青藏高原紅景天屬植物藥材中五種有效成分HPLC分析
遲曉峰,矯曉麗,董 琦,肖遠燦,胡風(fēng)祖*
1中國科學(xué)院西北高原生物研究所,西寧810008;2中國科學(xué)院研究生院,北京100049
Introduction
Rhodiola rosea,also known as“golden root”or“roseroot”belongs to the plant family Crassulaceae,grows at elevated altitudes in the Arctic and in mountainous regions throughout Europe and Asia[1].The genus Rhodiola L.(Crassulaceae)consists of nearly 200 species in which at least 20 species are commonly used in traditional medical practice in Eastern Europe and Asia especially in traditional Tibetan medicine in China[2-4].R.rosea has been most intensively studied in Russia and Scandinavia,where it has been purported to stimulate the nervous system,decrease depression,enhance work performance,eliminate fatigue,and prevent high altitude sickness[5-10].
In China,Rhodiola crenulata H.Ohba is the only authorized herb according to Chinese Pharmacopoeia[11].However,in recent years,increased commercial demands of the source had seriously threatened its survival.In view of this,to find suitable substitute in the Qinghai-TibetPlateau becomes extremely crucial.Rhodiola rosea contains flavonoids,monoterpenes,triterpenes,phenolic acids,and phenylethanol derivatives such as salidroside and tyrosol specific to this plant.Significant antioxidant activities have been documented for the extracts of various Rhodiola species,which have been attributed to a variety of antioxidant compounds including gallic acid,catechin and epocatechin[12].Based on this,in this paper Salidroside,tyrosol,gallic acid,catechin and epicatechin were selected as the typical and pharmacologically components to evaluate the quality of several medicinal plants belonging to the Rhodiola L.Genus from Qinghai-Tibetan Plateau.In this paper a fast and precise HPLC-DAD method was developed for simultaneous determination of total Salidroside,tyrosol,gallic acid,catechin and epicatechin in several medicinal plants belonging to the Rhodiola L.Genus from Qinghai-Tibetan Plateau aiming to identify distributional differences and explore the potential resources of the Rhodiola.
Experimental
Chemicals and materials
The reference standard of Salidroside,tyrosol,gallic acid,catechin and epicatechin were purchased from the National Institute for the Control of Pharmaceutical and Biological Products(Beijing,China).
Methanol was of HPLC grade and obtained from Merck (Germany),while all other chemicals used were of analytical grade.Water was purified on a Milli-Q system (Millipore,Bedford,MA)and used throughout the study.Thirteen samples of different species of Rhodiola were selected for analysis(Table 1).Samples used in this study were collected directly at different parts of Qinghai-Tibetan Plateau.At each sampling site the sample was acquired from different positions more than 1m apart,and at least five individual plants were collected randomly to ensure representative sampling.The samples were identified by Professor Chen shi-long in Northwest Institute of Plateau Biology of Chinese Academy of Sciences.

Table 1 Description of the Rhodiola samples
Apparatus and chromatographic conditions
Five bioactive compounds in fourteen species of Rhodiola were analyzed by HPLC.The HPLC system consisted of a Waters(Milford,MA,USA)515 pumps coupled to a Waters 2996 diode array detector.Chromatographic separation of five bioactive compounds was achieved on a Phenomenex Luna C18(250×4.6 mm,5 μm).The temperature of the column was maintained at 30°C.The mobile phase consisted of solvent A(methanol)and B(deionized water).A gradient elution program was used as follows:0-5 min 10%-20%A(v/v,linear gradient),5-40 min 20%-70%A(v/v,linear gradient)with the flow rate of 1.0 mL min-1.

Fig.1 Typical HPLC chromatograms of(A)mixed standards and(B)Rhodiola crenula.Gallic acid(1),salidroside (2),tyrosol(3),epicatechin(4)and catechin(5).
Preparation of standard solutions
The standard of five bioactive contents were accurately weighed and then dissolved in methanol to produce stock solutions,which were diluted to appropriate concentration for the construction of calibration curves.All standard and sample solutions were kept at 4°C in the refrigerator.
Preparation of sample solutions
The radix of plant material was powdered and sieved through a 0.315 mm sieve.An accurately weighed sample(200 mg)was put into a conical flask and then extracted respectively with 40 mL of 50%aqueous methanol by sonication for 40 min at 40°C.The solutions were filtered through a 0.45 μm nylon membrane filter before subjecting 10 μL aliquots to HPLC analysis.Determinations were performed after three separate extractions of each sample,and each extract was injected in triplicate.
Results and Discussion
Method development
Several chromatographic columns including Phenomenex Luna-C18column(250×4.6 mm,5 μm),Phenomenex Gemini-C18column(250×4.6 mm,5 μm),Waters Symmetry-C18column(150×3.9 mm,5 μm) were tested,and the current column proved to be the best in this application.A variety of mobile phases were investigated to optimize the chromatographic conditions for the analysis of the five bioactive components.The methanol-deionized water system was chosen as mobile phases because of its better separation,resolution and shorter duration for the five contents than others.A gradient elution method was developed for the separation because of the differences in polarity,solubility and other characteristics of the five components.The gradient program was optimized as followed:10%-20% (methanol)in 0-5 min,20%-70%(methanol)in 5-40 min with the flow rate of 1.0 mL min-1.All the components can be separated within 20 min under this condition.
Validation of developed method
Linearity and Limit of Detection
Standard stock solutions containing the five analytes were prepared and diluted to appropriate concentrations for plotting the calibration curves.The regression curves were obtained from six concentration levels and then the calibration curves were constructed by plotting the peak areas versus the concentration of each analytes.The limit of detection(LOD)and the limit of quantification(LDQ),defined as the lowest concentration that could be measured with accuracy and precision.The minimum concentration,which could be calculated at S/N=3 and 10,respectively.The calculated results are summarized in Table 2.All the analytes showed good linearity(R>0.999)in the investigated ranges.The LOD and LOQ of the eleven analytes were 0.02-0.12 and 0.06-0.35 μg/mL,respectively.
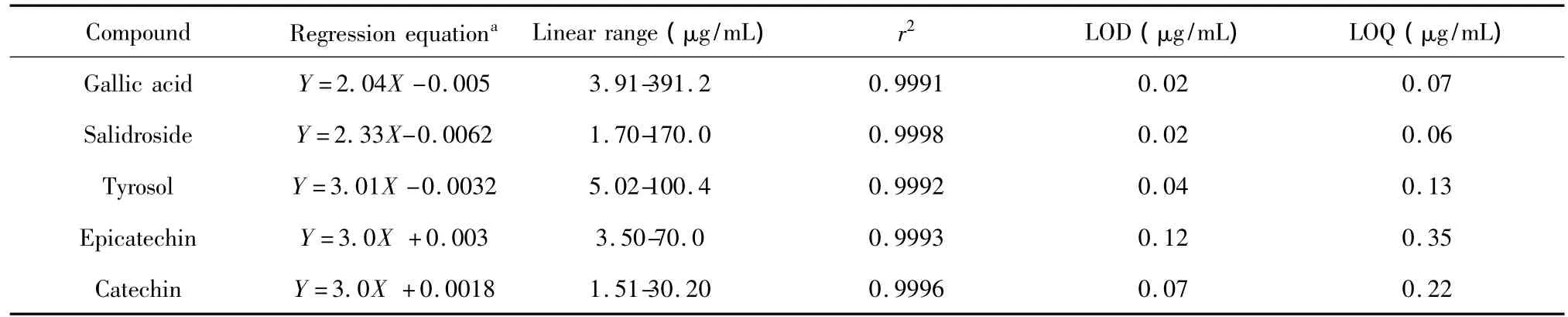
Table 2 Linear regression data,LOD and LOQ of five compounds
Accuracy and Precision
Recovery test using the addition of known amounts of standard mixture solution of the five bioactive components to the marked sample was applied to determine the accuracy of the method.These mixtures were analyzed by the proposed method and the experiment was performed in triplicate and recoveries(%),RSD(%) were calculated.The intra-day and inter-day precisions were investigated by determining a mixed standard solution in six replicates during a single day and by du-plicating the experiments on 3 consecutive days.All the data were shown in Table 3.
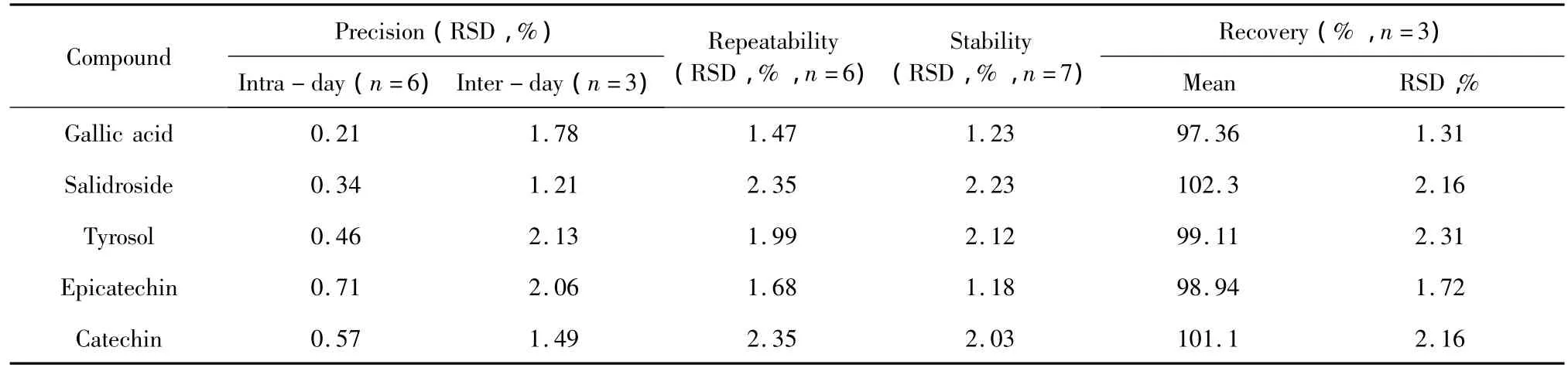
Table 3 Precision,repeatability,stability and recovery of 5 compounds
Repeatability and stability
To further evaluate the repeatability of the developed method,each extract was analyzed in six replicates.Stability of sample solution was analyzed at 0,2,4,8,12,24 and 48 h within 2 days at room temperature,respectively.No significant change was observed in the five components indicating the solution was stable for at least 48 h under room temperature.
Sample analysis The developed method was applied to the simultaneous quantification of Salidroside,tyrosol,gallic acid,catechin and epicatechin in Rhodiola of different species.The five contents in samples were identified by comparing the retention time and the UV-Vis spectra of standards to those in samples.The typical chromatograms are shown in Fig.1 and the contents of each sample were calculated and shown in Table 4.
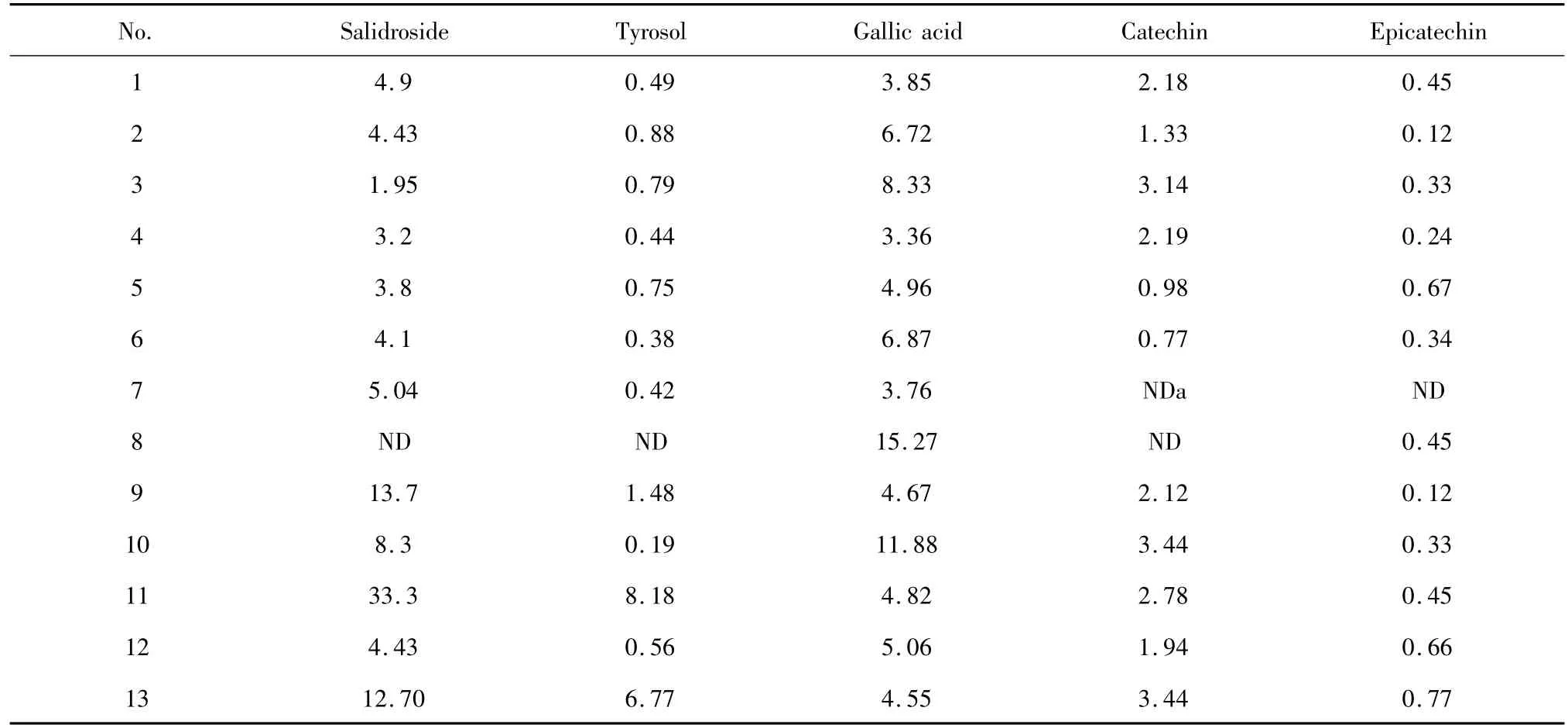
Table 4 Contents(mg/g)of five bioactive compounds in thirteen different species of Rhodiola
Table 4 shows that considerable differences were found for the five components within the 13 species of Rhodiola L.Salidroside(0-33.33 mg/g)and tyrosol(0-8.18 mg/g)were found in all the samples except sample 8.The highest content of Salidroside was found in sample 11 and the content was extremely higher than others,while the high content of tyrosol was also found in the high Salidroside samples.Gallic acid(3.36-15.27 mg/g)was found in all the 12 samples with the highest content found in sample 8 which lack of salidroside and tyrosol.Among the analyzed compounds,salidroside and gallic acid were the two major compounds while epicatechin(0-0.77 mg/g)was the least component.Catechin(0-3.44 mg/g),also an impor-tant component was not detected in sample 7 and 8.
Conclusion
A method has been established for estimation of five components in fourteen different species of Rhodiola.Chemical polymorphism was found in the fourteen species belonging to the Rhodiola L.Genus in the Qinghai-Tibetan Plateau.
1 Saratikov SA,Krasnov EA,Rhodiola rosea is a Valuable Medicinal Plant(Golden root).Monograph Tomsk State University Press,Tomsk,1987.252.
2 Bao W,et al.People’s Military Medical Press,China,2003.1-12.
3 Northwest institute of plateau biology of Chinese Academy of Sciences,Tibetan Medicine.Qinghai peoples’Press,China,1991.432-434.
4 Richard PB,Patricia LG,Zakir Ramazanov.Rhodiola rosea a Phytomedical Overview.Herbal Gram,2002,56:40-52.
5 Shevtsov VA,Zholus BI,Shervarly VI,et al.A randomized trial of two different doses of a SHR-5 extract versus placebo and control of capacity for mental work.Phytomedicine,2003,10:95-105.
6 Kanupriya,Prasad D,Sai RM,et al.Cytoprotective and antioxidant activity of Rhodiola imbricata against tert-butyl hydroperoxide induced oxidative injury in U-937 human macrophages.Mol Cell Biol,2005,275:1-6.
7 Lia TL,Xua GH,Wu LL,et al.Pharmacological studies on the sedative and hypnotic effect of salidroside from the Chinese medicinal plant Rhodiola sachalinensis.Phytomedicine,2007,14:601-604.
8 Qu ZQ,et al.Pretreatment with Rhodiola Rosea extract reduces cognitive impairment induced by intracerebroventricular streptozotocin in rats:implication of anti-oxidative and neuroprotective effects.Biomed Environ Sci,2009,22:318-326.
9 Chen X,et al.Salidroside attenuates glutamate-induced apoptotic cell death in primary cultured hippocampal neurons of rats.Brain Res,2008,1238:189-198.
10 Samuel ES,et al.Protection of human cultured cells against oxidative stress by Rhodiola rosea without activation of antioxidant defenses.Free Radical Biol Med,2009,47:577-584.
11 Pharmacopoeia Commission of PRC,Pharmacopoeia of the People’s Republic of China,vol.I.Beijin:Chemical Industry Press,2005.106.
12 Ohsugi M,et al.Active-oxygen scavenging activity of traditional nourishing-tonic herbal medicines and active constituents of Rhodiola sacra.J Ethnopharm,1999,1:111-119.

