過(guò)充電保護(hù)添加劑1,4-二甲氧基苯的反應(yīng)機(jī)理
李田田 王朝陽(yáng) 邢麗丹 李偉善,2,3,* 彭 彬 許夢(mèng)清,2,3
顧鳳龍1,2,3 胡社軍1,2,3
(1華南師范大學(xué)化學(xué)與環(huán)境學(xué)院,廣州510006;2華南師范大學(xué),廣東高校電化學(xué)儲(chǔ)能與發(fā)電技術(shù)重點(diǎn)實(shí)驗(yàn)室,廣州510006; 3華南師范大學(xué),電化學(xué)儲(chǔ)能材料與技術(shù)教育部工程研究中心,廣州510006)
過(guò)充電保護(hù)添加劑1,4-二甲氧基苯的反應(yīng)機(jī)理
李田田1王朝陽(yáng)1邢麗丹1李偉善1,2,3,*彭 彬1許夢(mèng)清1,2,3
顧鳳龍1,2,3胡社軍1,2,3
(1華南師范大學(xué)化學(xué)與環(huán)境學(xué)院,廣州510006;2華南師范大學(xué),廣東高校電化學(xué)儲(chǔ)能與發(fā)電技術(shù)重點(diǎn)實(shí)驗(yàn)室,廣州510006;3華南師范大學(xué),電化學(xué)儲(chǔ)能材料與技術(shù)教育部工程研究中心,廣州510006)
采用密度泛函理論(B3LYP/6-311+G(d,p))和MP2/6-311+G(d,p)方法,研究鋰離子電池過(guò)充電保護(hù)添加劑1,4-二甲氧基苯(p-DMOB)的作用機(jī)理.計(jì)算結(jié)果表明,在過(guò)充時(shí),p-DMOB優(yōu)先于溶劑分子(乙基甲基碳酸酯、二甲基碳酸酯、碳酸乙酯)發(fā)生氧化反應(yīng).用B3LYP和MP2計(jì)算所得的p-DMOB理論氧化電位接近,分別為4.12和4.05 V(vs Li/Li+).p-DMOB氧化時(shí)首先失去一個(gè)電子,生成p-DMOB+●正離子自由基,用B3LYP和MP2方法計(jì)算所得的相應(yīng)能量變化分別為701.24和728.27 kJ·mol-1.失去電子后苯環(huán)的共軛性受到破壞,隨后p-DMOB+●苯環(huán)上的C―H鍵發(fā)生斷裂,失去H+并形成p-DMOB·自由基.用B3LYP和MP2方法計(jì)算所得的相應(yīng)能量變化分別為1349.78和1810.99 kJ·mol-1.p-DMOB·自由基很不穩(wěn)定,會(huì)在電極表面發(fā)生聚合反應(yīng)形成聚合物膜,用B3LYP和MP2方法計(jì)算所得的相應(yīng)能量變化分別為-553.37和-1331.20 kJ·mol-1.
鋰離子電池;過(guò)充電保護(hù)添加劑;1,4-二甲氧基苯;反應(yīng)機(jī)理;密度泛函理論
1 Introduction
Lithium ion battery(LIB)has been widely used to power portable electronic devices such as camera,mobile phone,and computer.It is also demonstrated to be promising for largescale applications such as hybrid or pure electric vehicles.1-6One of the important limitations for the application of LIB is safety concern.7,8It is well known that solvents in LIB tend to decompose on anode or cathode surface when LIB is overcharged,9-12and LIB becomes thermally unstable at overcharged state,which sometimes smokes,ignites,and even explodes.13,14Much effort has been devoted to improving the safety characteristics of LIB in recent years.The use of solid polymer or ionic liquid electrolytes provides a safe solution to the decomposition of solvents that have been used in commercial LIBs,but the ionic conductivity of these electrolytes is too low to be used in practice.Alternatively,various types of electrolyte additives,such as polymerizable compounds,have been proposed as additives in electrolyte to prevent the LIBs from overcharging.15,16Polymerizable compounds,also called“shutdown additives”,are found to be effective for the overcharge protection of LIB.These additives are electrochemically polymerized forming an insulating polymer layer that blocks charge current from flowing further when the LIB is overcharged.
It has been reported by several research groups that many derivatives based on 1,4-dimethoxy benzene(p-DMOB),the compounds in which the hydrogen atoms on the benzene ring of p-DMOB are replaced by electron-donating or electron-withdrawing groups,are functioned as redox shuttles for overcharge protection of LIB.17-21A LIB is protected from overcharge by a redox shuttle through the reduction of oxidized shuttle on anode and the oxidation of reduced shuttle on cathode of LIB.The reaction process for the p-DMOB based derivatives as redox shuttles has been understood through the theoretical calculations.22Different from its derivatives,however, p-DMOB was found to function as a shutdown additive.23The aim of this paper is to understand the reaction mechanism of p-DMOB as a shutdown additive for lithium ion battery.
2 Computational details
All calculations have been performed using Gaussian 03 package.24The geometries are optimized by the B3LYP and MP2 methods in conjunction with the 6-311+G(d,p)basis set.25,26The solvent effects are considered by employing the integral equation formalism of the polarizable continuum model (IEFPCM).27A dielectric constant of 31.3 is adopted,which is a weighted average value between the dielectric constants of ethylene carbonate(EC:89),dimethyl carbonate(DMC:3), and ethylmethyl carbonate(EMC:2)(nEC/nDMC/nEMC=1:1:1).To confirm each optimized stationary point and make zero-point energy(ZPE)corrections,frequency analyses are done with the same basis set.Enthalpy and Gibbs free energy are obtained at 298.2 K.Charge distribution is analyzed by the natural bond orbital(NBO)theory.
The absolute potential(φabs)for the oxidation of p-DMOB is obtained based on the following equation:28

where IP is ionization potential,ΔS is the entropy difference between p-DMOB and p-DMOB+●in gas phase,ΔG0Sis the Gibbs free energy change of p-DMOB or p-DMOB+●between gas phase and solvent,and F is Faraday constant.The oxidation potential,φ(p-DMOB),of the compounds in this paper is given with respect to φabs(Li/Li+)that is the absolute potential of Li/ Li+(1.4 V).
3 Results and discussion
3.1 Oxidation activity and oxidative potential
The optimized geometric structures of p-DMOB,ethyl methyl carbonate,dimethyl carbonate,ethylene carbonate,and their radical cations are presented in Fig.1.
Table 1 lists the frontier molecular orbital energy and ionization potential(IP)of p-DMOB and solvents in gas phase,optimized by B3LYP and MP2.On the basis of the molecular orbital theory,the ability of a molecule to lose one electron depends on the energy level of the highest occupied molecular orbital (HOMO).There exists difference in calculated values of HOMO or IP between B3LYP and MP2,but the same conclusion on the difference among p-DMOB,EMC,DMC,and EC can be drawn from these two methods,i.e.,p-DMOB is oxidized prior to the solvents.The preferable reduction oxidation of p-DMOB is important for p-DMOB to be used as a shutdown additive.
Table 2 lists the thermodynamic properties of p-DMOB and its radical cation(p-DMOB+●)in gas phase(g)and solvent(s). With these data,we can obtain the theoretical oxidation potential(Ecal)of p-DMOB,which is 4.12 V(vs Li/Li+)by B3LYP and 4.05 V(vs Li/Li+)by MP2,respectively.Both theoretical values are in good agreement with the experimentally obtained oxidation potential,which is 3.9 V(vs Li/Li+).20
3.2 Oxidative process of p-DMOB

Fig.1 Optimized geometric structures of p-DMOB,EMC,DMC,EC and their radical cations

Table 1 The frontier molecular orbital energy and IPof p-DMOB and solvents in gas phase
The initial oxidation of p-DMOB involves a one-electron transfer,resulting in radical cation(p-DMOB+●).The optimized geometry is presented in Fig.1.The charge distributions on atoms in p-DMOB and p-DMOB+●obtained by natural population analysis(NPA)are presented in Table 3.It can be seen from Table 3 that the charge distributions on atoms in p-DMOB and p-DMOB+●are quite different.The charge changes on―C6H4,―O11CH3,―O12CH3are-0.589e,-0.205e,-0.205e by B3LYP and-0.687e,-0.154e,-0.155e by MP2,respectively.The results obtained by both B3LYP and MP2 indicate that the electron mainly bereaves from benzene ring and the charge conjugation of benzene ring is damaged after the initial oxidation of p-DMOB.
Scheme 1 shows the possible pathways for the further oxidation.The p-DMOB+●loses a hydrogen ion forming a radical p-DMOB·with the cleavage of the C1―H7bond and p-DMOB· copolymerizes by itself forming a polymer.The ΔE1,ΔE2,and ΔE3are defined as the activation energies in the reactions p-DMOB-e→p-DMOB+●,p-DMOB+●-H+→p-DMOB·,and p-DMOB·+p-DMOB·→p-DMOB-1,respectively.The optimized geometry structures of p-DMOB and p-DMOB+●are shown in Fig.1,and the optimized geometry structures of p-DMOB·and p-DMOB-1 are presented in Fig.2.
The energies of compounds involved in the polymerization of p-DMOB are presented in Table 4.The energy variation of p-DMOB+●with the bond length of C1―H7is shown in Fig.3.It can be found from Fig.3 that the energy of p-DMOB+●increases with increasing the bond length.This suggests that there is no transition state available during the breaking of C1―C7bond.The obtained ΔE1,ΔE2,ΔE3values are 701.24,1349.78,-553.37 kJ·mol-1by B3LYP and 728.27,1810.99,-1331.20 kJ·mol-1by MP2,respectively.Although ΔE2is large,the sum of ΔE2and ΔE3is small,compared with ΔE1.Therefore,the polymerization is preferable and p-DMOB+●functions as a shutdown additive for the overcharge protection of LIB.
From the obtained results above,it can be concluded that p-DMOB is preferably to be oxidized forming a polymerthrough the breaking of C―H bond on benzene ring.Differently,the C―H bond on benzene ring in the radical cations formed from the one-electron oxidation of p-DMOB derivatives cannot be broken.Since the radical cations of p-DMOB derivatives are hard to oxidize further,they are preferably reduced on anode and turn back to the derivative molecules.22This is why p-DMOB functions as a shutdown additive but its derivatives as shuttle additives.

Table 2 Thermodynamic properties of p-DMOB and its radical cation(p-DMOB+●)in gas phase(g)and solvent(s)
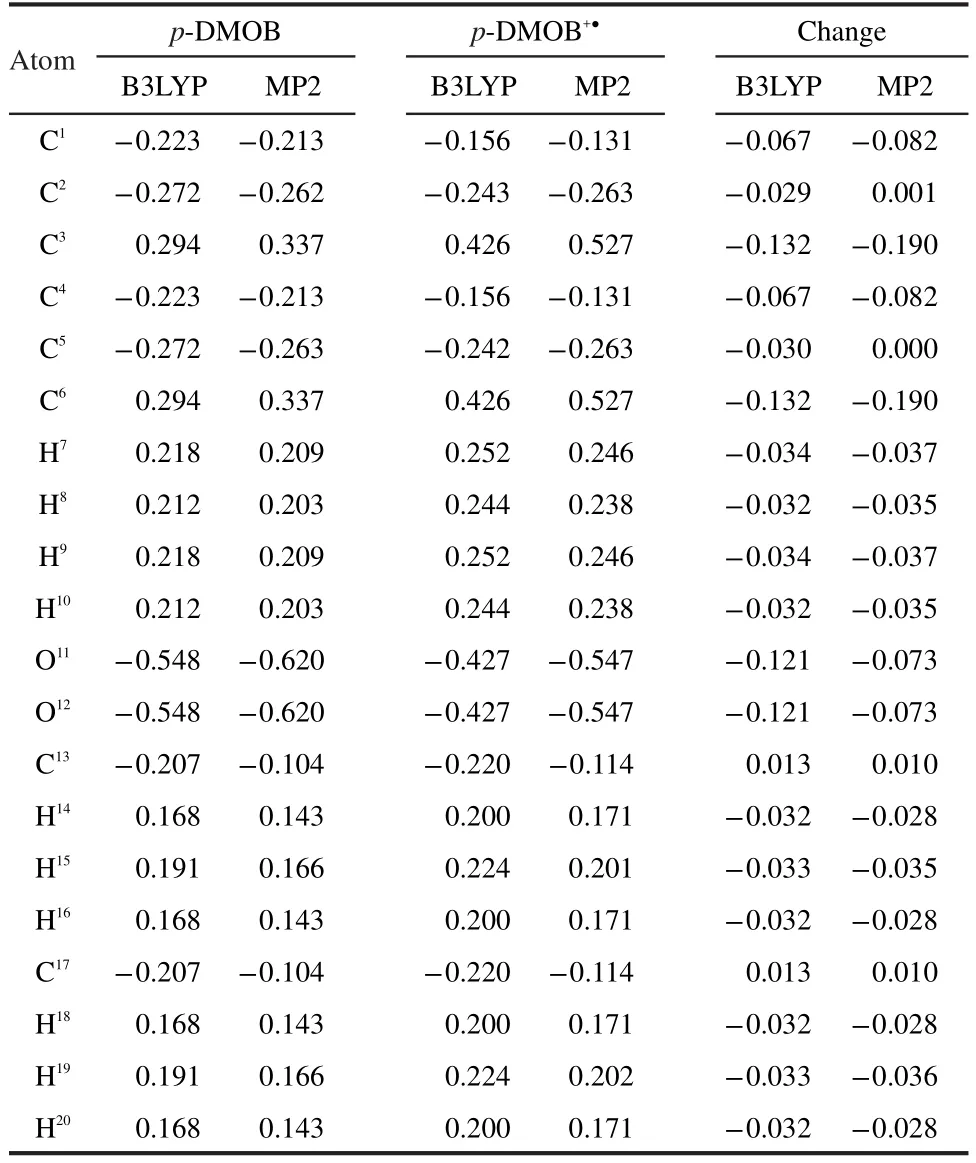
Table 3 Charge distribution(e)on atoms in p-DMOB and p-DMOB+●obtained by natural population analysis
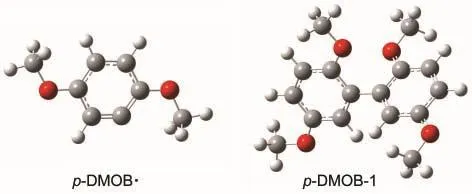
Fig.2 Optimized geometry structures of p-DMOB·and p-DMOB-1
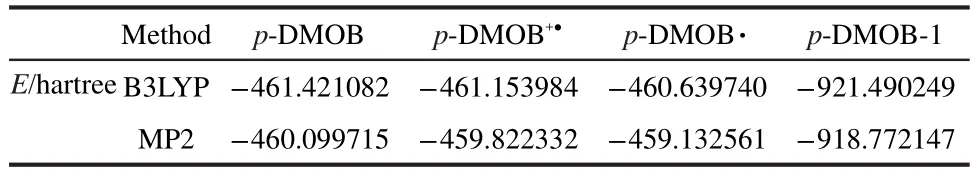
Table 4 Energy of compounds involved in the polymerization of p-DMOB
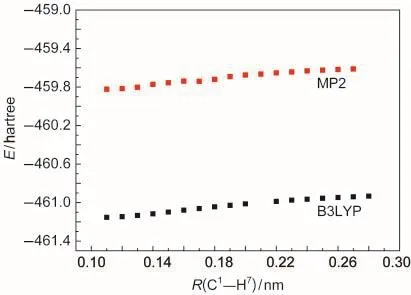
Fig.3 Energy variation of p-DMOB+●with the bond lengths of C1―H7
4 Conclusions
The reaction mechanism of p-DMOB as an overcharge protection additive for lithium ion battery was understood with the calculation based on B3LYP/6-311+G(d,p)and MP2/6-311+ G(d,p).Based on the calculated results,p-DMOB is oxidized prior to the solvents when the battery is overcharged.The initial oxidation of p-DMOB involves a one-electron transfer from p-DMOB resulting in a radical cation p-DMOB+●,the corresponding energy is 701.24 kJ·mol-1by B3LYP and 728.27 kJ·mol-1by MP2.The radical cation loses a hydrogen ion forming the radical p-DMOB·with the cleavage of the C―H bond on benzene ring and p-DMOB·copolymerizes forming a polymer.
(1)Ménard,L.;Fontès,G.;Astier,S.Energy Convers.Manage. 2010,327.
(2)Yu,Y.;Gu,L.;Zhu,C.;Aken,P.A.;Maier,J.J.Am.Chem.Soc. 2009,131,15984.
(3)Yao,Z.D.;Wei,W.;Wang,J.L.;Yang,J.;Nuli,Y.N.Acta Phys.-Chim.Sin.2011,27,1005. [姚真東,魏 巍,王久林,楊 軍,努麗燕娜.物理化學(xué)學(xué)報(bào),2011,27,1005.]
(4) Fergus,J.W.J.Power Sources 2010,195,939.
(5)Xu.J.;Yao,W.H.;Yao,Y.W.;Wang,Z.C.;Yang,Y.Acta Phys.-Chim.Sin.2009,25,201. [許 杰,姚萬(wàn)浩,姚宜穩(wěn),王周成,楊 勇.物理化學(xué)學(xué)報(bào),2009,25,201.]
(6)Xu,M.Q.;Zuo,X.X.;Li,W.S.;Zhou,H.J.;Liu,J.S.;Yuan, Z.Z.Acta Phys.-Chim.Sin.2006,22,335.[許夢(mèng)清,左曉希,李偉善,周豪杰,劉建生,袁中直.物理化學(xué)學(xué)報(bào),2006,22, 335.]
(7) Feng,J.K.;Ai,X.P.;Cao,Y.L.;Yang,H.X.Electrochem. Commun.2007,9,25.
(8)Chung,Y.S.;Yoo,S.H.;Kim,C.K.Ind.Eng.Chem.Res.2009, 48,4346.
(9) Matsuta,S.;Kato,Y.;Ota,T.;Kurokawa,H.;Yoshimura,S.; Fujitani,S.J.Electrochem.Soc.2001,148,A7.
(10) Moshkovich,M.;Cojocaru,M.;Gottlieb,H.E.;Aurbach,D. J.Electroanal.Chem.2001,497,84.
(11)Xing,L.D.;Wang,C.Y.;Li,W.S.;Xu,M.Q.;Meng,X.L.; Zhao,S.F.J.Phys.Chem.B 2009,113,5181.
(12)Xing,L.D.;Li,W.S.;Wang,C.Y.;Gu,F.L.;Xu,M.Q.;Tan, C.L.;Yi,J.J.Phys.Chem.B 2009,113,16596.
(13) Balakrishnan,P.G.;Ramesh,R.;Kumar,T.P.J.Power Sources 2006,155,401.
(14) Leising,R.A.;Palazzo,M.J.;Takeuchi,E.S.;Takeuchi,K.J. J.Power Sources 2001,97,681.
(15) Li,S.L.;Ai,X.P.;Feng,J.K.;Cao,Y.L.;Yang,H.X.J.Power Sources 2008,184,553.
(16)Xu,M.Q.;Xing,L.D.;Li,W.S.;Zuo,X.X.;Shu,D.;Li,G.L. J.Power Sources 2008,184,427.
(17) Chen,J.;Buhrmester,C.;Dahn,J.R.Electrochem.Solid-State Lett.2005,8,A59.
(18) Chen,Z.H.;Amine,K.Electrochim.Acta 2007,53,453.
(19)Taggougui,M.;Carré,B.;Willmann,P.;Lemordant,D. J.Power Sources 2007,174,1069.
(20)Wang,R.L.;Dahn,J.R.J.Electrochem.Soc.2006,153,A1922.
(21) Zheng,H.H.;Wang,X.J.;Li,B.;Qin,J.H.Chin.J.Power Sources 2006,30,511.[鄭洪河,王顯軍,李 苞,秦建華.電源技術(shù),2006,30,511.]
(22) Li,T.T.;Xing,L.D.;Li,W.S.;Peng,B.;Xu,M.Q.;Gu,F.L.; Hu,S.J.J.Phys.Chem.A 2011,115,4988.
(23) Demartinez,M.C.;Marquez,O.P.;Marquez,J.;Hahn,F.; Beden,B.;Crouigneau,P.;Rakotondrainibe,A.;Lamy,C.Syth. Met.1997,88,187.
(24) Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 03, Revision B.05;Gaussian Inc.:Pittsburgh,PA,2003.
(25)Abbotto,A.;Streitwieser,A.;Schleyer,P.R.J.Am.Chem.Soc. 1997,119,11255.
(26)Wang,Y.;Balbuena,P.B.J.Phys.Chem.A 2001,105,9972.
(27)Tomasi,J.;Mennucci,B.;Cammi,R.Chem.Rev.2005,105, 2999.
(28) Trasatti,S.Pure Appl.Chem.1986,58,955.
October 31,2011;Revised:January 3,2012;Published on Web:January 13,2012.
Reaction Mechanism of 1,4-Dimethoxy Benzene as an Overcharge Protection Additive
LI Tian-Tian1WANG Chao-Yang1XING Li-Dan1LI Wei-Shan1,2,3,*PENG Bin1
XU Meng-Qing1,2,3GU Feng-Long1,2,3HU She-Jun1,2,3
(1School of Chemistry and Environment,South China Normal University,Guangzhou 510006,P.R.China;2Key Laboratory of Electrochemical Technology on Energy Storage and Power Generation of Guangdong Higher Education Institutes,South China
Normal University,Guangzhou 510006,P.R.China;3Engineering Research Center of Materials and Technology for Electrochemical Energy Storage(Ministry of Education),South China Normal University,Guangzhou 510006,P.R.China)
The reaction mechanism of 1,4-dimethoxybenzene(p-DMOB)as an overcharge protection additive for lithium ion batteries was determined by theoretical calculation of density functional theory (DFT)at the level of B3LYP/6-311+G(d,p)and MP2/6-311+G(d,p).It was found that p-DMOB is oxidized prior to the solvents,ethyl methyl carbonate,dimethyl carbonate,and ethylene carbonate,when the lithium ion battery is overcharged.The calculated oxidative potentials of p-DMOB by B3LYP and MP2 methods are well in agreement at 4.12 and 4.05 V(vs Li/Li+),respectively.The initial oxidation of p-DMOB involves a one-electron transfer resulting in a radical cation p-DMOB+●.The corresponding energy variations were 701.24 and 728.27 kJ·mol-1from B3LYP and MP2 calculations,respectively.The p-DMOB+●species then loses one proton forming a radical p-DMOB·through the breaking of a C―H bond on the benzene ring, with the corresponding energy variations of 1349.78 and 1810.99 kJ·mol-1for B3LYP and MP2, respectively.The p-DMOB·species is unstable and copolymerizes forming an insulated polymer with the corresponding energy variations of-553.37 and-1331.20 kJ·mol-1for B3LYP and MP2,respectively.
Lithium ion battery;Overcharge protection additive;1,4-Dimethoxy benzene; Reaction mechanism;Density functional theory
10.3866/PKU.WHXB201201132
O646
?Corresponding author.Email:liwsh@scnu.edu.cn;Tel/Fax:+86-20-39310256.
The project was supported by the Joint Project of National Natural Science Foundation of China and Natural Science Foundation of Guangdong Province(U1134002)and Natural Science Foundation of Guangdong Province,China(10351063101000001).
國(guó)家自然科學(xué)基金-廣東省人民政府自然科學(xué)聯(lián)合基金(U1134002)和廣東省自然科學(xué)基金(10351063101000001)資助項(xiàng)目

