Diamond TG/DTA 6300(PerkinElmer,USA)at a heating rate of 10 °C·min-1and a nitrogen flow rate of 80 mL·min-1.Fieldemission scanning electron microscopy(FESEM)measurements were taken by using a Hitachi S-4800 scanning electron microscopy(Hitachi,Japan).Electrochemical measurements were carried out using CHI 660C electrochemical analyzer.Powder X-ray diffraction(XRD)measurements were conducted onX′PertPRO diffractometer(PANalytical,Netherlands).
2.2 Synthesis
A0,A1 were synthesized according to the reported procedure.24,25Described below are the synthesis and purification procedures forA2,P3,and the final products.
2.2.1 4-(2-bromo-9-(p-tolyl)-9H-fluoren-9-yl)-N-(4-(2-bromo-9-(p-tolyl)-9H-fluoren-9-yl)phenyl)-N-hexylaniline(A2)
A solution of triflic acid(1 mL)in 1,4-dioxane(50 mL)was added to a mixture of 2-bromo-9-(p-tolyl)-9H-fluoren-9-ol(A0)(1.5 g,4.2 mmol),N-hexyl-N-phenylaniline(A1)(0.5 g,2 mmol).The mixture was refluxed for 24 h under the nitrogen.After the solids were filtered off,the product was extracted with dichloromethane and dried with anhydrous MgSO4.The solvent was removed by rotary evaporation,and the residue was purified by column chromatography using ethyl acetate/petroleum ether(1:100)(V/V)as eluent to obtain the white solid(1.51 g,82.4%).1H NMR(500 MHz,CDCl3,δ):7.73(t,J=9.6 Hz,2H),7.61(dd,J=8.0,3.9 Hz,2H),7.53(t,J=3.8 Hz,2H),7.47(dd,J=8.1,1.7 Hz,2H),7.36(m,4H),7.05(m,14H),6.78(m,4H),3.59(dd,J=15.0,7.1 Hz,2H),2.31(s,6H),1.27(m,8H),0.86(t,J=8.3 Hz,3H).13C NMR(125 MHz,CDCl3,δ):153.76,151.52,146.41,142.32,139.11,138.91,137.50,136.39,130.51,129.33,129.01,128.87,128.04,127.94,127.45,126.15,121.40,121.27,120.37,120.11,64.71,58.47,52.37,31.54,27.43,26.65,22.63,20.92,18.43,13.97.Elementanal.calcd.for C58H49Br2N(%):C,75.73;H,5.37;Br,17.37;N,1.53.Found(%):C,75.75;H,5.35;Br,17.36;N,1.54.
2.2.2 2-(pyren-1-yl)-9-(p-tolyl)-9H-fluoren-9-ol(P3)
2-Bromo-9-(p-tolyl)-9H-fluoren-9-ol(A0)(0.87 g,2.27 mmol),1-pyrenylboronic acid(0.92 g,3.71 mmol),Pd(Pph3)4(0.05 g,0.05 mmol),aqueous K2CO3(2.0 mol·L-1,3 mL),tetrahydrofuran(30 mL),and toluene(50 mL)were mixed in a flask.The mixture was degassed and the reaction was then heated at 90°C while stirring under the nitrogen.After 48 h,the solvent was evaporated under vacuum and the product was extracted with dichloromethane and dried with MgSO4.The solvent was removed by rotary evaporation,and the residue was purified by column chromatography using ethyl acetate/petroleum ether(1:50)as eluent to obtain the white solid(0.91g,78.3%).1H NMR(500 MHz,CDCl3,δ):8.24-8.15(m,4H),8.10(s,2H),8.01(m,J=17.2 Hz,3H),7.86(d,J=7.7 Hz,1H),7.79(d,J=7.4 Hz,1H),7.69-7.64(m,2H),7.47-7.41(m,2H),7.39(d,J=8.2 Hz,2H),7.33(td,J=7.5,0.9 Hz,1H),7.11(d,J=8.1 Hz,2H),2.58(s,1H),2.32(s,3H).13C NMR(125 MHz,CDCl3,δ):151.01,150.86,141.51,140.23,139.46,138.72,137.47,137.04,131.68,131.55,131.02,130.71,129.28,129.07,128.62,128.49,127.66,127.61,127.52,127.47,127.06,126.08,125.47,125.21,125.06,124.92,124.90,124.69,120.31,120.02,83.81,26.99,25.36,21.14.EIMS(m/z):472.2[M+].Element anal.calcd.for C36H24O(%):C,91.50;H,5.12;O,3.38.Found(%):C,91.46;H,5.14;O,3.40.
2.2.3N-hexyl-4-(2-(10-phenylanthracen-9-yl)-9-(p-tolyl)-9H-fluoren-9-yl)-N-(4-(2-(10-phenylanthracen-9-yl)-9-(p-tolyl)-9H-fluoren-9-yl)phenyl)aniline(FAn)
Following the synthetic procedure of P3 described above,we obtained FAn as white solid(0.95 g,75.1%).1H NMR(500 MHz,CDCl3,δ):7.98(dd,J=7.7,1.8 Hz,2H),7.87(t,J=8.8 Hz,2H),7.87(m,2H),7.74-7.65(m,4H),7.62(m,J=13.4 Hz,4H),7.59-7.50(m,8H),7.44(m,J=21.5 Hz,8H),7.36-7.28(m,6H),7.19(m,J=14.2 Hz,6H),7.14-7.07(m,6H),7.02(d,J=8.2 Hz,4H),6.80(d,J=8.7 Hz,4H),3.57(t,2H),2.29(s,6H),1.58(s,2H),1.24(dd,J=15.9,12.4 Hz,6H),0.82(t,3H).13C NMR(125 MHz,CDCl3,δ):152.19,154.94,146.24,142.77,139.88,139.43,138.03,137.26,136.07,131.29,130.64,129.79,129.63,128.95,128.91,128.87,128.40,128.06,127.77,127.44,127.01,126.95,126.32,124.94,120.37,120.23,119.93,64.76,31.55,27.34,26.66,22.63,20.91,13.98.MALDI-TOF MS(m/z):1266.6[M+].Element anal.calcd.for C98H75N(%):C,92.93;H,5.97;N,1.10.Found(%):C,92.97;H,5.92;O,1.11.
2.2.4N-hexyl-4-(2-(pyren-1-yl)-9-(p-tolyl)-9H-fluoren-9-yl)-N-(4-(2-(pyren-1-yl)-9-(p-tolyl)-9H-fluoren-9-yl)phenyl)aniline(FPy)
Following the synthetic procedure of A2 described above,we obtained FPy as white solid(0.68 g,83.5%).1H NMR(500 MHz,CDCl3,δ):8.18-8.12(m,J=11.5 Hz,6H),8.06(dd,J=7.7,6.0 Hz,6H),7.97(d,J=7.8 Hz,4H),7.93-7.87(m,4H),7.84(d,J=7.5 Hz,2H),7.70(s,2H),7.65-7.61(m,J=7.6 Hz,2H),7.45(dd,J=7.5,3.9 Hz,2H),7.42-7.37(m,2H),7.30(dd,J=8.0,3.8 Hz,2H),7.20(dd,J=8.2,3.2 Hz,4H),7.16(dd,J=8.4,4.8 Hz,4H),7.05(t,J=7.0 Hz,4H),6.92-6.79(m,4H),3.59(s,2H),2.28(s,6H),1.56(s,2H),1.23(s,6H),0.80(t,J=5.9 Hz,3H).13C NMR(125 MHz,CDCl3,δ):139.79,139.18,130.48,129.83,128.99,128.90,128.58,128.08,127.71,127.32,126.29,125.93,125.29,124.99,124.70,124.58,120.29,64.76,26.91,26.68,22.62,20.93,13.95.MALDI-TOF MS(m/z):1161.5[M+].Element anal.calcd.for C90H67N(%):C,92.99;H,5.81;N,1.20.Found(%):C,92.95;H,5.83;N,1.22.
3 Results and discussion
3.1 Synthetic procedure and characterization
Scheme 1 illustrates the synthetic procedures for the compounds.2-bromo-9-(p-tolyl)-9H-fluoren-9-ol(A0)andN-hexyl-N-phenylaniline(A1)were prepared according to literature25procedures.Acid-promoted Friedel-Crafts type substitution of A0 with A1 afforded 4-(2-bromo-9-(p-tolyl)-9H-fluoren-9-yl)-N-(4-(2-bromo-9-(p-tolyl)-9H-fluoren-9-yl)phenyl)-N-hexylaniline(A2)in high yield.Then the Suzuki coupling reaction of A2 and 9-phenylanthracen-10-yl-10-boronic acid yielded FAn in 75.1%yield.A0 and 1-pyrenylboronic acid yielded 2-(pyren-1-yl)-9-(p-tolyl)-9H-fluoren-9-ol(P3).Then the FPy was synthesizedviathe Friedal-Crafts reaction of A1 and P3 in 83.5%yield.1H and13C NMR,MS,and elemental analyses were employed to confirm the chemical structures of the final compounds.
3.2 Thermal properties
The thermal properties of the materials were evaluated by thermal gravimetric analysis and differential scanning calorimetry.As shown in Fig.1,the glass transition temperature of FAn was up to 207°C,and the thermal decomposition temperatures(Td,corresponding to 5%mass loss)of FAn and FPy were as high as 439 and 366°C,respectively,which can be ascribed to the effect of polyaromatic anthracene and pyrene.The results indicated that the existence of the rigid polyaromatic end-cappers effectively restrained the intermolecular interaction and increased theTdvalues of the compounds.
The XRD and SEM were measured to further confirm the high morphological stability of FAn and FPy.The film was preparedviaspin coating in toluene solution at a speed rate of 1600 r·min-1for 60 s,and the typical thickness was about 60 nm.A weak and broad peak was observed in the thin film of FAn and FPy(Fig.2).More importantly,there was no obvious change on the FAn and FPy films after annealed at 100°C for 10 h under air atmosphere,exhibited that the introduction of fluorene-arylamine group could restrain the crystallization of anthracene or pyrene.As seen from Fig.3,no agglomeration occurred after anneal for both FAn and FPy,the results were in agreement with the XRD curves above.The results of XRD and SEM revealed that the FAn and FPy possessed excellent morphological stability and thermal stability.
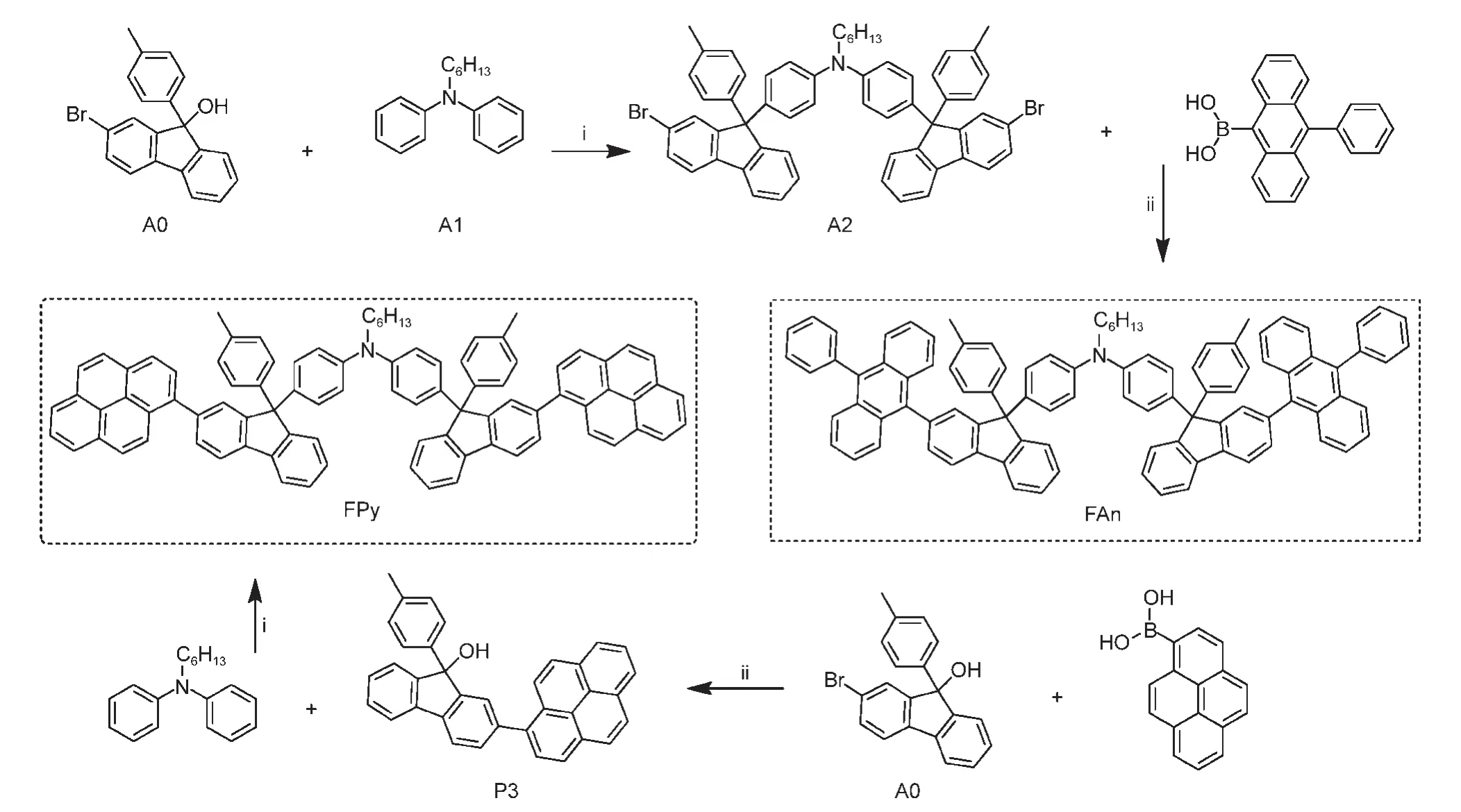
Scheme 1 Synthetic routes to the compounds
3.3 Photophysical properties
Fig.4 and Fig.5 represent the absorption and PL spectra of FAn and FPy in dilute solution and film state.As seen from Fig.4,The absorption spectrum of FAn in chloroform(10-5mol·L-1)displays an absorption band at 314 nm.Additionally,characteristic vibronic patterns of anthracene could also be found in the region of 357-398 nm.The absorption of FAn solid film exhibited slight redshift of 1-3 nm compared with that in dilute solution.The FAn emission maximum occurs at 431 nm in chloroform and 449 nm in solid film.The minor redshifts both in absorption and emission indicates that theπ-πinteraction between molecules in film state is relatively weak,as a result of twisting molecular structure caused by the introduction of non-planar diphenylamine.The absorption and PL spectra of FPy were dramatically different from those of FAn.The absorption peak of FPy in solid film is similar to those in solution and exhibits slightly red-shifted of 3 nm.Upon excitation,the solid film shows an emission maximum at 465 nm,while the solution displays two emission peaks of 425 and 489 nm.The phenomenon was probably caused by intermolecular interaction in solid state.Both of the energy gaps(Eg)of FAn and FPy were estimated from onset absorption(Eg=1240/λonset)to be 2.79 and 2.80 eV,respectively.
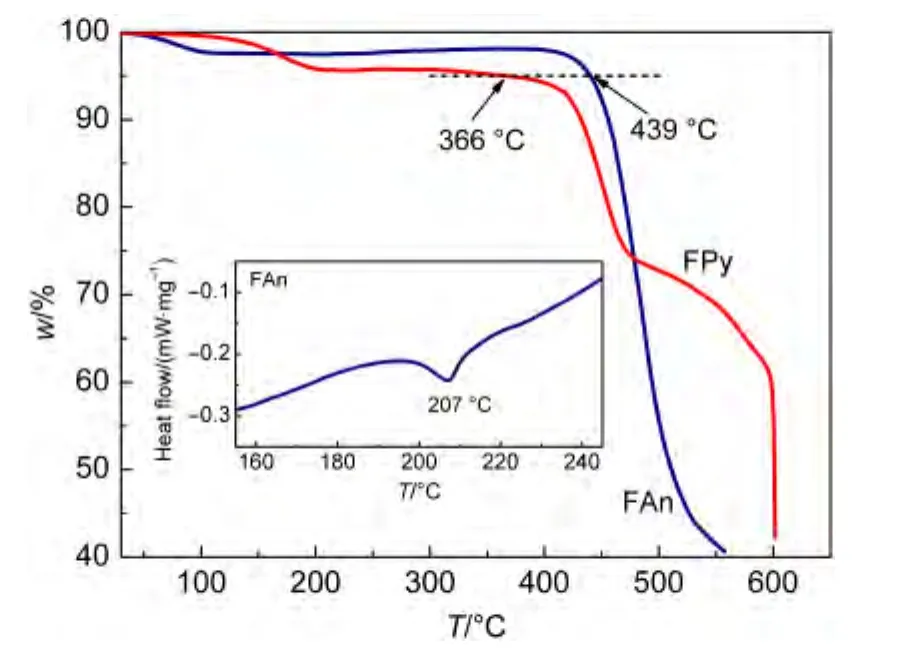
Fig.1 Thermogravimetric analyses of FAn and FPy and differential scanning calorimetry of FAn(inset)under a nitrogen atmosphere with a heating rate of 10 °C·min-1
3.4 Electrochemical properties
The electrochemical properties of FAn and Fpy were characterized in chloroform in a three-electrode electrochemical cellwith tetrabutylammonium perchlorate(0.1 mol·L-1)as a supporting electrolyte.The electrochemical properties are shown in Fig.6.Two reversible anodic redox couples for FAn and FPy were obtained.The anode scans demonstrated similar values of onset oxidation potential(EOX)for both emitters(0.99 V for FAn and 0.98 V for FPy).To assess the carrier injecting properties,the estimated HOMO energy levels were as high as-5.37 and-5.36 eV for FAn and FPy,respectively(EHOMO=-(EOX+4.38 eV)).The result indicated that the introduction of diphenylamine at the C2 position was beneficial to decrease the holeinjection barriers.Such a high HOMO energy level greatly reduced the energy barrier for hole injection from indium tin oxide(ITO)(work function=-4.8 eV)to the emissive fluorene derivatives.As a result,FAn and FPy were allowed to be used as hole-injection materials.The LUMO energy levels for FAn and FPy were calculated to be-2.58 and-2.26 eV,respectively.
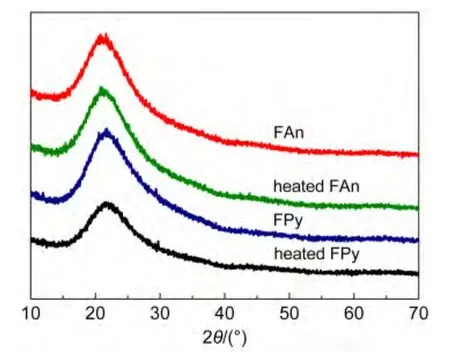
Fig.2 Powder X-ray diffraction patterns of FAn,FPy,heated FAn,and heated FPy
Fig.3 SEM images of FAn(a,c)and FPy(b,d)
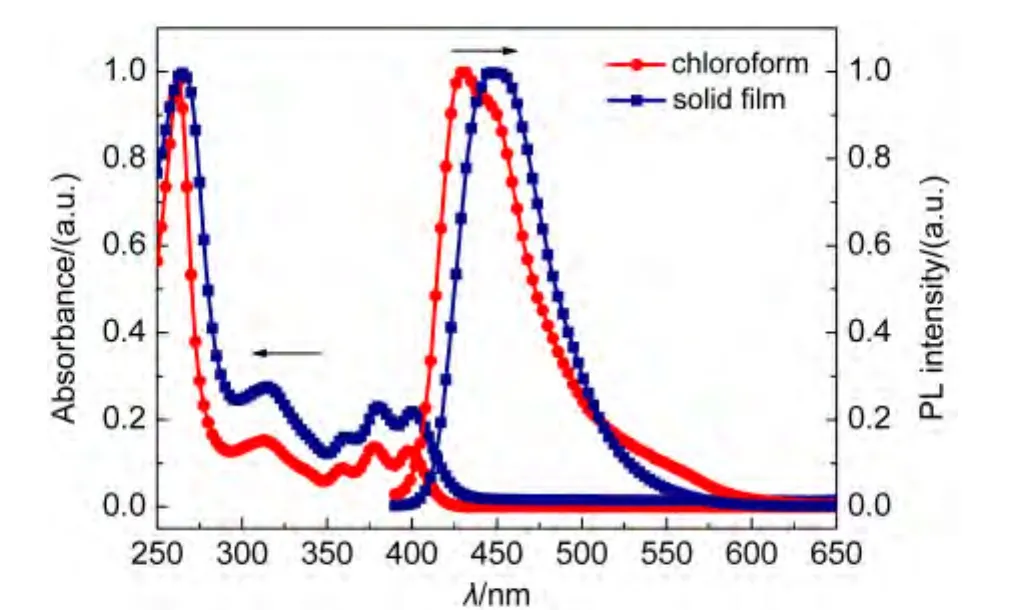
Fig.4 Absorption and photoluminescence spectra of FAn in solution and in film
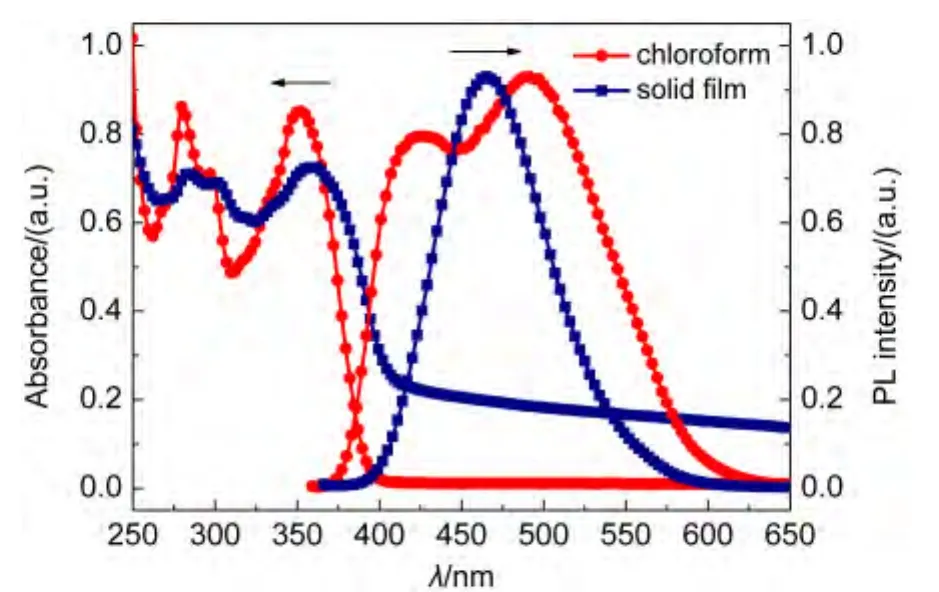
Fig.5 Absorption and photoluminescence spectra of FPy in solution and in film
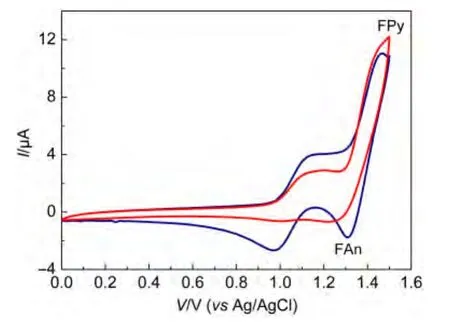
Fig.6 Cyclic voltammograms of compounds FAn and FPy
4 Conclusions
In summary,two novel blue fluorescent emitters containing fluorene-diphenylamine unit as the core and polyaromatic anthracene and pyrene as the peripheries were synthesized by Suzuki coupling and Friedel-Crafts reactions.The presence of non-planar diphenylamine restrained the forming long-wavelength aggregates effectively and enhanced the hole-injection ability of the compounds.Moreover,the introduction of hexyl improved solubility in common solvents for the purpose of solution processed of devices.Also,the two compounds exhibited good thermal stability.The studies on the introduction ofN-hexyl-N-diphenylamine provide an approach for the design of blue materials with easily hole-injection and solution processing properties.Further studies to use these materials as emitters in solution processed electrofluorescent devices are currently being pursued.
(1) Tang,C.W.;VanSlyke,S.A.Appl.Phys.Lett.1987,51(12),913.doi:10.1063/1.98799
(2)Uoyama,H.;Goushi,K.;Shizu,K.;Nomura,H.;Adachi,C.Nature2012,492(7428),234.doi:10.1038/nature11687
(3) Kido,J.;Kimura,M.;Nagai,K.Science1995,267(5202),1332.doi:10.1126/science.267.5202.1332
(4)Muller,C.D.;Falcou,A.;Reckefuss,N.;Rojahn,M.;Wiederhirn,V.;Rudati,P.;Frohne,H.;Nuyken,O.;Becker,H.;Meerholz,K.Nature2003,421(6925),829.doi:10.1038/nature01390
(5)Liu,C.;Li,Y.H.;Li,Y.F.;Yang,C.L.;Wu,H.B.;Qin,J.G.;Cao,Y.Chem.Mat.2013,25(16),3320.doi:10.1021/cm401640v
(6) Trattnig,R.;Pevzner,L.;Jager,M.;Schlesinger,R.;Nardi,M.V.;Ligorio,G.;Christodoulou,C.;Koch,N.;Baumgarten,M.;Mullen,K.;List,E.J.W.Adv.Funct.Mater.2013,23(39),4897.doi:10.1002/adfm.v23.39
(7)Wang,C.F.;Hung,W.Y.;Cheng,M.H.;Hwang,J.S.;Leung,M.K.;Wong,K.T.Org.Electron.2013,14(8),1958.doi:10.1016/j.orgel.2013.04.047
(8)Zuniga,C.A.;Barlow,S.;Marder,S.R.Chem.Mat.2011,23(3),658.doi:10.1021/cm102401k
(9)Okumoto,K.;Kanno,H.;Hamaa,Y.;Takahashi,H.;Shibata,K.Appl.Phys.Lett.2006,89(6),063504.doi:10.1063/1.2266452
(10) Zhu,M.R.;Yang,C.L.Chem.Soc.Rev.2013,42(12),4963.doi:10.1039/c3cs35440g
(11) Chen,C.T.Chem.Mat.2004,16(23),4389.doi:10.1021/cm049679m
(12)Xiao,L.X.;Hu,S.Y.;Kong,S.;Chen,Z.J.;Qu,B.;Gong,Q.H.Acta Phys.-Chim.Sin.2011,27(4),977.[肖立新,胡雙元,孔 勝,陳志堅,曲 波,龔旗煌.物理化學學報,2011,27(4),977.]doi:10.3866/PKU.WHXB20110325
(13) Zhang,L.;Lin,Z.Q.;Gu,J.F.;Yin,C.R.;Hou,X.Y.;Liu,F.;Liu,Y.Y.;Xie,L.H.;Chen,S.F.;Huang,W.Acta Phys.-Chim.Sin.2010,26(7),1934.[張 龍,林宗瓊,顧菊芬,殷成蓉,侯曉雅,劉 烽,劉玉玉,解令海,陳淑芬,黃 維.物理化學學報,2012,26(7),1934.]doi:10.3866/PKU.WHXB20100739
(14)Chu,Z.Z.;Wang,D.;Zhang,C.;Zou,D.C.Acta Phys.-Chim.Sin.2012,28(8),2000.[初增澤,王 丹,張 超,鄒德春.物理化學學報,2012,28(8),2000.]doi:10.3866/PKU.WHXB201206071
(15)Wu,K.C.;Ku,P.J.;Lin,C.S.;Shih,H.T.;Wu,F.I.;Huang,M.J.;Lin,J.J.;Chen,I.C.;Cheng,C.H.Adv.Funct.Mater.2008,18(1),67.
(16) Chan,K.L.;Lim,J.P.F.;Yang,X.H.;Dodabalapur,A.;Jabbour,G.E.;Sellinger,A.Chem.Commun.2012,48(42),5106.doi:10.1039/c2cc30995e
(17)Hu,J.Y.;Era,M.;Elsegood,M.R.J.;Yamato,T.Eur.J.Org.Chem.2010,2010(1),72.doi:10.1002/ejoc.200900806
(18)Kim,R.;Lee,S.;Kim,K.H.;Lee,Y.J.;Kwon,S.K.;Kim,J.J.;Kim,Y.H.Chem.Commun.2013,49(41),4664.doi:10.1039/c3cc41441h
(19) Park,H.;Lee,J.;Kang,I.;Chu,H.Y.;Lee,J.I.;Kwon,S.K.;Kim,Y.H.J.Mater.Chem.2012,22(6),2695.doi:10.1039/c2jm16056k
(20) Kumar,D.;Thomas,K.R.J.;Chen,Y.L.;Jou,Y.C.;Jou,J.H.Tetrahedron2013,69(12),2594.doi:10.1016/j.tet.2013.01.046
(21)Yao,L.;Sun,S.H.;Xue,S.F.;Zhang,S.T.;Wu,X.Y.;Zhang,H.H.;Pan,Y.Y.;Gu,C.;Li,F.H.;Ma,Y.G.J.Phys.Chem.C2013,117(27),14189.doi:10.1021/jp403463k
(22) Zhang,Y.J.;Jin,Y.X.;Bai,R.;Yu,Z.W.;Hu,B.;Ouyang,M.;Sun,J.W.;Yu,C.H.;Liu,J.L.;Zhang,C.J.Photochem.Photobiol.A-Chem.2012,227(1),59.doi:10.1016/j.jphotochem.2011.11.003
(23) Taguchi,Y.;Uyama,H.;Kobayashi,S.J.Polym.Sci.Pol.Chem.1996,34(4),561.
(24)Hong,Y.;Liao,J.Y.;Cao,D.;Zang,X.;Kuang,D.B.;Wang,L.;Meier,H.;Su,C.Y.J.Org.Chem.2011,76(19),8015.doi:10.1021/jo201057b
(25)Vougioukalakis,G.C.;Roubelakis,M.M.;Orfanopoulos,M.J.Org.Chem.2010,75(12),4124.doi:10.1021/jo100277v

