Synthesis of Sub-micrometer Lithium Iron Phosphate Particles for Lithium Ion Battery by Using Supercritical Hydrothermal Method
(張艷潔)*(楊艷芳) WANG Xueyong(王學(xué)勇)(李帥三)
1Mudanjiang Medical University, Mudanjiang 157011, China
2The 209th Hospital of People’s Liberation Army, Mudanjiang 157011, China
Synthesis of Sub-micrometer Lithium Iron Phosphate Particles for Lithium Ion Battery by Using Supercritical Hydrothermal Method
ZHANG Yanjie(張艷潔)1,*, YANG Yanfang(楊艷芳)1, WANG Xueyong(王學(xué)勇)2and LI Shuaisan(李帥三)1
1Mudanjiang Medical University, Mudanjiang 157011, China
2The 209th Hospital of People’s Liberation Army, Mudanjiang 157011, China
A supercritical hydrothermal method was employed to prepare sub-micrometer LiFePO4particles with high purity and crystallinity. The structure and morphology of LiFePO4particles were characterized by X-ray diffraction and scanning electron microscope. The electrochemical tests were carried out to determine the reversible capacity, rate and cycling performance of the LiFePO4particles as cathode material for lithium ion battery. Experimental results show that solvent and calcining time have significant effects on purity, size and morphology of LiFePO4particles. Mixed solvent contained deionized water and ethanol is conducive to synthesize smaller and more uniform particles. The size of LiFePO4particles as-prepared is about 100-300 nm. The specific discharge capacities of the LiFePO4particles are 151.3 and 128.0 mA·h·g?1after first cycle at the rates of 0.1 and 1.0 C, respectively. It retains 95.0% of the initial capacity after 100 cycles at 1.0 C.
LiFePO4, supercritical hydrothermal synthesis, lithium iron
1 INTRODUCTION
Since the pioneering work of Padhi et al. in 1997 [1], lithium iron phosphate (LiFePO4) has been considered as one of the most promising cathode materials for lithium-ion batteries. Because of its high theoretical capacity of 170 mA·h·g?1, intermediate voltage of 3.4-3.5 V, excellent cycle life, safety, low cost and environmental friendliness [2], LiFePO4has attracted much attention. The most common synthesis method is solid phase method [3], but it needs multi-step mixed ball milling, which really wastes energy, and the diameter of particles is more than 10 μm. The low ionic conductivity (10?9S·cm?1) [4] and smaller lithium ion diffusion ratio (<10?14cm2·S?1) [5] limit its use for high rate. In order to improve the electronic conductivity, carbon coating and super-valent cation doping processes have been investigated by many research groups. To overcome the drawback of low lithium ion diffusion coefficient, various strategies have been explored, including minimizing particle size, optimizing the electrolyte and preparing materials with porous or hollow structure.
Yang et al. [6] have prepared LiFePO4through sol-gel and hydrothermal methods at relatively low temperature 393K, but the method also suffers from long synthesis time (6-24 h) and low production rate. Compared with sol-gel and hydrothermal method, subcritical or supercritical hydrothermal method can reduce synthesis time. For example, Lee and Teja [7] have synthesized LiFePO4under subcritical or supercritical hydrothermal conditions in a batch reactor. This paper presents a facile and controllable route for synthesis of submicro-sized LiFePO4particles by using supercritical hydrothermal synthesis in a batch reactor. The effects on the morphology, particle size and electrochemical performance of LiFePO4synthesized in different solvents (deionized water, deionized water + ethanol) are investigated and the electrochemical performance is examined.
2 EXPERIMENTAL
2.1 Materials
The precursor solution of FeSO4-7H2O, H3PO4and LiOH with a molar ratio at 1︰1︰3 was first prepared. Ascorbic acid was used as a reducing agent, and polyvinyl pyrrolidone (PVP) was used as templates and dissolved in solvent by magnetic stirring. The solvents used in the experiment were deionized water and ethanol-deionized water solution (volume ratio 1︰1). The mixed solution was transferred into the autoclave. After the reaction, the mixture solution was rapidly cooled to room temperature and collected after filtering, the products were washed with deionized water and dried in a vacuum oven at 700 °C for 1 h under Ar-H2(H2volume ratio 10%) atmosphere. Finally LiFePO4particles were obtained.
2.2 Characterization
The structure and morphology of the as-prepared materials were examined by powder X-ray diffraction (XRD, PW1825, powder diffractometer using Cu Kαradiation at 40 kV and 40 mA at steps of 0.020°) and field emission scanning electron microscopy (SEM, JEOL JSM-6390 scanning electron microscope).
2.3 Electrochemical performance test
To evaluate the electrochemical properties of the synthesized particles, the active materials were mixed with acetylene black and polyvinylidene fluoride (PVDF) in N-methyl pyrrolidone (NMP) in a ratio of 80︰10︰10 to form slurry. The slurry was coated onto aluminum foil, followed by drying at 110 °C for 1 h in a vacuum oven. The electrodes were punched under 15 MPa for 1 min. The cathodes were assembled into CR2025-type coin cell with lithium foil anode and Celgard 2400 as separator in a dry glove box under argon. The electrolyte was 1 mol·L?1LiPF6in EC (ethylene carbonate)︰DMC (dimethyl carbonate)︰EMC (methyl ethyl carbonate)=1︰1︰1 (volume ratio) solvent. The cells were tested by a commercial multichannel NEWARE Battery testing system in the charge-discharge cycle in the potential range of 2.0-4.2 V versus Li+/Li at different rates.
3 RESULTS AND DISCUSSION
3.1 Effect of pressure
Figure 1 shows the XRD patterns of LiFePO4/C particles prepared at different pressures, with deionized water as the solvent. The main diffraction patterns can be indexed to orthorhombic LiFePO4olivine-type phase (JCPDS PDF number 83-2092). No impurity peaks are observed. As the pressure increases, the intensity of diffraction peaks is higher and clearer.
Figure 2 shows the SEM patterns of these LiFePO4/C particles. The samples are uniformly distributed and irregular particles. Compared with the samples prepared at 20 and 25 MPa, the particles prepared at 30 MPa are smaller, more regular, without obvious agglomeration. As the pressure increases, the particles are smaller with the size generally in the range of 1 μm. Thus higher pressure gives smaller particle size and prevents particles from agglomeration.
3.2 Effect of temperature
Figure 3 shows the SEM patterns of LiFePO4samples prepared at different temperatures, with deionized water as the solvent. LiFePO4prepared at 380 °C, with obvious agglomeration and irregular particles, has larger particle size than LiFePO4prepared at 360 °C and 400 °C, with average particle size of 200-600 nmand more uniform size, but without obvious agglomeration. The results show that the temperature influences the morphology, size, and crystallinity of LiFePO4. The morphology and size of particles are better controlled at 400 °C. The sample synthesized at 30 MPa and 700 °C is labeled as S1.

Figure 1 X-ray diffraction patterns of LiFePO4particles prepared at different pressures (360 °C)

Figure 2 SEM images of LiFePO4samples prepared at different pressures (360 °C)

Figure 3 SEM images of LiFePO4samples prepared at different temperatures (30 MPa)
3.3 Effect of solvent
The sample prepared with deionized water and ethanol (volume ratio 1︰1) at 30 MPa and 400 °C is labeled as S2. Fig. 4 shows its SEM patterns. The particles are spherical and the size is in the range of 100-300 nm, smaller than that with deionized water as the solvent. Thus solvent influences the morphology and size of LiFePO4. There is no obvious agglomeration when the material is prepared with deionized water and ethanol. Ethanol is good for controlling particle morphology and size.

Figure 4 SEM of LiFePO4/C prepared with deionized water and ethanol (volume ratio 1︰1) as solvent
3.4 The electrochemical performance of LiFePO4/C
Figure 5 shows charge-discharge curves of LiFePO4/C at 0.1 C. The first discharge capacity of samples S1 and S2 are 141.2 and 151.3 mA·h·g?1, charge and discharge efficiency are 94.6% and 96.2%, respectively. Ethanol will increase the electronic conductivity and improve the rate performance.
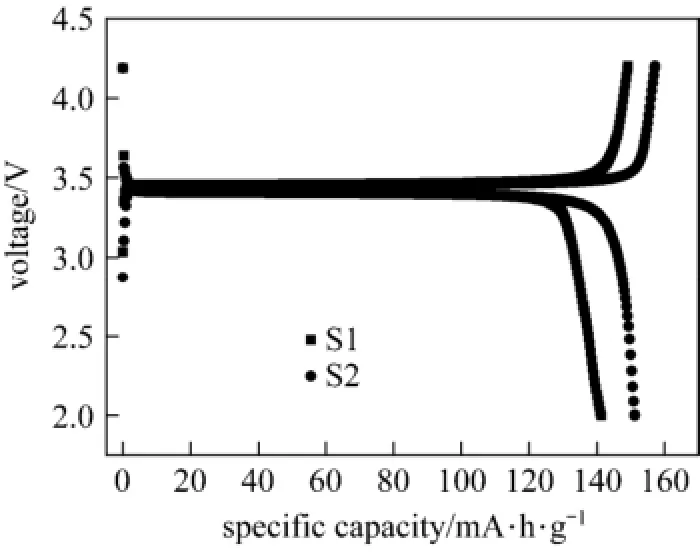
Figure 5 Charge-discharge curves of LiFePO4/C prepared in different solvents at 0.1 C

Figure 6 Cycling performance of LiFePO4/C prepared in different solvents at 0.1 C
Figure 6 shows the cycling performance curves of LiFePO4/C prepared at 0.1 C. The cycle performance of the two samples, S1 and S2, are fine. The capacity of S1 is from 142 mA·h·g?1to 141 mA·h·g?1after 30 cycles and the capacity retention is 99.3%. The capacity of S2 faded from 151.42 mA·h·g?1to 142.9 mA·h·g?1and the capacity retention is 94.4% after 30 cycles, and it is 138.1 mA·h·g?1after 50 cycles and the capacity retention is 91.2%.
Figure 7 shows the cycling performance curves of LiFePO4/C prepared at 1 C. After 100 cycles, the capacity of S1 faded from 113.6 mA·h·g?1to 109 mA·h·g?1and the capacity retention is 96%; the capacity of S2 is from 128 mA·h·g?1to 121.5 mA·h·g?1and the capacity retention is 95%. The samples have different specific capacity, but they show good cycling performance.

Figure 7 Cycling performance of LiFePO4/C prepared in different solvents at 1 C

Figure 8 CV curves of LiFePO4/C prepared in different solvents
3.5 CV test of LiFePO4/C in different solvents
Figure 8 shows the cyclic voltammograms (CV) of LiFePO4/C particles. The sharp peaks of LiFePO4/C prepared in the two solvents show excellent kinetics. The lithium extraction and intercalation peaks of S1 prepared in water locate at 3.546 V and 3.326 V vs. Li+/Li, while those of S2 prepared in water and ethanol are at 3.548 V and 3.348 V, respectively. The peak distance of LiFePO4/C prepared in water and ethanol is less than that synthesized in water, which suggests that the former has better electrochemical reversibility.
REFERENCES
1 Padhi, A.K., Nanjundaswamy, K.S., Goodenough, J.B., “Phosphoolivines as positive-electrode materials for rechargeable lithium batteries”, J. Electrochem. Soc., 144 (4), 1188-1194 (1997).
2 Aimable, A., Aymes, D., Bernard, F., Le Cras, F., “Characteristics of LiFePO4obtained through a one step continuous hydrothermal synthesis process working in supercritical water”, Solid State Ionics, 180 (11-13), 861-866 (2009).
3 Oh, S.W., Myung, S.T., Bang, H.J., Yoon, C.S., Amine, K., Sun, Y.K.,“Nanoporous structured LiFePO4with spherical microscale particles having high volumetric capacity for lithium batteries”, Electrochem. Solid St., 12 (9), A181-A185 (2009).
4 Chung, S.Y., Bloking, J.T., Chiang, Y.M., “Electronically conductive phospho-olivines as lithium storage electrodes”, Nat. Mater., 1 (2), 123-128 (2002).
5 Prosini, P.P., Lisi, M., Zane, D., Pasquali, M., “Determination of the chemical diffusion coefficient of lithium in LiFePO4”, Solid State Ionics, 148 (1/2), 45-51 (2002).
6 Yang, S.F., Zavalij, P.Y., Whittingham, M.S., “Hydrothermal synthesis of lithium iron phosphate cathodes”, Electrochem. Commun., 3 (9), 505-508 (2001).
7 Lee, J., Teja, A.S., “Characteristics of lithium iron phosphate (LiFePO4) particles synthesized in subcritical and supercritical water”, Supercrit. Fluids, 35 (1), 83-90 (2005).
Received 2012-08-22, accepted 2013-02-05.
* To whom correspondence should be addressed. E-mail: zyj730916@163.com
 Chinese Journal of Chemical Engineering2014年2期
Chinese Journal of Chemical Engineering2014年2期
- Chinese Journal of Chemical Engineering的其它文章
- Kinetics of Glucose Ethanolysis Catalyzed by Extremely Low Sulfuric Acid in Ethanol Medium*
- Hydrogenation of Silicon Tetrachloride in Microwave Plasma
- Effects of Solvent and Impurities on Crystal Morphology of Zinc Lactate Trihydrate*
- Large-eddy Simulation of Ethanol Spray-Air Combustion and Its Experimental Validation*
- Kinetic and Thermodynamic Studies of Acid Scarlet 3R Adsorption onto Low-cost Adsorbent Developed from Sludge and Straw*
- Performance of EDAB-HCl Acid Blended System as Fracturing Fluids in Oil Fields*
