釩改性鋰離子電池正極材料LiFe0.5Mn0.5PO4/C電化學(xué)性能
龔 強 王 紅 廖小珍 麻 微 何雨石 馬紫峰
(上海交通大學(xué)化學(xué)化工學(xué)院化學(xué)工程系,電化學(xué)與能源技術(shù)研究所,上海200240)
釩改性鋰離子電池正極材料LiFe0.5Mn0.5PO4/C電化學(xué)性能
龔 強 王 紅 廖小珍*麻 微 何雨石 馬紫峰
(上海交通大學(xué)化學(xué)化工學(xué)院化學(xué)工程系,電化學(xué)與能源技術(shù)研究所,上海200240)
采用高溫固相反應(yīng),以NH4VO3為釩源合成了化學(xué)計量式為(1-x)LiFe0.5Mn0.5PO4-xLi3V2(PO4)3/C(x=0, 0.1,0.2,0.25,1)的釩改性磷酸錳鐵鋰正極材料.電化學(xué)測試表明釩改性能明顯提高磷酸錳鐵鋰材料的充放電性能,其中x=0.2時得到的0.8LiFe0.5Mn0.5PO4-0.2Li3V2(PO4)3/C(標(biāo)記為LFMP-LVP/C)材料電化學(xué)性能最好,其0.1C倍率時的放電比容量為141 mAh·g-1.X射線衍射(XRD)分析指出LFMP-LVP/C材料的微觀結(jié)構(gòu)為橄欖石型LiFe0.5Mn0.5PO4/C和NASICON型Li3V2(PO4)3組成的雙相結(jié)構(gòu).能量色射X射線譜(EDS)分析結(jié)果指出,Fe、Mn、V、P元素在所合成材料中的分布非常均勻,表明所制備材料成分的均一性.Li3V2(PO4)3改性使材料的電導(dǎo)率明顯提高.LiFe0.5Mn0.5PO4的電導(dǎo)率為1.9×10-8S·cm-1,而LFMP-LVP材料電導(dǎo)率提高到2.7×10-7S·cm-1.與純Li3V2(PO4)3的電導(dǎo)率(2.3×10-7S·cm-1)相近.電化學(xué)測試表明釩改性使LFMP-LVP/C材料充放電過程電極極化明顯減小,從而電化學(xué)性能得到顯著提高.本文工作表明Li3V2(PO4)3改性可成為提高橄欖石型磷酸鹽鋰離子電池正極材料電化學(xué)性能的一種有效方法.
鋰離子電池; LiFe0.5Mn0.5PO4; Li3V2(PO4)3改性; 正極材料
1 Introduction
Over the past few years,there have been considerable activities devoted to finding new cathode materials for next-generation lithium batteries,particularly for potential use in hybrid electric or pure electric vehicles.1-5Olivine-structured LiFePO4compound has been well investigated as promising cathode materials for rechargeable lithium-ion batteries due to its relatively high theoretical capacity of 170 mAh·g-1and good environmental compatibility and safety.3Recently,another olivinestructured compound LiMnPO4has also attracted much attention due to its high discharge potential versus Li/Li+(4.1 V).6-9However,the practical application of LiMnPO4is limited owing to its very low electrical conductivity and the intrinsically unstable characteristic of pure MnPO4due to Jahn-Teller distortions around trivalent Mn.9In order to overcome these restrictions,much effort has been devoted to the mixed-metal olivine compounds Li(FeyMn1-y)PO4.10-15It is suggested that the ionic and electrionic conductivities were enhanced in mixed-metal olivine structures. However, the rate performance of Li(FeyMn1-y)PO4is still needed to be further improved.
NASICON structure Li3V2(PO4)3presents high theoretical capacity(197 mAh·g-1)and higher electronic conductivity compared with olivine type phosphates.16,17Partly introducing the NASICON structure Li3V2(PO4)3into LiFePO4has recently been investigated as an alternative way to enhance the rate performance and cycling stability of this cathode material.18-23It was reported that the electrochemical activity of LiFePO4can be improved significantly when Li3V2(PO4)3was used as an additive in LiFePO4-based materials.The NASICON-type structure of Li3V2(PO4)3enables Li+ions to transport in a three-dimensional pathway,which leads to fast Li+diffusion.16This makes the LiFePO4-based materials with the addition of Li3V2(PO4)3display more excellent high-rate performance.The aim of the present article is to investigate the effect of vanadium modification on the structure and electrochemical properties of LiFe0.5Mn0.5PO4/C material(labeled as LFMP/C)by preparing composite materials with the nominal composition of (1-x)LiFe0.5Mn0.5PO4-xLi3V2(PO4)3/C(x=0,0.1,0.2,0.25,1)using a solid-state reaction.
2 Experimental
Vanadium modified LiFe0.5Mn0.5PO4/C cathode materials with the nominalcomposition of (1-x)LiFe0.5Mn0.5PO4-xLi3V2(PO4)3/C(x=0,0.1,0.2,0.25,1)were prepared using a solid-state reaction process.Stoichiometric amounts of FeC2O4· 2H2O(99%,Aldrich),Mn(AC)2·4H2O(99%,Sinopharm Chemical Reagent Co.,Ltd.),NH4VO3(99%,Sinopharm Chemical Reagent Co.,Ltd.),LiH2PO4(99%,Sinopharm Chemical Reagent Co.,Ltd.),and oxalic acid(99%,Sinopharm Chemical Reagent Co.,Ltd.;used as reducing agent)were used as source materials.Citric acid(C6H8O7·H2O,6%(mass fraction,w)with respect to the total amount of above raw materials)was added as the carbon source.The powders were mixed thoroughly by high energy ball-milling using acetone as medium.The obtained slurry was dried at 80°C and then calcinated at 750°C for 8 h in Ar atmosphere to yield the(1-x)LiFe0.5Mn0.5PO4-xLi3V2(PO4)3/C(x=0,0.1,0.2,0.25,1)composites.LiFePO4/C sample was also prepared using the same method in order to conduct the comparative study.The sample products were characterized by powder X-ray diffraction(XRD,D/max-2200/PC, Rigaku,Japan).The grain size and morphology were investigated using a field emission scanning electron microscope (FE-SEM,JSM-7401F,JEOL Ltd.,Japan).The carbon content of the product sample was analyzed by a CHNS/O analyzer (PE 2400 II,Perkin Elmer,America).
Electrochemical tests for the samples were carried out using coin cells(R2016)with Li metal foil as the counter electrode and 1.0 mol·L-1LiPF6/EC+DMC+EMC(1:1:1,volume ratio) electrolyte.The cathode consisted of 75%(w)active material, 15%(w)carbon black,and 10%(w)PVDF binder.The active material loaded on the electrode disks was about 3.5 mg·cm-2. Charge-discharge performance of the cells was evaluated using a battery test system(LAND CT2001 A model,Wuhan Jinnuo Electronic Co.,Ltd.).The cyclic voltammetry of the cells was performed on Solartron SI1287.Electrochemical impedance spectroscopy(EIS)was performed using a Solatron 1260 electrochemical interface combined with a Solatron 1287 frequency response analyzer with a frequency ranging from 105to 1 Hz.
3 Results and discussion
Fig.1 presents the charge-discharge profiles of the prepared (1-x)LiFe0.5Mn0.5PO4-xLi3V2(PO4)3/C(x=0,0.1,0.2,0.25,1)samples at a current density of 16 mA·g-1(0.1C rate).It can be seen that Li3V2(PO4)3/C cathode showed obviously higher discharge potential than LiFe0.5Mn0.5PO4/C cathode.Vanadium modification not only enhanced the discharge potentials but also increased the specific capacities of the LiFe0.5Mn0.5PO4-based materials.The specific capacity of(1-x)LiFe0.5Mn0.5PO4-xLi3V2(PO4)3/C sample increased with increasing of Li3V2(PO4)3content till x=0.2,and then the specific capacity decreased with further increasing of Li3V2(PO4)3content.The 0.8LiFe0.5Mn0.5PO4-0.2Li3V2(PO4)3/C sample demonstrated the best electrochemical performance with the highest discharge capacity of 141 mAh·g-1,while the specific capacities of LiFe0.5Mn0.5PO4/C and Li3V2(PO4)3/C were 133 and 120 mAh·g-1,respectively.Thus, in the following part of the paper,we focus on the investigation of 0.8LiFe0.5Mn0.5PO4-0.2Li3V2(PO4)3/C sample(labeled as LFMP-LVP/C)compared with pristine LiFe0.5Mn0.5PO4/C(labeled as LFMP/C).
Fig.2 shows the powder XRD patterns of the LFMP-LVP/C, LiFe0.5Mn0.5PO4/C,Li3V2(PO4)3/C(LVP/C),and LiFePO4/C samples.The XRD pattern of LiFe0.5Mn0.5PO4/C is in good agreement with the pattern of LiFePO4/C,except very slightly shifts of diffraction peaks to smaller 2θ angles.It was reported that LiFe1-xMnxPO4(0≤x≤1)compounds showed single phase sol-id-solution characteristics at the whole manganese substitution range(0≤x≤1),24which are isostructural with their LiFePO4and LiMnPO4end-members.For LFMP-LVP/C sample,a dual phase including LiFe0.5Mn0.5PO4and Li3V2(PO4)3was detected according to the XRD pattern.It is obvious that the resulting LFMP-LVP/C sample is not a solid solution of multi-transition-metal phosphates,but a mixture of LiFe0.5Mn0.5PO4and Li3V2(PO4)3.In addition,no diffraction peaks were observed from crystalline carbon(graphite).Therefore the carbon was in an amorphous state.The residual carbon content was 3.6%(w) measured by CHNS/O analyzer.
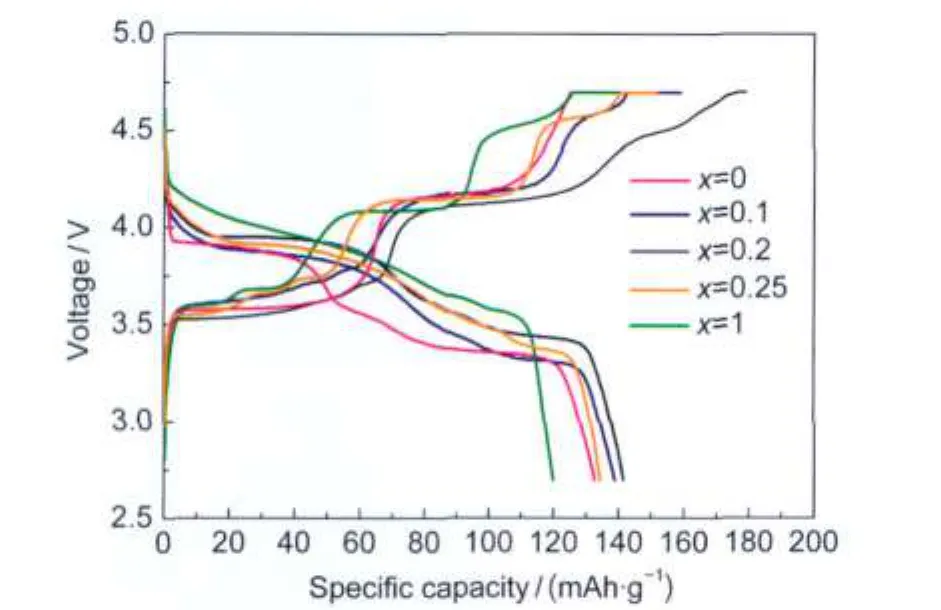
Fig.1 Charge-discharge profiles of(1-x)LiFe0.5Mn0.5PO4-xLi3V2(PO4)3/C(x=0,0.1,0.2,0.25,1)samples at 0.1C rate
Scanning electron micrographs of the LFMP/C and LFMPLVP/C samples are shown in Fig.3.It can be seen that most of the particle sizes of LFMP-LVP/C sample(Fig.3b)are distributed in the range of 200-500 nm.It is interesting to find that the LFMP/C sample(Fig.3a)shows finer particle size than LFMP-LVP/C sample.EDS mapping results corresponding to Fe Kα,Mn Kα,V Kα,and P Kαin LFMP-LVP/C presented in Fig.3c show fairly uniform distribution of these elements in LFMP-LVP/C composite,indicating that the LiFe0.5Mn0.5PO4and Li3V2(PO4)3phases in LFMP-LVP/C were well dispersed and mixed.
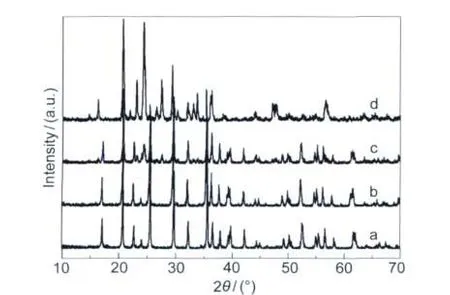
Fig.2 XRD patterns of(a)LiFePO4/C,(b)LFMP/C, (c)LFMP-LVP/C,and(d)LVP/C
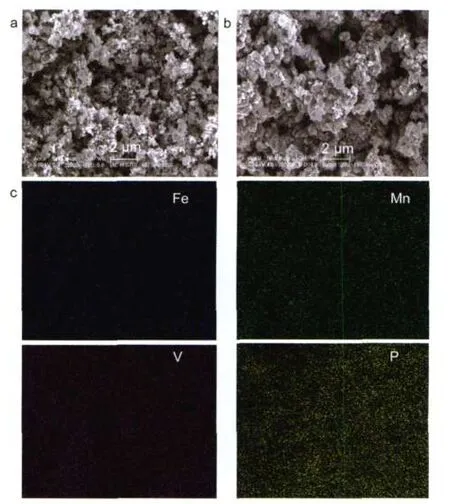
Fig.3 SEM images of(a)LFMP/C and(b)LFMP-LVP/C; (c)EDS mapping results for Fe,Mn,V,Pelements in LFMP-LVP/C sample
The cyclic voltammetries(CV)of LFMP-LVP/C and LFMP/ C samples at a scan rate of 0.2 mV·s-1between 2.7 and 4.7 V was conducted in order to study the effect of vanadium modification on the LiFe0.5Mn0.5PO4-based material.As shown in Fig.4,the CV curve of LFMP/C presents two oxidation peaks at around 3.85 and 4.31 V,corresponding to the Fe2+/Fe3+and Mn2+/Mn3+redox couples,respectively.During the cathodic sweep,Mn3+is reduced to Mn2+at around 3.77 V while Fe3+is reduced to Fe2+at around 3.17 V.For LFMP-LVP/C sample, the voltammetry curve shows three oxidation peaks at 3.76, 4.25,and 4.59 V.It was reported in literature25that LVP/C showed four oxidation peaks at 3.60,3.72,4.09,and 4.57 V. The first three current peaks were assigned to the V3+/V4+redox couple based on the removal of two Li+ions.The peak at 4.57 V was associated to the last lithium extraction,which led to the formation of the completely lithium free phase V2(PO4)3.Here in the voltammetry curve for LFMP-LVP/C sample,the volt-age values of oxidation and reduction for V3+/V4+redox couple overlapped with those of Fe and Mn ions,which are corresponding to the two broad oxidation peaks around 3.76 and 4.25 V,and two broad reduction peaks around 3.33 and 3.78 V. The LFMP-LVP/C sample showed slightly higher peak current density and decreased potential separations between the anodic and cathodic peaks.This result indicates a reduced electrode polarization during electrochemical reaction process for LFMPLVP/C sample.
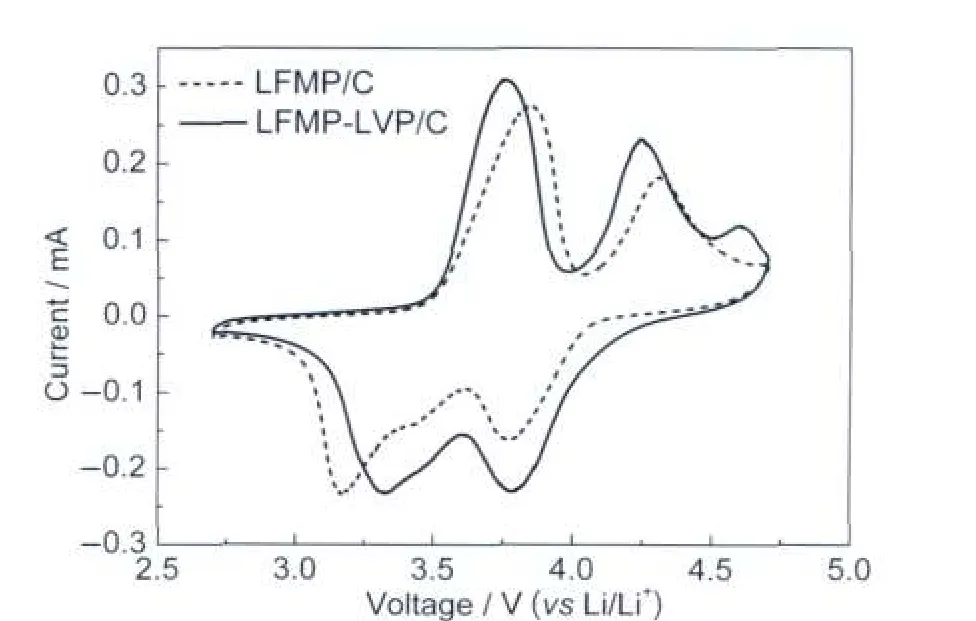
Fig.4 Cyclic voltammetries of LFMP-LVP/C and LFMP/C samples at a scanning rate of 0.2 mV·s-1
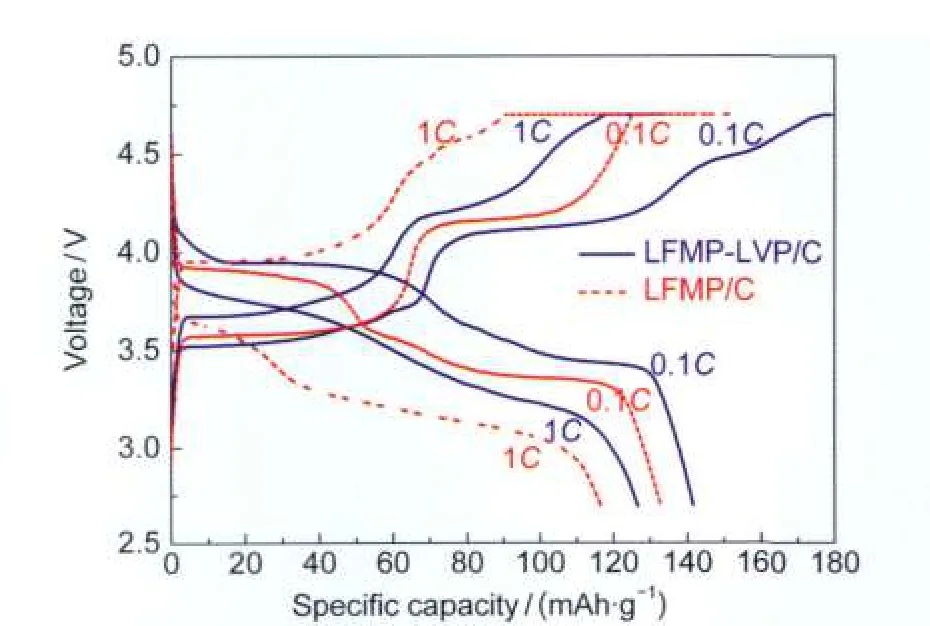
Fig.5 Charge-discharge profiles of the first cycle at 0.1C and 1C rates
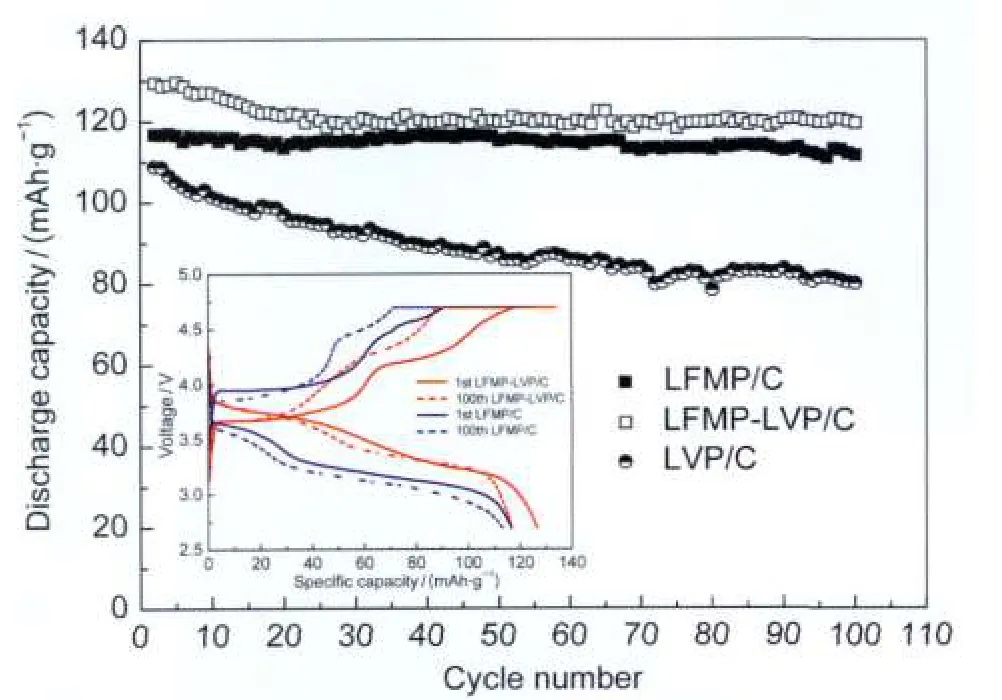
Fig.6 Cycling behaviors of Li|LFMP-LVP/C and Li|LFMP/C cells at 1C rate
The charge-discharge and cycling behaviors of Li|LFMPLVP/C and Li|LFMP/C cells were compared as shown in Fig.5 and Fig.6,respectively.The cells were first activated at 0.1C rate for two cycles and then cycled at 1C rate.A constant current-constant voltage(CC-CV)charging mode was used.The potential limits for the tests were 2.7 and 4.7 V.Fig.5 displays the initial charge-discharge profiles of the cells at 0.1Cand 1C rates,respectively.It is clear that the LFMP-LVP/C sample shows obviously improved charge-discharge performance.The initial discharge capacities of LFMP-LVP/C are 141 mAh·g-1(0.1C)and 127 mAh·g-1(1C),while the LFMP/C sample shows a capacity of 133 mAh·g-1(0.1C)and 117 mAh·g-1(1C).The discharge potential of LFMP-LVP/C cathode was 0.3-0.5 V higher than that of LFMP/C cathode under 1Crate. Fig.6 shows the cycling performances of the Li|LFMP-LVP/C, Li|LFMP/C,and Li|LVP/C cells at 1C charge-discharge rate.It can be seen that both LFMP-LVP/C and LFMP/C samples exhibit satisfying long term cycling stabilities,which are superior to that of LVP/C.The slightly capacity decrease in the first 20 cycles for the LFMP-LVP/C sample is due to the irreversible extraction of the third Li+ion in the Li3V2(PO4)3phase at high charge potential corresponding to the irreversible anodic peak at 4.59 V on the CV curve.After 20 cycles the LFMP-LVP/C sample shows very good cycling stability with discharge capacity of 120 mAh·g-1after 100 cycles,which is higher than the value(111 mAh·g-1)of LFMP/C sample.The most noticeable effect of vanadium modification is the obvious enhancement of the discharge potential for LFMP-LVP/C cathode with respect to pristine LFMP/C as shown in the inset figure in Fig.6.
To further understand the effect of vanadium modification, electrochemical impedance spectroscopy(EIS)was also applied.Fig.7 shows the Nyquist plots of the Li|LFMP-LVP/C and Li|LFMP/C cells in the completely charged state after ten cycles at 0.1C.The Nyquist plots for the cells are comprised of a depressed semicircle in high-to-medium frequency range and a line inclined at constant angle to the real axis in the lower frequency range.The depressed semicircle in the higher frequency range is mainly related to the complex reaction process at the electrolyte/electrode interface.The inclined line in the lower frequency range is attributed to the Warburg impedance, which is associated with lithium-ion diffusion through the cathode material.It can be seen that the semicircle of the LFMPLVP/C sample is obviously smaller than that of the LFMP/C sample.It means that the charge transfer resistance of LFMPLVP/C is reduced compared with that of LFMP/C,which indicates an easier Li+insertion/extraction in the LFMP-LVP/C cathode.
In order to clarify the influence of Li3V2(PO4)3additive on the LiFe0.5Mn0.5PO4-based material,the electrical conductivities of LiFe0.5Mn0.5PO4and LFMP-LVP were measured by four-probe dc technique.Pristine LiFe0.5Mn0.5PO4showed an electrionic conductivity of 1.9×10-8S·cm-1.The conductivity of LFMP-LVP reached a value of 2.7×10-7S·cm-1,which is similar to the value(2.3×10-7S·cm-1)of Li3V2(PO4)3.It is clear that the improved performance of LFMP-LVP/C sample is contributed from the substantial increase in electronic conductivity of LFMP-LVP dual phase and the chemical effect of LVP on the LFMP compound.
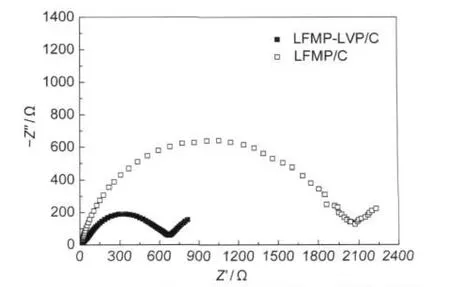
Fig.7 Nyquist plots of the Li|LFMP-LVP/C and Li|LFMP/C cells in the completely charged state after ten cycles at 0.1C
4 Conclusions
Vanadium modified LiFe0.5Mn0.5PO4-based cathode materials were synthesized by a solid-state reaction route.The introduction of Li3V2(PO4)3led to an increased reversible capacity and reduced polarization of LiFe0.5Mn0.5PO4-based materials.The discharge potential of the 0.8LiFe0.5Mn0.5PO4-0.2Li3V2(PO4)3/C cathode was 0.3-0.5 V higher than that of LiFe0.5Mn0.5PO4/C cathode operated at 1Crate with an initial discharge capacity of 127 mAh·g-1.Vanadium modification could be an effective method for improving electrochemical performance of the olivine-type cathode materials.
(1)Tarascon,J.M.;Armand,M.Nature 2008,451,652.
(2)Whittingham,M.S.Chem.Rev.2004,104,4271.
(3)Padhi,A.K.;Nanjundaswamy,K.S.;Goodenough,J.B. J.Electrochem.Soc.1997,144,1188.
(4) Delacourt,C.;Poizot,P.;Morcrette,M.;Tarascon,J.M.; Masquelier,C.Chem.Mater.2004,16,93.
(5) Liao,X.Z.;Ma,Z.F.;Wang,L.;Zhang,X.M.;Jiang,Y.;He,Y. S.Electrochem.Solid-State Lett.2004,7,A552.
(6) Li,G.H.;Azuma,H.;Tohda,M.Electrochem.Solid-State Lett. 2002,5,A135.
(7) Delacourt,C.;Laffont.L.;Bouchet,R.;Wurm,C.;Leriche,J. B.;Morcrette,M.;Tarascon,J.M.;Masquelier,C. J.Electrochem.Soc.2005,152,A913.
(8) Kim,J.K.;Shin,C.R.;Ahn,J.H.;Matic,A.;Jacobsson,P. Electrochem.Commun.2011,13,1105.
(9)Yonemura,M.;Yamada,A.;Takei,Y.;Sonoyama,N.;Kanno,R. J.Electrochem.Soc.2004,151,A1352.
(10)Yamada,A.;Kudo,Y.;Liu,K.Y.J.Electrochem.Soc.2001, 148,A747.
(11) Zhang,Y.;Sun,C.S.;Zhou,Z.Electrochem.Commun.2009, 11,1183.
(12) Honma,T.;Nagamine,K.;Komatsu,T.Ceramics International 2010,36,1137.
(13) Hong,J.;Wang,F.;Wang,X.L.;Graetz,J.J.Power Sources 2011,196,3659.
(14)Oh,S.M.;Jung,H.G.;Yoon,C.S.;Myung,S.T.;Chen,Z.H.; Aminee,K.;Sun,Y.K.J.Power Sources 2011,196,6924.
(15) Burba,C.M.;Frech,R.J.Power Sources 2007,172,870.
(16) Jiang,T.;Pan,W.C.;Wang,J.;Bie,X.F.;Du,F.;Wei,Y.J.; Wang,C.Z.;Chen,G.Electrochim.Acta 2010,55,3864.
(17) Yin,S.C.;Strobel,P.S.;Grondey,H.;Nazar,L.F.Chem.Mater. 2004,16,1456.
(18)Wang,L.;Li,Z.C.;Xu,H.J.;Zhang,K.L.J.Phys.Chem.C 2008,112,308.
(19)Yang,M.R.;Ke,W.H.;Wu,S.H.J.Power Sources 2007,165, 646.
(20) Chen,X.J.;Cao,G.S.;Zhao,X.B.;Tu,J.P.;Zhu,T.J.J.Alloy. Compd.2008,463,385.
(21) Xiang,J.Y.;Tu,J.P.;Zhang,L.;Wang,X.L.;Zhou,Y.;Qiao,Y. Q.;Lu,Y.J.Power Sources 2010,195,8331.
(22) Zheng,J.C.;Li,X.H.;Wang,Z.X.;Niu,S.S.;Liu,D.R.;Wu, L.;Li,L.J.;Li,J.H.;Guo,H.J.J.Power Sources 2010,195, 2935.
(23)Ma,J.;Li,B.H.;Du,H.D.;Xu,C.J.;Kang,F.Y. J.Electrochem.Soc.2011,158,A26.
(24) Li,G.H.;Azuma,H.;Tohda,M.J.Electrochem.Soc.2002, 149,A743.
(25) Bini,M.;Ferrari,S.;Capsoni,D.;Massarotti,V.Electrochim. Acta 2011,56,2648.
July 20,2011;Revised:October 13,2011;Published on Web:October 19,2011.
Electrochemical Performance of Vanadium Modified LiFe0.5Mn0.5PO4/C Cathode Materials for Lithium-Ion Batteries
GONG Qiang WANG Hong LIAO Xiao-Zhen*MA Wei HE Yu-Shi MA Zi-Feng
(Institute of Electrochemical and Energy Technology,Department of Chemical Engineering,School of Chemistry and Chemical Engineering,Shanghai Jiao Tong University,Shanghai 200240,P.R.China)
Vanadium modified LiFe0.5Mn0.5PO4/C cathode materials with a nominal composition of (1-x)LiFe0.5Mn0.5PO4-xLi3V2(PO4)3/C(x=0,0.1,0.2,0.25,1)were prepared by a solid-state reaction using NH4VO3as the vanadium source.The electrochemical performance of the LiFe0.5Mn0.5PO4-based compounds improved upon vanadium modification.The 0.8LiFe0.5Mn0.5PO4-0.2Li3V2(PO4)3/C(LFMP-LVP/C) sample exhibited the highest discharge capacity of 141 mAh·g-1at 0.1C rate.X-ray diffraction analyses revealed a dual phase of the LFMP-LVP/C composite with the coexistence of an olivine-type LiFe0.5Mn0.5PO4/C phase and a NASICON-type Li3V2(PO4)3phase.Energy dispersive X-ray spectroscopy (EDS)analysis indicates a uniform distribution of Fe,Mn,V,and P in the composite.The electronic conductivity of LFMP-LVP was found to be 2.7×10-7S·cm-1,which is much higher than the value(1.9×10-8S·cm-1)of LiFe0.5Mn0.5PO4and similar to the value(2.3×10-7S·cm-1)of pure Li3V2(PO4)3.Vanadium modification remarkably reduced the electrode polarization of the LFMP-LVP/C cathode during the charge-discharge procedure.This suggests that vanadium modification is an effective method to improve the electrochemical performance of olivine-type cathode materials.
Lithium-ion battery;LiFe0.5Mn0.5PO4;Li3V2(PO4)3-modified;Cathode material
10.3866/PKU.WHXB201228100
*Corresponding author.Email:liaoxz@sjtu.edu.cn;Tel:+86-21-54744533;Fax:+86-21-54757717.
The project was supported by the National Natural Science Foundation of China(21073120,20773087,21006063)and Science and Technology Commission of Shanghai Municipality,China(09DZ1203603,10DZ1202702).
國家自然科學(xué)基金(21073120,20773087,21006063)和上海市科學(xué)技術(shù)委員會科技支撐項目(09DZ1203603,10DZ1202702)資助項目
O646;O649

