Synthesis and Photolum inescence of Mn-Mg Co-doped AlON Phosphors
ZHONG Hong-mei,LIU Qian,ZHOU Yao,ZHUANG Jian-dong,ZHOU Hu
(State Key Laboratory of High Performance Ceramics and Superfine Microstructure,Shanghai Institute of Ceramics,Chinese Academy of Sciences Shanghai200050,China)
*Corresponding Author,E-mail:xljin1213@gmail.com
1 Introduction
Oxynitride or nitride phosphors have been considered as excellent phosphormaterials at high temperature forwhite LED applications due to their thermal and chemical stability,stable luminescence properties[1-5].Up to date,some oxynitride materials have been developed in recently years,such as rare-earth(RE=Eu,Ce)-activatedα-,β-SiAlON,AlN, MSiN, AlON[6-15]. Aluminum oxynitride(AlON),a solid solution in the binary system of Al2O3-AlN,is well known as an important window material due to its optical transparency and high thermomechanical properties.It is also used as candidate phosphors for high-powder LED.There are few works about luminescence of AlON-based powder.AlON phosphor doped with Mn2+was synthesized by a solid-state reaction(SSR)and the crystal structure and luminescence properties[13]were studied.Kikkawa et al[14]prepared Eu2+doped spineltype AlON by ammonia nitridation and Yin et al[15]also synthesized Eu-Mg co-doped AlON phosphor by SSR,but a pure phase of AlON was not obtained.These reports indicate it is difficult to obtain a pure phase of AlON and the mechanism of luminescence still needs to be further investigated.
In this paper,we synthesized undoped and only Mg-doped and a series of Mn-doped AlON phosphors,investigated the effect of Mn concentration on phosphors photoluminescence properties.The results show the blue emission appears in both the undoped and all Mn-Mg co-doped samples and the green emission is only observed in samples.The green emission is assigned to the transition of 3d electrons of Mn2+ion and the blue emission might be attributed to impurities or Al vacancy.
2 Experiments
AlON(Al23O27N5)phosphors were synthesized by a solid-state reaction.The initial powder composition is based on the formula of Al23O27N5.The initial high purity powder of Al2O3,AlN,MgO,MnCO3weremilled in absolute alcohol for 24 h in a plastic jar,using Si3N4milling media.The mixed powder was then dried at 80℃ for 24 h.Then it was calcinated in BN crucibles under a nitrogen atmosphere at 1 800~1 900℃ for 2 h.The mole fractions of Mn with respect to Al are varied from 5%to 15%,and themole fraction of Mg is fixed to 10%for all doping samples.
The crystal structure and phases of samples were performed by X-ray diffraction(XRD)with Cu Kα radiation(λ =0.154 1 nm).The photoluminescence spectra of the phosphors under UV light were recorded on a Hitachi F-4600 spectrometer.
3 Results and Discussion
Fig.1 shows the XRD patterns of undoped and different concentration Mn-Mg co-doped AlON sampleswhich calcined at different temperatures for 2 h.A pure phase of AlON was obtained by calcination at 1 900℃for undoped samples and 1 850℃for only Mg-doped samples.As Mn doping mole fraction increased up to 10%,the temperature for achieving AlON pure phase decreases from 1 850℃ to 1 800℃.When Mn doping dopingmole fraction increased up to 10%,the Al2O3phase appeared,which indicating the saturation of Mn.Fig.2 shows the lattice parameter of AlON varies from a=0.795 to 0.80 nm due to Mg and Mn doping.Comparing with undoped sample,the lattice parameter increased for Mg-doped or all Mn-Mg co-doped samples.It indicates the doping of Mg2+and Mn2+result in the lattice expansion.
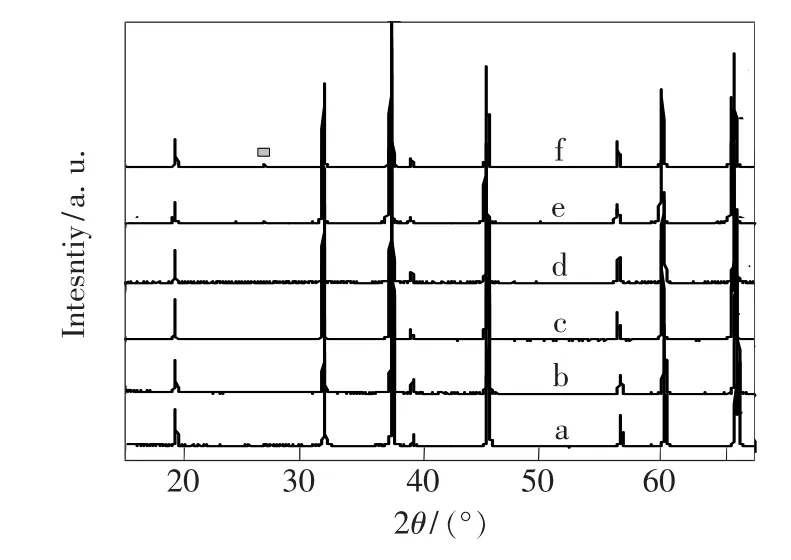
Fig.1 XRD diffraction patterns of samples calcined at(a)undoped,1 900℃ for 2 h,(b)10%Mg,1 850℃for 2 h,(c)5%Mn,1 850 ℃ for 2 h,(d)7%Mn,1 850 ℃ for 2 h,(e)10%Mn,1 800 ℃ for 2 h,(f)15%Mn,1 800℃ for 2 h.The square indicates the peak from Al2 O3.
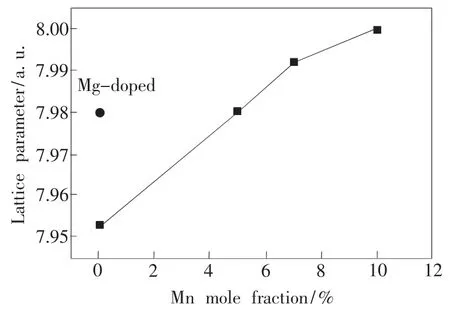
Fig.2 Dependence of lattice parameter on Mnmole fractions
The room temperature excitation spectra of all of undoped,Mg-doped and Mn-Mg co-doped AlON samples calcined at different temperature are shown in Fig.3.The excitation spectral from 220 nm to 370 nm consists of two shoulder peaks at about 263 and 290 nm for all samples,monitored at386 and 393 nm for undoped and all doping samples,respectively.The strong absorption peaks at 260 and 265 nm are observed for undoped sample and all doping samples,respectively.Fig.4 shows the broad-band emission spectra from 350 to 600 nm for all samples under the excitation of260 and 265 nm for undoped and doping AlON,respectively.The emission band is asymmetric,which suggests amultiple-emission-peak composition.Fig.5 shows the fitting results by Gaussian line distributions for all undoped and only Mg-doped and Mn-Mg co-doped samples.Table 1 gives the position of all emission peaks of all of samples.The emission band of undoped and only Mg-doped sample contains two blue emission sub-bands(P1 and P2),and two blue emission bands are observed in Mn-Mg co-doped samples.Moreover,the green emission
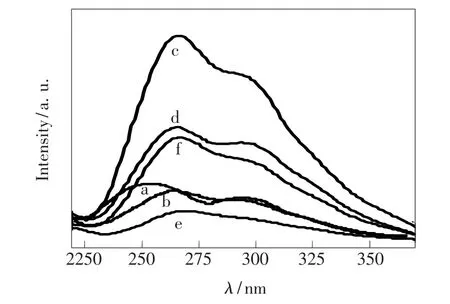
Fig.3 Excitation spectra of(a)undoped,(b)Mg-doped,(c)5%Mn-doped,(d)7%Mn-doped,(e)10%Mn-doped,(f)15%Mn-doped AlON samples.
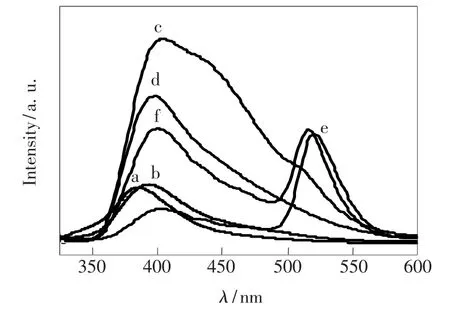
Fig.4 Emission spectra of(a)undoped,(b)Mg-doped,(c)5%Mn-doped,(d)7%Mn-doped,(e)10%Mn-doped,(f)15%Mn-doped AlON samples under the excitation of 260 and 265 nm,respectively.

Fig.5 Fitting emission spectra of undoped and doped samples
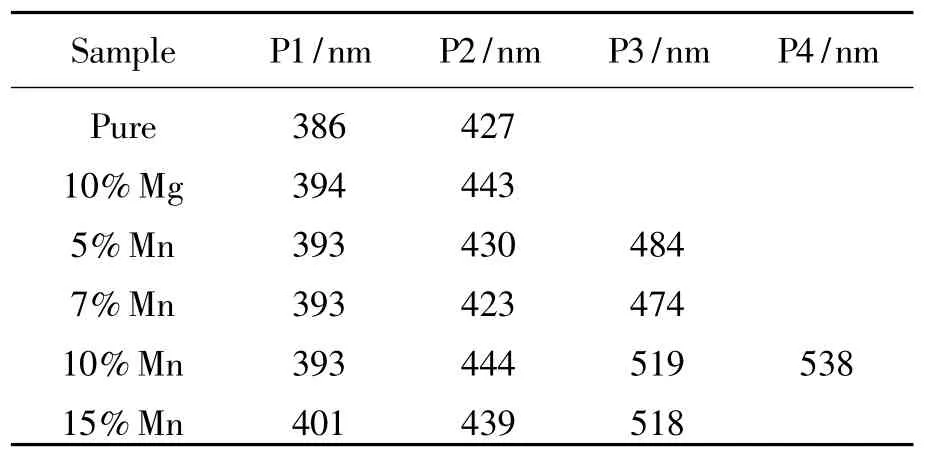
Table 1 Observed peak position of fitting em ission spectra
centered at 518 nm(P3)is present in 10%and 15%Mn-Mg co-doped samples and P4 peak at538 nm in 10%Mn-Mg co-doped sample.P1 and P2 peak may be related to Al vacancy or impurities because they are also found in undoped sample.From Fig.6,we find it is interested that the ratio of P2 to P1 in-creases only for Mg doping and 5%Mn-Mg co-doping phosphor,while the ratio begins to decrease when the Mn mole fraction over 5%.This suggests that the origin of P1 and P2 is different.P1 peak is probably related to Al vacancies.After incorporation of Mg and Mn,the number of Al vacancies is decreased due to partial Mg and Mn replacing Al sites so that the ratio of the P2 to P1 integral intensities is increased.We consider P2 peak may originate from impurities in AlON because the intensity of this peak of all of doping samples is stronger than thatof undoped sample,which Mg and Mn doping may induce more impurities.On the other hand,the position of P1 and P2 peak of only Mg and Mn-Mg co-doped samples shifts to long wavelength comparing with that of undoped sample.It indicates Mg and Mn doping can make the energy level changed.P3 peak only presents in 10%and 15%Mn-Mg co-doped samples and this peak shifts from blue to green with the increase of Mn concentration.It strongly suggests this peak is attributed to Mn doping.Hence,P3 and P4 peaks in Mn-Mg co-doped samples should
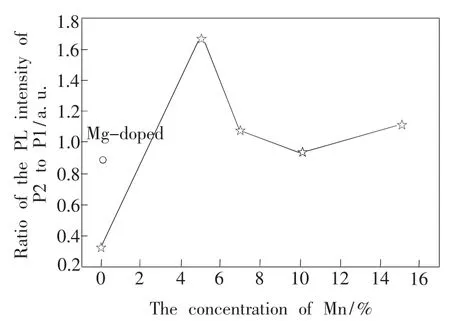
Fig.6 The relation between the concentration of Mn to the ration of PL intensity of P2 to P1
be of3d electrons assigned to the transition of Mn2+ion.It is consistentwith the Ref.[13].It is noted that P2 and P3 in 7%Mn doped sample shift to short wavelength comparing with the 5%sample.The reason is not clear.It is also noticed that P4 peak centered at 538 nm appears in 10%Mn-Mg doped samples and disappears in 15% Mn-Mg doped sample.Therefore,we can exclude that this peak is caused by the increased Mn concentration.Themost probable reason is that there are two kinds of surroundings for Mn2+ions in 10% Mn-Mg doped sample.One is responsible for the emission centered at519 nm and the other is responsible for emission centered at538 nm.The detail origination need further study.
4 Conclusion
In conclusion,undoped,Mg-doped and Mn-Mg co-doped AlON samples were successfully synthesized by solid-state reaction.XRD results show that a pure phase of AlON was obtained by calcinations at1 900℃for undoped samples and 1 850℃for 10%Mn-doped samples and 1 800℃for 10%Mndoped samples.The spectra analyses reveal the two blue emission centered at 386~394 mm and 423~444 nm are observed in undoped and all doped samples.The former is from impurities in AlON and the latter is attributed to Al vacancies.After incorporation of Mn,the emission shifts from blue emission(474 nm)to green emission(519 nm)as Mn mole fraction increase from 5%to 15%.This two green emission should be assigned to the transition of3d electrons of Mn2+ion.
[1]Yamamoto H,White LED phosphors:The next step[J].SPIE,2010,7598:759808-1-10.
[2]He X H,Lian N,Sun JH,etal.Dependence of luminescence properties on composition of rare-earth actived(oxy)nitrides phosphors for white-LEDs applications[J].J.Mater.Sci.,2009,44(18):4763-4775.
[3]Xie R J,Hirosaki N,Mitomo M,et al.Strong green emission from α-SiAlON activated by divalent ytterbium under blue light irradiation[J].J.Phys.Chem.B,2005,109(19):9490-9494.
[4]Xie R J,HirosakiN,Mitomo M,etal.Photoluminescencealof rare-earth-doped Ca-α-SiAlON phosphors:Composition and concentration dependence[J].J.Am.Ceram.Soc,2005,88(10):2883-2888.
[5]Kikkawa S,Nagasaka K,Takeda T,et al.Preparation and lithium doping of gallium oxynitride by ammonia nitridation viaa citrate precursor route[J].Solid State Chem.,2007,180(3):1984-1989.
[6]Li H L,Xie R,Hirosaki N,et al.Phase purity and luminescence properties of fine Ca-α-SiAlON∶Eu phosphors synthesized by gas reduction nitridationmethod [J].J.Electrochem.Soc.,2008,155(6):J175-J179.
[7]Xie R J,Hirosaki N,Li H L,et al.Synthesis and photoluminescence properties of β-sialon∶Eu2+(Si6-zAlzOzN8-z∶Eu2+):A promising green oxynitride phosphor for white light-emitting diodes[J].J.Electrochem.Soc.,2007,154(10):J314-J319.
[8]Liu L H.,Xie R J,Hirosaki N,et al.Optical properties of blue-emitting CexSi6-zAlz-xOz+1.5xN8-z-xfor white light-emitting diodes[J].J.Electrochem.Soc.,2010,157(1):H50-H54.
[9]Zhu X W,Masubuchi Y,Motohashi T,et al.The z value dependence of photoluminescence in Eu2+-dopedβ-SiAlON(Si6-zAlzOzN8-z)with 1≤z≤4 [J].J.Alloys Compds.,2010,489(1):157-161.
[10]Inoue K,HirosakiN,Xie R J,etal.High efficient thermally stable blue-emitting AlN∶Eu2+phosphor for ultravioletwhite light-emitting diodes[J].J.Phys.Chem.C,2009,113(21):9392-9397.
[11]Li H L,Xie R J,Hirosaki N,et al.Synthesis and photoluminescence properties of Sr2Si5N8∶Eu2+red phosphor by a gasreduction and nitridation method[J].J.Electrochem.Soc.,2008,155(12):J378-J381.
[12]Duan C J,Delsing A CA.,Hintzen H T,Red emission from Mn2+on a tetrahedral site in MgSiN2[J].J.Lumin.,2009,129(3):645-649.
[13]Xie R J,Hirosaki N,Liu X J,et al.Crystal structure and photoluminescence of Mn2+-Mg2+codoped gamma aluminum oxynitride(γ-AlON):A promising green phosphorforwhite light-emitting diodes[J].Appl.Phys.Lett.,2008,92(20):201905-1-3.
[14]Kikkawa S,Hatta N,Takeda T,etal.Preparation of aluminum oxynitride by nitridation of a precursor derived from aluminum-glycine gel and the effects of the presence of europium[J].J.Am.Ceram.Soc.,2008,91(3):924-928.
[15]Yin L J,Xu L Y,Hao Y,et al.Synthesis and photoluminescence of Eu2+-Mg2+co-doped γ-AlON phosphors[J].Matter.Lett.,2009,6(17):1511-1513.
- 發(fā)光學(xué)報的其它文章
- 《發(fā)光學(xué)報》入選“2012年中國國際影響力優(yōu)秀學(xué)術(shù)期刊”
- 金錫共晶互連對HP-LED光熱性能的改善
- Performance Improvement of Blue InGaN Light-em itting Diode w ith A Special Designed Electron-blocking Layer
- Effects of Experimental Conditions on The Morphology and Photocurrent Density of TiO2 Nanorods
- 金納米粒子摻雜DNA-CTMA-DPFP薄膜的表面增強拉曼散射特性
- 跑道型結(jié)構(gòu)光子晶體波導(dǎo)定向耦合器

