Spontaneous Deposition of Pt Nanoparticles on Poly(diallyldimethylammonium chloride)/Carbon Nanotube Hybrids and Their Electrocatalytic Oxidation of Methanol
CUI Ying KUANG Yin-Jie ZHANG Xiao-Hua LIU Bo CHEN Jin-Hua,*
(1State Key Laboratory of Chemo/Biosensing and Chemometrics,College of Chemistry and Chemical Engineering,Hunan University,Changsha 410082,P.R.China; 2Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation,School of Chemistry and Biological Engineering,Changsha University of Science&Technology,Changsha,410114,P.R.China; 3Xi'an Taijin Industrial Electrochemical Technology Co.,Ltd.,Northwest Institute for Non-ferrous Metal Research,Xi'an 710016,P.R.China)
1 lntroduction
Due to its high power density,relatively quick start-up,rapid response to varying loading,and low operating temperatures,direct methanol fuel cell(DMFC)has been regarded as an ideal power source for electric vehicles and electronic portable devices.1So far,Pt and Pt-based alloy are the most common catalysts for the anodic oxidation of methanol.However,the high cost of the Pt-based catalysts,together with their limited reserves on earth,is one of the main barriers for the large-scale commercialization of DMFCs.2In order to lower the cost of noble-metal nanocatalysts,carbon materials with high surface area are usually used as supports to enhance the dispersion of metal nanoparticles(NPs)and thus to increase the efficiency and utilization of the noble-metal nanocatalysts.3Carbon nanotubes(CNTs)have been considered as an attractive material for supporting noble-metal NPs owing to their high specific surface area,excellent electronic conductivity,outstanding chemical and electrochemical stability,and so on.4Unfortunately,the metal NPs on the pristine CNTs usually have poor dispersion,because the CNT surface does not have enough binding sites to anchor the precursors of metal ions or metal NPs.
In order to obtain high dispersion of noble-metal NPs on CNTs,CNTs is usually modified by the covalent5or noncovalent6methods to introduce lots of binding sites,which can adsorb the metal precursors by electrostatic attraction and coordination effect.Finally,the metal precursors are reduced to metal NPs by the reductant in solution.Whereas,this kind of methods has some disadvantages including more affecting factors on metal deposition,the waste of the metal precursors and reductant.Chenet al.7reported the spontaneous deposition of Pt NPs on the surface of CNTs by oxidizing CNTs in strong acid solution to introduce lots of carboxyl and hydroxyl groups.But the acid-oxidation treatment in strong acid will destroy the structure of CNTs and decrease their conductivity and anti-corrosion ability.
On the other hand,the durability of the electrocatalyst is another issue in DMFCs.At high potential and during long operating period,the dissolution/agglomeration of noble-metal NPs and the corrosion of carbon supports are generally responsible for the degradation of electrocatalytic performance.8Colmenareset al.9reported that the graphitized carbon had stronger resistance towards carbon corrosion at high potential.However,the graphitized process needs extra high temperature.
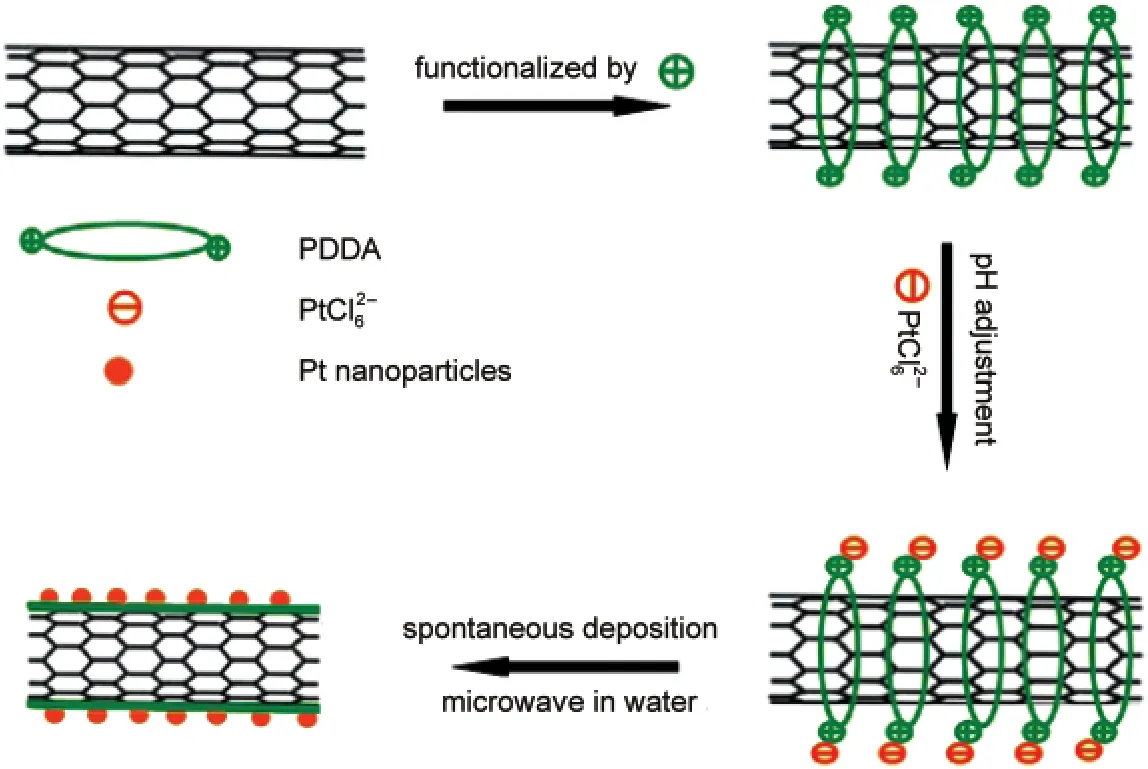
Fig.1 Scheme of the functionalization of CNTs with PDDAand the dispersion of Pt NPs on CNTs-PDDA
In this paper,we reported an alternative strategy to spontaneously deposit noble metal NPs on CNTs with high dispersion and to improve the resistance to the corrosion of CNTs.Poly(diallyldimethylammonium chloride)(PDDA),an environment-friendly polymer,10has been used widely in advanced catalysts and chemo/biosensors.11It can easily coat on the CNT surface byπ-πstacking interaction,12introducing sufficient binding sites to anchor the metal precursorviaelectrostatic self-assembly(Fig.1).Herein,it not only acts as a reductant13to reduce the metal precursor,its oxidation product but also serves as a stabilizer to anchor thein-situproduced metal NPs on CNTs by the coordination action of plentiful nitrogen atoms.Furthermore,the outstanding film-forming ability of PDDA will make it easy to form a thin film on the CNT surface,12awhich will inhibit the corrosion of CNTs in fuel cell operating conditions and thus improve the operating stability of noble-metal NPs/CNTs electrocatalysts.It is expected that Pt NPs will be dispersed uniformly on the PDDA-functionalized CNTs(CNTs-PDDA)and the resulting catalysts(Pt NPs/CNTs-PDDA)will show excellent electrocatalytic performance for the methanol oxidation.For comparison,the pristine CNTs-supported Pt NPs(Pt NPs/CNTs)were prepared.
2 Experimental
2.1 Materials
Multi-walled carbon nanotubes(CNTs)(length 1-2 μm,diameter 20-60 nm)were purchased from Shenzhen Nanotech Port Co.Ltd.,China.PDDA(20%(w)in water,MW=200000-350000)was purchased from Sigma Aldrich.Aqueous solutions were prepared with double-distilled water.Other chemicals were of analytical grade and used as received.
2.2 Functionalization of CNTs with PDDA
The procedure for the noncovalent functionalization of CNTs with PDDA was as follows:CNTs(50 mg)were first ultrasonicated in double-distilled water(200 mL)containing PDDA(0.5%(w))and NaCl(0.5%(w))for 3 h.Then,the obtained suspensions were stirred overnight,filtered using a nylon membrane and washed with vast double-distilled water to remove excess PDDA and NaCl.The final product,denoted as CNTs-PDDA,was dried at 60°C in vacuum for 24 h.
2.3 Preparation of Pt NPs/CNTs-PDDA and Pt NPs/CNTs nanohybrids
Deposition of Pt NPs on the CNTs-PDDA was carried out by a microwave-assisted reduction process in water and the details were as follows:CNTs-PDDA(5 mg)was ultrasonicated in double-distilled water(10 mL)for 30 min to yield stable suspensions.Then,H2PtCl6(19.3 mmol·L-1,332 μL)was added to the suspensions and mixed under stirring for 30 min.After the pH value of the solution was adjusted to 8-9 with 1.0 mol·L-1NaOH aqueous solution,the mixture was then placed in a microwave oven and heated by microwave irradiation(800 W)at 80°C for 20 min.The products were centrifuged and washed three times with double-distilled water.The obtained CNTs-PDDA supported Pt NPs,denoted as Pt NPs/CNTs-PDDA,were dried in vacuum oven at 60°C for 12 h.For comparison,Pt NPs supported on the pristine CNTs,labeled as Pt NPs/CNTs,were prepared under the same procedure as described above.
2.4 Characterization
Fourier transform infrared(FTIR)spectrometry(Nicolet,6700,USA)was employed to analyze the surface chemical compositions of the CNTs-PDDA.The mass fraction of the PDDA in the CNTs-PDDA was determined by thermogravimetric analysis(TGA)(NETZSCH STA 409PC,Germany).The Raman spectrum(Labram-010,France)was also used to study the integrity and electronic structure of the CNTs and CNTs-PDDA.The morphology of the samples was characterized by transmission electron microscopy(TEM,JEM-3010,Japan)with an accelerating voltage of 200 kV.The metal Pt loading mass for the Pt NPs/CNTs-PDDA and Pt/CNTs catalysts was determined by Inductively Coupled Plasma-Atom Emission Spectroscopy(ICP-AES,Spectro Ciros,Germany).
2.5 Electrochemical measurements
For electrochemical investigation,a glassy carbon(GC,5 mm diameter)electrode was polished with the slurry of 0.50 and 0.03 μm alumina successively and washed ultrasonically in double-distilled water prior to use.The catalyst ink was prepared by dispersing 5 mg of catalyst in 5 mL of water by sonication.Then,40 μL of the ink was dropped onto the GC electrode using micro-syringe.After dried in air,the electrode was coated with 5 μL of 0.05%(w)Nafion ethanol solution.The electrochemical surface area(ESA)and the electrochemical performance of the electrocatalysts were evaluated by cyclic voltammetry(CV)and chronoamperometry.All electrochemical measurements were performed on a CHI660B electrochemical workstation(Chenhua Instrument Company of Shanghai,China).A conventional three-electrode glass cell was used with a platinum wire as the counter electrode and a saturated calomel electrode(SCE)as the reference electrode.All the potentials reported herein were with respect to SCE.Double-distilled water was used throughout.
3 Results and discussion
3.1 CNTs-PDDA characterization
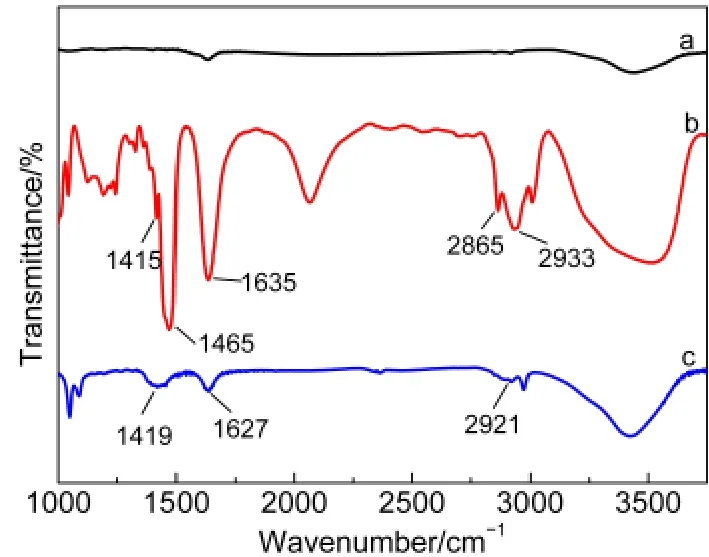
Fig.2 FTIR spectra of(a)pristine CNTs,(b)PDDA,and(c)CNTs-PDDA
Surface-functionalization of CNTs with PDDA was characterized by FTIR spectroscopy.Fig.2 shows the FTIR spectra of the pristine CNTs,PDDA,and CNTs-PDDA.The FTIR spectrum of PDDA(curve b)shows some characteristic peaks at 2865 and 2933 cm-1(C―H stretching vibration from methylene),1465 cm-1(C―H asymmetrical deformation vibration from N―CH3),and 1415 cm-1(C―H symmetrical deforma-tion vibration from N―CH3).The absorption peak at 1635 cm-1in curve b is ascribed to the stretching vibration of C=C,which comes from the unsaturated impurity existing in PDDA chain.12b,14These characteristic peaks are absent in the FTIR spectrum of the pristine CNTs(curve a).However,The mainly characteristic peaks of PDDA are also observed in the spectrum of the CNTs-PDDA nanohybrids(curve c)and slightly shift to lower wavenumbers,which might be attributed to the interaction between PDDA and CNTs.12bThis confirms the presence of PDDAon the CNT surface.
The mass fraction of the PDDA film coated on the CNT surface was further determined by TGA.TGA was performed on a NETZSCH STA 409PC at a heating rate of 10 °C·min-1in N2atmosphere and the corresponding results are shown in Fig.3.For the pristine CNTs,there is almost no weight loss in the measured temperature range of 300-1000 K.However,for the PDDA-functionalized CNTs,an obvious weight loss can be observed in temperature range of 300-1000 K,which is due to the decomposition of PDDA functionalized on CNT surface.From the difference in weight loss of the CNTs without and with PDDA at 1000 K,the mass fraction(w)of PDDA in the CNTs-PDDAis estimated to be about 21.7%.
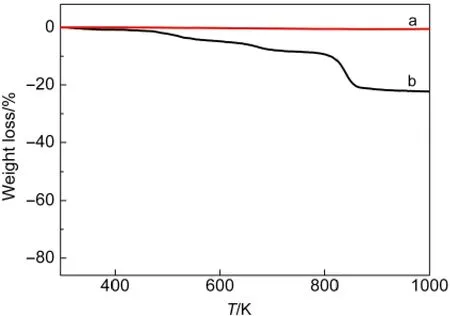
Fig.3 TGAcurves of(a)pristine CNTs and(b)CNTs-PDDA
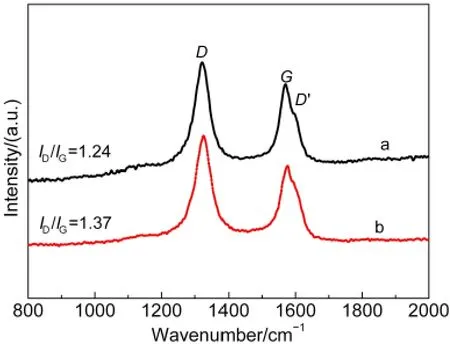
Fig.4 Raman spectra of(a)CNTs-PDDAand(b)pristine CNTs
The Influence of the PDDA film on the structure of the CNTs was also investigated by Raman spectroscopy.As shown in Fig.4,the peak at 1321 cm-1is assigned to the stretching mode of the poor crystallization of graphite(disorder band,D-band).The peak at~1570 cm-1is ascribed to the stretching mode of the crystal graphite(graphitic band,G-band),reflecting the structure of thesp2hybridized carbon atoms.An additional side band at~1600 cm-1was also observed,which is assigned as theD′band.Both theDand theD'bands are due to the defect sites in the hexagonal framework of graphite materials.15From Fig.4,it can be calculated that the intensity ratio ofDandGbands(ID/IG)of CNTs-PDDA is 1.24 and lower than that of the pristine CNTs(1.37),which is consistent with the results of Wanget al.6aThis result indicates that the functionalization of CNTs with PDDA has no detrimental effect on the structure of CNTs.Taking into account the good film-forming ability of PDDA,the results from Fig.4 imply that the CNTs-PDDA may have good anti-corrosion ability during the long-term operating process of methanol electro-oxidation.
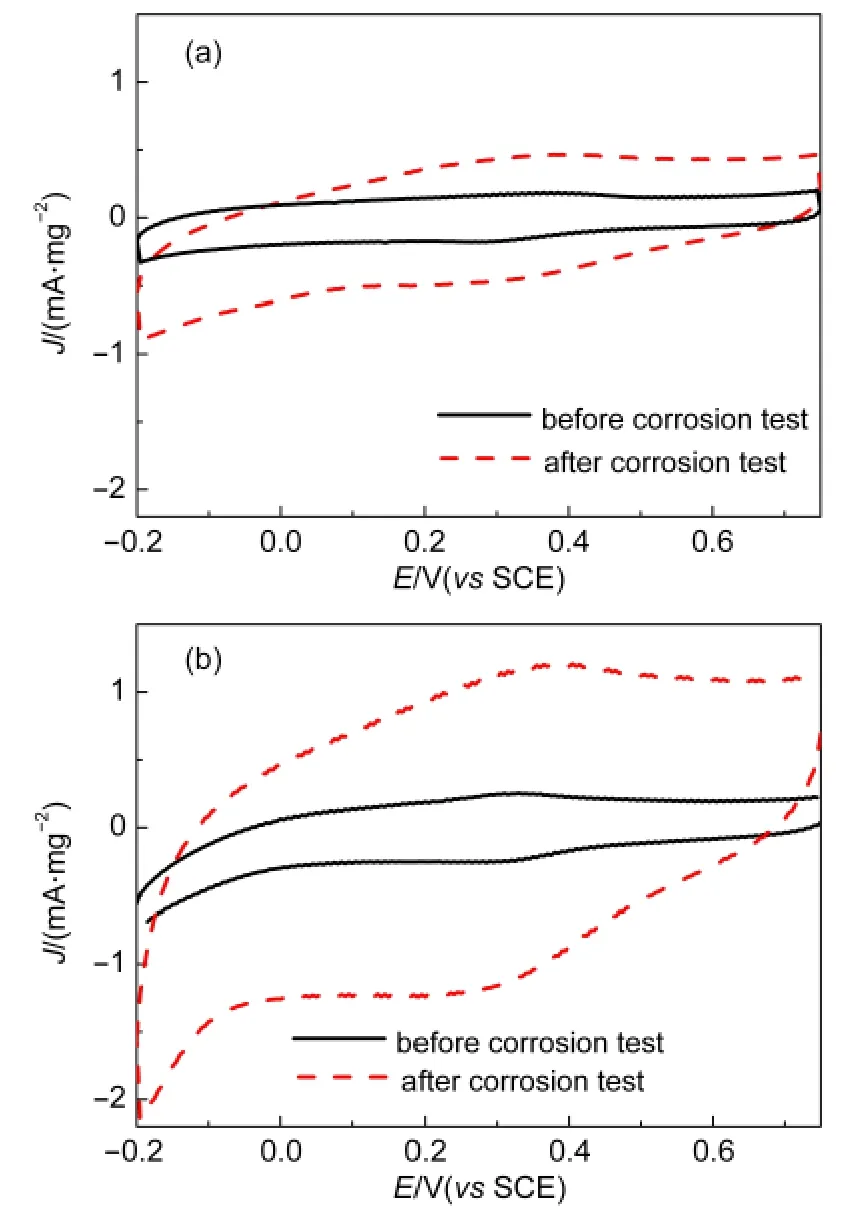
Fig.5 Cyclic voltammogramms of(a)PDDA-CNTs and(b)CNTs electrodes in nitrogen-saturated 0.5 mol·L-1H2SO4solution before and after carbon corrosion tests at a scan rate of 20 mV·s-1
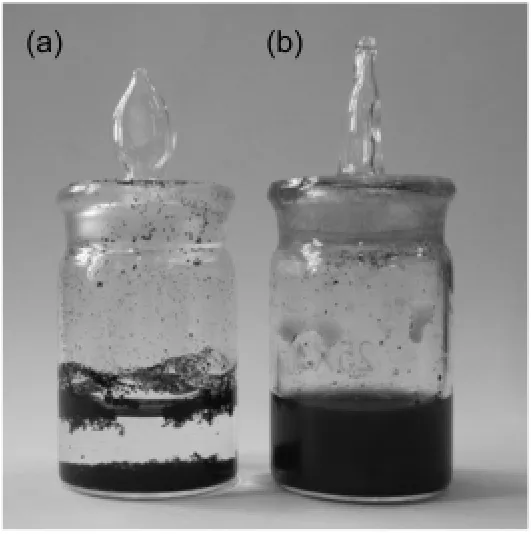
Fig.6 Pictures of the dispersion of(a)CNTs and(b)CNTs-PDDA in aqueous solution(1 mg·mL-1)standing for 8 h
It is well known that the corrosion of carbon is an important factor that the performance of carbon-supported catalyst degrades.The investigation of carbon corrosion was carried out at a constant potential of 1.25 V for 8 h.16Carbon corrosion can be clearly identified with the related cyclic voltammograms before and after corrosion test.Fig.5 shows the CV results before and after carbon corrosion tests in a nitrogen-saturated 0.5 mol·L-1H2SO4aqueous solution.It is noted that the capacitive currents of both CNTs and PDDA-CNTs increase after the carbon corrosion test,implying that both CNTs and PDDA-CNTs occur oxidation.However,the increment of the capacitive current of CNTs is much larger than that of PDDA-CNTs,indicating that the oxidation degree of CNTs is much higher than that of PDDACNTs.On the other hand,it can be seen obviously that there are redox current peaks at about 0.35 V on both CNTs and PDDA-CNTs electrodes.The faradic peak indicates the surface oxide formation due to the hydroquinone-quinone(HQ-Q)redox couple on the carbon nanotube surface.16,17The amount of surface oxides on the carbons can be quantified by the charge due to the above reaction.It is noted that the surface oxides of CNTs were obviously increased by 322.8%after corrosion test(Fig.5(a))and the increment is much higher than that occurred on the PDDA-CNTs(Fig.5(b))(61.6%),indicating further a higher oxidation degree on the CNT surface.These indicate that PDDA-CNTs have higher resistance to electrochemical corrosion than CNTs.It is widely recognized that the electrochemical oxidation of CNTs preferentially occurs at the defects on the CNTs.16,17On the surface of the PDDA-CNTs,PDDA molecules cover the original defect sites of CNTs and easily form a thin film to prevent the invasion of corrosion medium.The results from Fig.5 imply that the PDDA-CNTs may be the good catalyst support for the electrocatalysts with good longterm stability.
On the other hand,the dispersibility of CNTs in solvents is one of the key issues for the applications of CNTs.Here,the comparison of the dispersibility of CNTs-PDDA and pristine CNTs in water was carried out and the corresponding results are shown in Fig.6.It is noted that CNTs-PDDA can easily and uniformly disperse in water(Fig.6(a)).In comparison,it is hard for pristine CNTs to uniformly disperse in water and obvious aggregation and precipitation are observed(Fig.6(b)).This implies that the dispersibility of CNTs in water is greatly improved by the surface functionalization with PDDA because of the excellent hydrophilicity of PDDA.Moreover,the presence of PDDA molecules make the surface of CNTs create positive charge,which prevents aggregation of CNTs by electrostatic repulsion and induces stable CNT suspension in water.
Based on the above results,the excellent anti-corrosion ability,good dispersibility in aqueous solution and nitrogen-containing groups make PDDA-CNTs be the promising catalyst supports for noble metal NPs in fuel cells.
3.2 Deposition of Pt NPs on CNTs
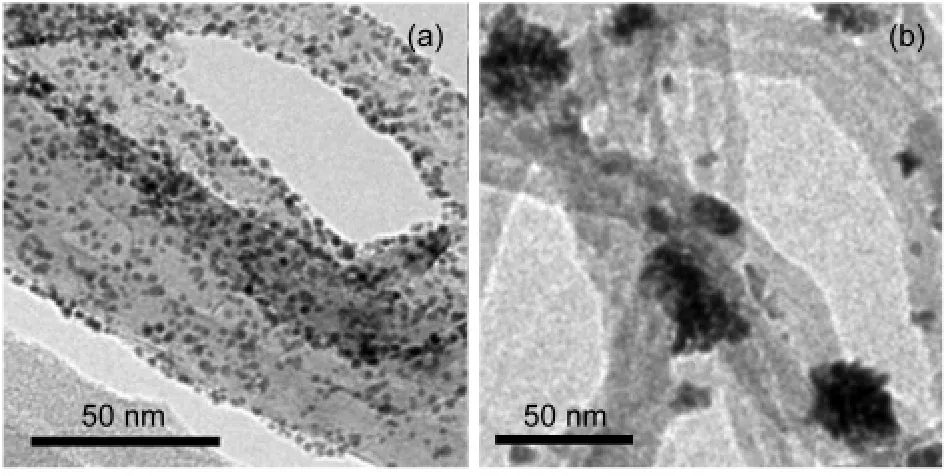
Fig.7 TEM images of Pt NPs in(a)Pt NPs/CNTs-PDDAand(b)Pt NPs/CNTs nanohybrids
Fig.7 shows the TEM images of the Pt NPs/CNTs-PDDA and Pt NPs/CNTs nanohybrids.As shown in Fig.7(a),lots of Pt NPs with an average particle size of(2.0±0.5)nm are uniformly dispersed on the surface of the CNTs-PDDA.It is noted that no aggregation of NPs is clearly observed on the CNT surface.In contrast,for the Pt NPs/CNTs nanohybrids,Pt NPs have a poor dispersion on the CNT surface and the diameter is much longer than that of Pt NPs in Pt NPs/CNTs-PDDA(Fig.7(b)).The reasons should be as follows:The pristine CNTs usually have some uneven defects generated during the growth and post-synthesis treatment of the CNTs.When PtCl62-is reduced by the defects of CNTs,Pt NPs tend to deposit on these localized defect sites,thus resulting in poor dispersion and aggregation.However,for the PDDA-functionalized CNTs,lots of PDDA molecules on the CNT surface introduce a uniform distribution of the ammonium groups that serve as functional groups for the self-assembly of Pt precursors.Furthermore,PDDA acts as a reductant10,13for thein-situreduction of PtCl62-and itself is simultaneously oxidized to another polymer containing higher valence of nitrogen element.10,13Therefore,a much more uniform distribution of Pt NPs is observed on the surface of the CNTs-PDDA.The ICP-AES results show that the amount of Pt loading in Pt NPs/CNTs-PDDA nanohybrids(17.5%)is higher than that in Pt NPs/CNTs nanohybrids(13.5%),implying the more effective reducing ability of CNTs-PDDA than that of the pristine CNTs.
3.3 Electrochemical properties
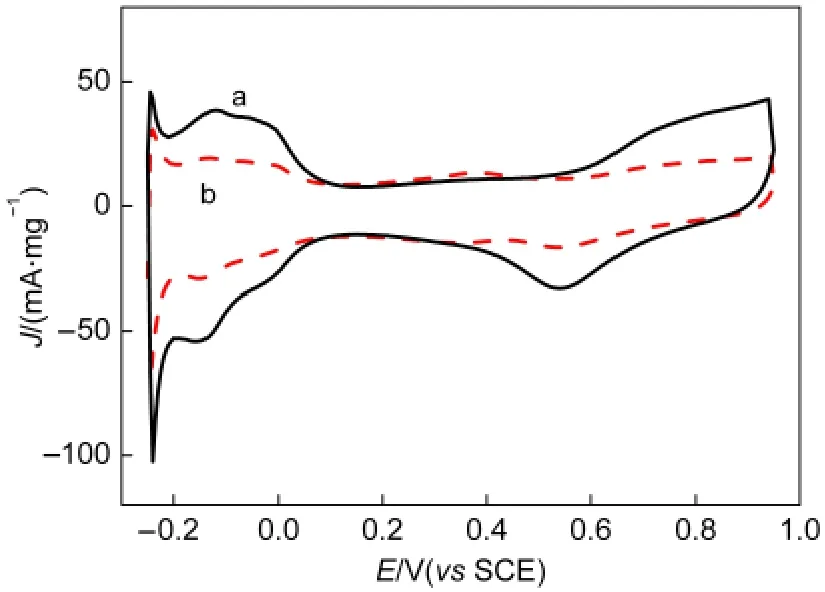
Fig.8 Cyclic voltammograms of(a)Pt NPs/CNTs-PDDAand(b)Pt NPs/CNTs nanohybrids in nitrogen-saturated 0.5 mol·L-1 H2SO4solution at a scan rate of 50 mV·s-1
Fig.8 shows the cyclic voltammograms of the Pt NPs/CNTs-PDDA and Pt NPs/CNTs nanohybrids in nitrogen-satu-rated 0.5 mol·L-1H2SO4solution.Based on the hydrogen adsorption-desorption charges,the values of the ESA of Pt NPs supported on the CNTs-PDDA and pristine CNTs could be calculated according to the following equation:18

where,QH(mC·cm-2)represents the mean value between the amounts of charge exchanged during the electro-adsorption and desorption of H2on Pt sites,[Pt]is the Pt loading(mg·cm-2)on the electrode and 0.21(mC·cm-2)represents the charge required to oxidize a monolayer of H2on bright Pt.The calculated ESA value of Pt NPs/CNTs-PDDA catalyst is 47.8 m2·g-1,higher than that of Pt NPs/CNTs catalyst(30.6 m2·g-1),most likely due to the much smaller particle size and better dispersion of Pt NPs on CNTs-PDDA.This also demonstrates that the Pt NPs deposited on the CNTs-PDDA are electrochemically more accessible,which is very important for electrochemical oxidation of methanol.
The electrochemical performance of the Pt NPs/CNTs-PDDA(curve a)and Pt NPs/CNTs catalysts(curve b)for methanol oxidation was investigated by cyclic voltammetry in nitrogen-saturated 0.5 mol·L-1H2SO4+1.0 mol·L-1CH3OH solution and the corresponding results are shown in Fig.9.Comparing Pt NPs/CNTs catalyst,the significant enhancement of the peak current of methanol oxidation can be observed on Pt NPs/CNTs-PDDA catalyst.It is noted that the forward peak current of methanol oxidation on the Pt NPs/CNTs-PDDA catalyst is 333 mA·mg-1and almost 1.6 times higher than that on the Pt NPs/CNTs catalyst(211 mA·mg-1).This indicates that Pt NPs/CNTs-PDDA catalyst has higher mass activity for methanol oxidation than Pt NPs/CNTs catalyst,due to the optimum particle diameter(ca 2 nm),19better dispersion and higher ESA of the Pt NPs on the Pt NPs/CNTs-PDDAcatalyst.
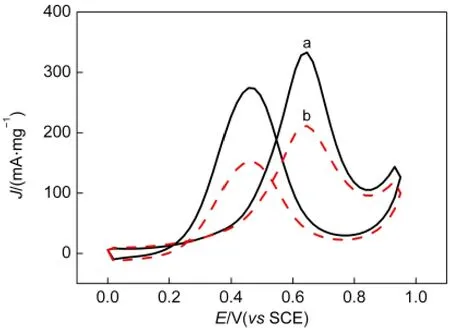
Fig.9 Cyclic voltammograms of(a)Pt NPs/CNTs-PDDAand(b)Pt NPs/CNTs nanohybrids in nitrogen-saturated 0.5 mol·L-1 H2SO4+1.0 mol·L-1CH3OH solution at a scan rate of 50 mV·s-1
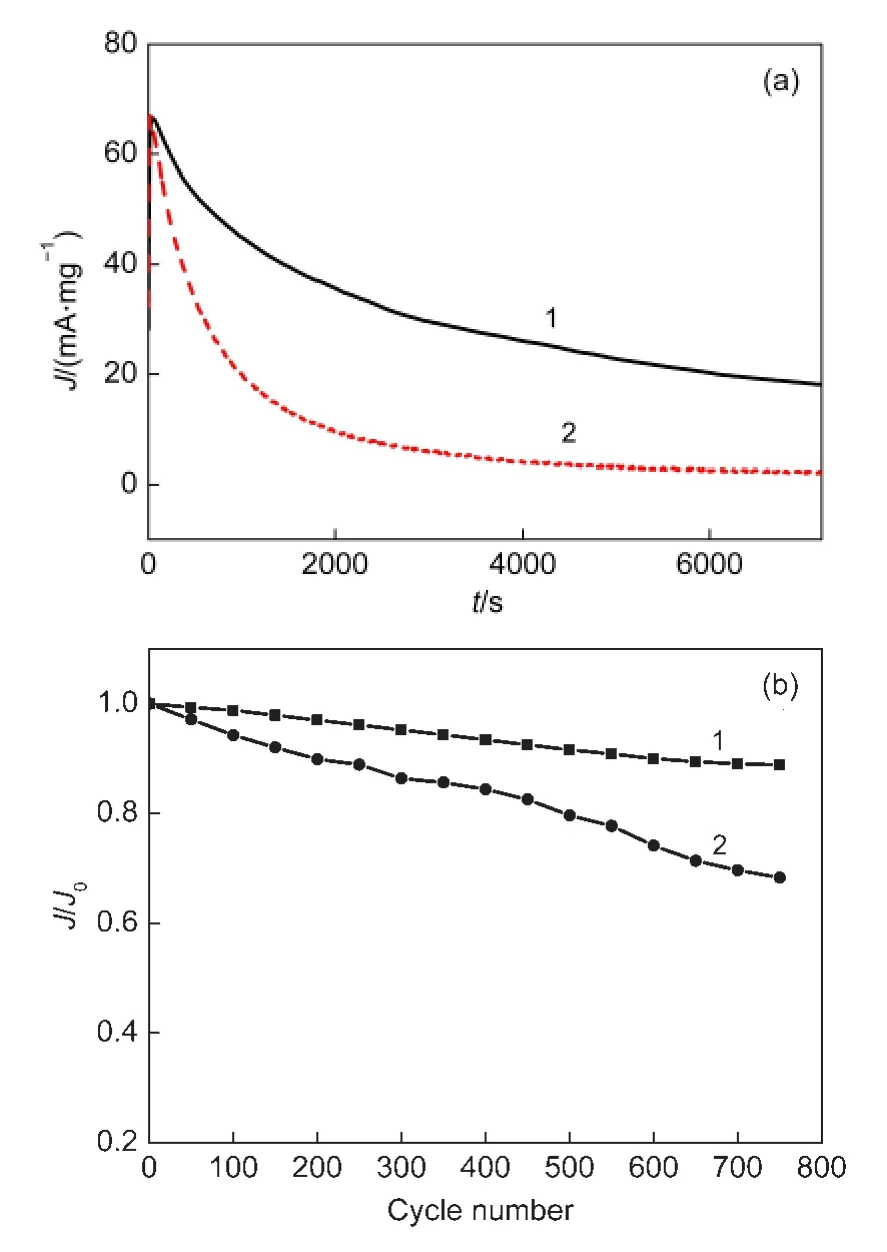
Fig.10 (a)Chronoamperometric curves of the different electrocatalysts at a fixed potential of 0.4 V,(b)the long-term cycle stability of the different electrocatalysts at a scan rate of 50 mV·s-1
In practical application,the long-term stability of the catalysts is of great importance and the chronoamperometry is a useful method for the evaluation of the stability of the electrocatalysts in fuel cells.The electrochemical stabilities of the Pt NPs/CNTs-PDDA and Pt NPs/CNTs catalysts were investigated and the results are shown in Fig.10(a).Within 2 h of operation,although the current gradually decays for both catalysts,the Pt NPs/CNTs-PDDA catalyst always shows a much larger methanol oxidation current than Pt NPs/CNTs.At the end,the steady-state current on the Pt NPs/CNTs-PDDA(18.1 mA·mg-1)is 8.8 times larger than that on the Pt NPs/CNTs(2.1 mA·mg-1),indicating a much better electrochemical stability of Pt NPs/CNTs-PDDAcatalyst.20
On the other hand,the long-term cycle stabilities of the Pt NPs/CNTs-PDDA and Pt NPs/CNTs catalysts were further investigated by cyclic voltammetry and the corresponding results are shown in Fig.10(b).The ratio between the peak current obtained from the forward anodic peak(J)and that at the first cycle(J0)was used to evaluate the long-term stability of the electrocatalysts.It can be observed that the value ofJ/J0decreases gradually with the successive scanning.After 750 cycles of CV,the value ofJ/J0is 88.8%for the Pt NPs/CNTs-PDDA nanohybrids,which is much higher than that for the Pt NPs/CNTs catalysts(67.3%).It implies that the electrochemical durability of the Pt NPs/CNTs-PDDA nanohybrids is much better than that of the Pt NPs/CNTs catalysts.The reason may be as follows:The interaction between Pt NPs and PDDA prevents Pt NPs from migrating/agglomerating on the carbon support and from detaching from the carbon support.20Moreover,a thin film of PDDA coated on CNTs is helpful to inhibit the electrochemical corrosion of CNTs under high potential and thus to re-strain the detachment of Pt NPs from CNTs.
4 Conclusions
In summary,We have developed an alternative strategy for the spontaneous deposition of Pt NPs on CNTs by noncovalent functionalization of the CNT surface with PDDA,which acts as the reductant for the Pt precursor(PtCl62-)and film-forming reagent to increase the corrosion resistance of CNTs,its oxidation products also stabilize the reduced Pt NPsin situ.In comparison with Pt NPs deposited on the pristine CNTs,the Pt NPs on the CNTs-PDDA showed a smaller particle size and better dispersion,which resulted in much-better electrocatalytic performance towards methanol electro-oxidation.
(1) (a)McGrath,K.M.;Prakash,G.K.S.;Olah,G.A.J.Ind.Eng.Chem.2004,10(7),1063.
(b)Aricò,A.S.;Srinivasan,S.;Antonucci,V.Fuel Cells2001,1(2),133.
(c)Liu,H.S.;Song,C.J.;Zhang,L.;Zhang,J.J.;Wang,H.J.;Wilkinson,D.P.J.Power Sources2006,155(2),95.
(2) (a)Colón-Mercado,H.R.;Kim,H.;Popov,B.N.Electrochem.Commun.2004,6(8),795.doi:10.1016/j.elecom.2004.05.028
(b)Liu,Z.L.;Ling,X.Y.;Su,X.D.;Lee,J.Y.J.Phys.Chem.B2004,108(24),8234.
(3) (a)Wang,J.J.;Yin,G.P.;Shao,Y.Y.;Zhang,S.;Wang,Z.B.;Gao,Y.Z.J.Power Sources2007,171(2),331.doi:10.1016/j.jpowsour.2007.06.084
(b)Rao,V.;Simonov,P.A.;Savinova,E.R.;Plaksin,G.V.;Cherepanova,S.V.;Kryukova,G.N.;Stimming,U.J.Power Sources2005,145(2),178.
(c)Wang,Z.B.;Yin,G.P.;Shi,P.F.Carbon2006,44(1),133.
(d)Zhao,Y.;E.Y.;Fan,L.Z.;Qiu,Y.F.;Yang,S.H.Electrochim.Acta2007,52(19),5873.
(e)Xu,Q.J.;Zhou,X.J.;Li,Q.X.Acta Phys.-Chim.Sin.2010,26(8),2135.[徐群杰,周小金,李巧霞,李金光.物理化學學報,2010,26(8),2135.]doi:10.3866/PKU.WHXB20100802
(4) Quinn,B.M.;Dekker,C.;Lemay,S.G.J.Am.Chem.Soc.2005,127(17),6146.doi:10.1021/ja0508828
(5) (a)Hernadi,K.;Siska,A.;Thiên-Nga,L.;Forró,L.;Kiricsi,I.Solid State Ionics2001,141-142,203.
(b)Li,Y.L.;Hu,F.P.;Wang,X.;Shen,P.K.Electrochem.Commun.2008,10(7),1101.
(c)Xu,H.;Zeng,L.P.;Xing,S.J.;Shi,G.Y.;Xian,Y.Z.;Jin,L.T.Electrochem.Commun.2008,10(12),1839.
(6) (a)Wang,S.Y.;Jiang,S.P.;Wang,X.Nanotechnology2008,19(26),265601.doi:10.1088/0957-4484/19/26/265601
(b)Wang,S.Y.;Jiang,S.P.;White,T.J.;Guo,J.;Wang,X.J.Phys.Chem.C2009,113(43),18935.
(c)Sanles-Sobrido,M.;Correa-Duarte,M.A.;Carregal-Romero,S.;Rodríguez-González,B.;álvarez-Puebla,R.A.;Hervés,P.;Liz-Marzán,L.M.Chem.Mater.2009,21(8),1531.
(7) Chen,J.;Wang,M.;Liu,B.;Fan,Z.;Cui,K.;Kuang,Y.J.Phys.Chem.B2006,110(24),11775.
(8) (a)Shrestha,S.;Liu,Y.;Mustain,W.E.Catal.Rev.2011,53(3),256.doi:10.1080/01614940.2011.596430
(b)Shao,Y.;Yin,G.;Gao,Y.J.Power Sources2007,171(2),558.
(9) Colmenares,L.C.;Wurth,A.;Jusys,Z.;Behm,R.J.J.Power Sources2009,190(1),14.
(10)Zhang,S.;Shao,Y.Y.;Liao,H.G.;Engelhard,M.H.;Yin,G.P.;Lin,Y.H.ACS Nano2011,5(3),1785.
(11) (a)He,W.;Zou,L.L.;Zhou,Y.;Lu,X.J.;Li,Y.;Zhang,X.G.;Yang,H.Chem.J.Chin.Univ.2012,33(1),133.[何 衛,鄒亮亮,周 毅,盧向軍,李 媛,張校剛,楊 輝.高等學校化學學報,2012,33(1),133.]
(b)He,W.;Jiang,H.J.;Zhou,Y.;Yang,S.D.;Xue,X.Z.;Zou,Z.Q.;Zhang,X.G.;Akins,D.L.;Yang,H.Carbon2012,50(1),265.
(c)Shen,X.F.;Chen,Q.;Pang,Y.H.;Cui,Y.;Qian,H.Sci.China Chem.2011,41(7),1184. 沈曉芳,陳 沁,龐月紅,崔 燕,錢 和.中國科學:化學,2011,41(7),1184.]
(d)Qin,X.;Wang,H.;Wang,X.;Miao,Z.;Chen,L.;Zhao,W.;Shan,M.;Chen,Q.Sensors and Actuators B:Chemical2010,147(2),593.
(12) (a)Chakraborty,S.;Raj,C.R.Carbon2010,48(11),3242.doi:10.1016/j.carbon.2010.05.014
(b)Yang,D.Q.;Rochette,J.F.;Sacher,E.J.Phys.Chem.B2005,109(10),4481.
(13)Chen,H.J.;Wang,Y.L.;Wang,Y.Z.;Dong,S.J.;Wang,E.Polymer2006,47(2),763.doi:10.1016/j.polymer.2005.11.034
(14)Wang,S.Y.;Yu,D.S.;Dai,L.M.J.Am.Chem.Soc.2011,133(14),5182.
(15)Hsin,Y.L.;Hwang,K.C.;Yeh,C.T.J.Am.Chem.Soc.2007,129(32),9999.
(16) Li,L.;Xing,Y.J.Electrochem.Soc.2006,153(10),A1823.
(17)(a)Wang,J.;Yin,G.;Shao,Y.;Wang,Z.;Gao,Y.J.Power Sources2008,176(1),128.doi:10.1016/j.jpowsour.2007.10.057
(b)Li,L.;Xing,Y.J.Power Sources2008,178(1),75.
(18) Pozio,A.;De Francesco,M.;Cemmi,A.;Cardellini,F.;Giorgi,L.J.Power Sources2002,105(1),13.
(19) (a)Leger,J.M.;Lamy,C.Berichte der Bunsengesellschaft für Physikalische Chemie1990,94(9),1021.doi:10.1002/bbpc.v94:9
(b)Hamnett,A.Catal.Today1997,38(4),445.
(20)Zhang,S.;Shao,Y.Y.;Yin,G.P.;Lin,Y.H.J.Mater.Chem.2009,19(42),7995.doi:10.1039/b912104h

