Coexistence of Oligonucleotide/Single-Chained Cationic Surfactant Vesicles with Precipitates
GUO Xia LI Hua GUO Rong
(School of Chemistry and Chemical Engineering,Yangzhou University,Yangzhou 225002,Jiangsu Province,P.R.China)
Coexistence of Oligonucleotide/Single-Chained Cationic Surfactant Vesicles with Precipitates
GUO Xia*LI Hua GUO Rong
(School of Chemistry and Chemical Engineering,Yangzhou University,Yangzhou 225002,Jiangsu Province,P.R.China)
It is well known that DNA(including oligonucleotide)and cationic surfactant can form insoluble complex.In this study,by turbidity measurement and TEM image,we found that the single-chained cationic surfactant could transform the oligonucleotide/single-chained cationic surfactant precipitates into vesicles and the vesicles coexist with the insoluble complex.The hydrophobic interaction between the cationic surfactant and the precipitates plays a key role in vesicle formation.Moreover,when the temperature reaches a specific value where the oligonucleotide begins to melt,the oligonucleotide/single-chained cationic surfactant vesicles form far easier.Thus,the more extended the oligonucleotide,the much easier for vesicle formation.As far as we know,the study about the oligonucleotide/cationic surfactant vesicle formation is very limited.Therefore,considering the growing importance and significance of DNA(including oligonucleotide)/amphiphile systems in medicine,biology,pharmaceutics,and chemistry,this study should provide some helpful information in further understanding these systems.
Surfactant;Self-assembly;Vesicle; Oligonucleotide;Transmission electron microscope
Surfactants have the ability to form organized assemblies such as micelles,vesicles,and lamellar structures in solution[1-4].DNA,including oligonucleotide,is a kind of important natural polyelectrolyte.So far,there have been many studies about the interactions between DNA and vesicles and/or cationic surfactants because of their biological and technological significances[5-13].It has been concluded that DNA can become compacted in the presence of cationic surfactant or positively charged vesicles[14-17].In DNA/cationic surfactant system,when the surfactant concentration is much lower than its critical micelle concentration (cmc)but reaches the critical aggregation concentration(cac), surfactant molecules can aggregate into micelle-like structure along the DNA chain[14-15],followed by precipitate formation[14,16-17]; and when the surfactant concentration is much higher than the cmc,DNA can induce micelles to elongate into rod-like structures[18].Moreover,depending on the composition of the complex, the insoluble DNA/cationic surfactant complex can exhibit hexagonal structure,cubic structure,and/or lamellar structure[18-21]and the DNA/vesicle complex can self-assemble into a condensed multilamellar mesophase and an inverted hexagonal phase[21-23].
Recently,we observed that in the single-chained cationic surfactant/oligonucleotide system,when the surfactant concentration was close to or higher than the cmc,all the precipitates could become soluble and the oligonucleotide/cationic surfactant vesicles could form[24].Furthermore,the facilitation efficiencyofoligonucleotideonvesicle formation depends on its size and base composition;the oligonucleotide with a bigger size or with a hairpin structure favors vesicle formation more[25].The increases in the size of the head group and/or the length of the alkyl group of surfactant decrease the facilitation efficiency of oligonucleotide[25].Since so far,there is very limited report about the vesicle formation in DNA/cationic surfactant solution,this study may be expected to increase the efficiency and applicability for DNA/amphiphile system.However,whether oligonucleotide/ cationic surfactant vesicles only form with surfactant concentration close to or higher than the cmc is left unknown.In the present paper,we will report that the oligonucleotide/cationic surfactant vesicles can form when the surfactant concentration is much lower than the cmc,i.e.,the oligonucleotide/cationic surfactant vesicles coexist with the precipitates.
1 Materials and methods
1.1 Materials
Dodecyl triethyl ammonium bromide(DEAB)was synthesized from dodecyl bromide and triethylamine and the crude product was recrystallized 5 times from acetone/ethanol.Its purity was examined and no surface tension minimum was found in the surface tension curve(1H NMR(600 MHz,D2O,25.0℃),δ:0.777 (t,3J(H,H)=7.2 Hz,3H;CH3),1.192(br,25H),1.275(br,2H), 1.575(br,2H;CH2),3.061(t,3J(H,H)=7.8 Hz,2H;CH2),3.201 (q,3J(H,H)=7.2 Hz,6H;3CH2)).Dodecyl trimethyl ammonium bromide(DTAB)andcetyltrimethylammoniumbromide(CTAB) were bought from Amresco Co.,USA (>99%purity).Oligonucleotides were purchased from Takara Co.(Dalian,China).Propidium iodide(PI)was bought from BD Biosciences(Pharmingen, USA).The water used is ultrapure prepared by Milli-Q system (Millipore Corporation,USA).
1.2 Sample preparation
The stock solutions of oligonucleotide and surfactant were prepared in water and the oligonucleotide concentration was determined from UV spectrum at 260 nm and expressed as nucleotide or phosphate(1 mol·L-1nucleotide or phosphate is ca 330 g·L-1).
The surfactant/oligonucleotide mixtures were prepared by adding water and the desired amounts of the stock solutions of surfactant and oligonucleotide successively in a test tube.
All the samples,except those used to determine the turbidity with varying temperature,were equilibrated for 1 h at experimental temperature before measurements.
1.3 Methods
Turbidity measurements were carried out using a Lambda 850 spectrophotometer(PerkinElmer,USA)at 514 nm at(25.0±0.1)℃or at varying temperatures.In the latter case,the temperature ranges from 5-28℃with an increment of 2℃and an equilibration time of 20 min at each temperature was used throughout the experiment.
The images of surfactant/oligonucleotide aggregates were observed with a transmission electron microscope(TECNAI 12, Philip Apparatus Co.,The Netherlands)by negative staining technique;the negative staining reagent was uranyl acetate aqueous solution.
Circular dichroism(CD)spectra were collected on a Jasco J-810 spectrometer at(25.0±0.1)℃.The path length of the quartz cuvette used was 1 cm and four scans were averaged.The step interval is 0.5 nm,the integration time 0.5 s,the bandwidth 1.0 nm,and the scanning rate 20 nm·min-1.The thermal melting analysis was monitored at 288 nm from 10.0 to 75.0℃(±0.1℃) at 2-3℃increments.An equilibration time of 15 min at each temperature was used throughout the melt.
Dynamic light scattering(DLS)measurements were performed withan ALV 5022 laser light-scattering instrument equipped with a 22 mW He-Ne laser at 632 nm(JDS model 1145P,Germany)in combination with an ALV-5000 digital correlator with a sampling time range from 1.0 μs to 100 ms.The scattering angle was 90°and the intensity autocorrelation functions were analyzed by using CONTIN method.The experimental temperature was 25.0℃.It should be noted here that before we measured the CD spectra and DLS plots for oligonucleotide/cationic surfactant systems,the samples were filtered through a filter paper with a pore size(bigger than 2 μm)to remove the precipitates.
All the experiments were repeated at least 3 times.
2 Results and discussion
2.1 Oligonucleotide/cationic surfactant vesicles coexist with precipitates
Just as described above,with the increase of the concentration of cationic surfactant,the oligonucleotide/cationic surfactant system is first clear(region A),then becomes turbid and the precipitates can be observed(region B),and finally,when the surfactant concentration is close to or higher than the cmc,all the precipitates become soluble(region C).Take oligo d(C)25/DEAB system as example.The DEAB concentrations in regions A,B,and C are lower than 0.1 mmol·L-1,between 0.1 and 10 mmol·L-1, and higher than 10 mmol·L-1,respectively[24].Fig.1 shows the tur-bidity of oligo d(C)25/DEAB system in region B,from which it can be seen that the readings become obvious and are increased linearly when DEAB concentration reaches 1 mmol·L-1.The monotonic increase in region B(Fig.1)is surprising and interesting because(i)the turbidity readings for a system with precipitates should be very unstable due to precipitation[26],(ii)when DEAB concentration is close to 10 mmol·L-1,the precipitates are very few,however,the turbidity under this condition is most obvious in region B,and(iii)after we remove the precipitates by centrifugation with an Eppendorf microcentrifuge at 5000 r· min-1for 30 min or by using a filter paper with a pore size larger than 2 μm,the turbidity is still obvious(although it becomes smaller).Therefore,the monotonic increase in region B(Fig.1) suggests that some aggregates other than precipitates should exist.
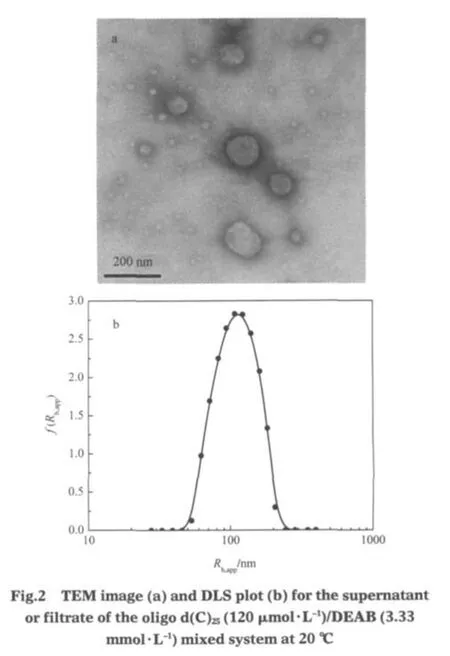
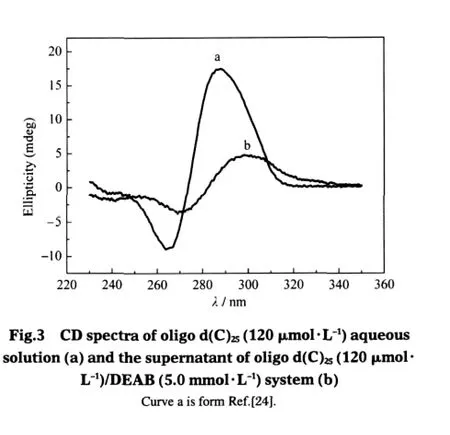
Fig.2 shows the TEM image and DLS plot of the supernatant (obtained by centrifugation)or the filtrate(obtained by filtering) of oligo d(C)25(120 μmol·L-1)/DEAB(3.33 mmol·L-1)system. Noaggregatescanbeobservedfor3.33mmol·L-1of DEAB aqueous solution since the cmc of DEAB is 16 mmol·L-1[24].However,in the presence of oligo d(C)25,vesicles can be seen(Fig.2a). The DLS plot(Fig.2b)indicates that the aggregates in the supernatant or the filtrate have an average hydrodynamic radius(Rh,app) of about 100 nm,coincident with the vesicle size from Fig.2a. Here,it should be mentioned that(1)when the DEAB concentration is lower than 1 mmol·L-1,although precipitates are observed, no vesicles can be found,and(2)vesicles are easily observed when all the precipitates become soluble(in region C,see Ref. [24]).Therefore,vesicles should be transformed from precipitates.This also explains why the turbidity in region B is most obvious(although the precipitates are very few)when DEAB concentration is close to 10 mmol·L-1.Fig.3 presents the CD spectra of the oligonucleotide aqueous solution(curve a)and of the filtrate of oligonucleotide/DEAB system(curve b).The smaller CD signal in curve b than that in curve a is reasonable since some of the oligonucleotide molecules exist in the precipitates. However,what should be noted is that compared with the case in water,when DEAB is present,both the positive and the negative Cotton effects for the oligonucleotide shift to longer wavelengths.The bathochromic shift of the Cotton effect suggests the oligonucleotide conformation should become more extended[24-25,27-29].

Moreover,the same phenomenon is also observed when we use oligo d(A)15and oligo d(C)15or change the cationic surfactant from DEAB to CTAB or DTAB.

2.2 Temperature effect on vesicle formation in region B
It is well known that temperature shows a great effect on DNA conformation.To better understand the vesicle formation, we measured the turbidity of oligonucleotide/cationic surfactant system in region B with temperature(exemplified by the case for oligo d(C)25/DEAB system,Fig.4).
From Fig.4,it can be seen that when the temperature is higher than 29℃,the turbidity is increased with temperature.Fig.5 further elucidates the TEM images of the supernatant or the filtrate of oligo d(C)25/DEAB system in region B at 37℃.By comparing with the case at 25℃(shown in Fig.2),it is easily seen that more vesicles can be found at higher temperature.
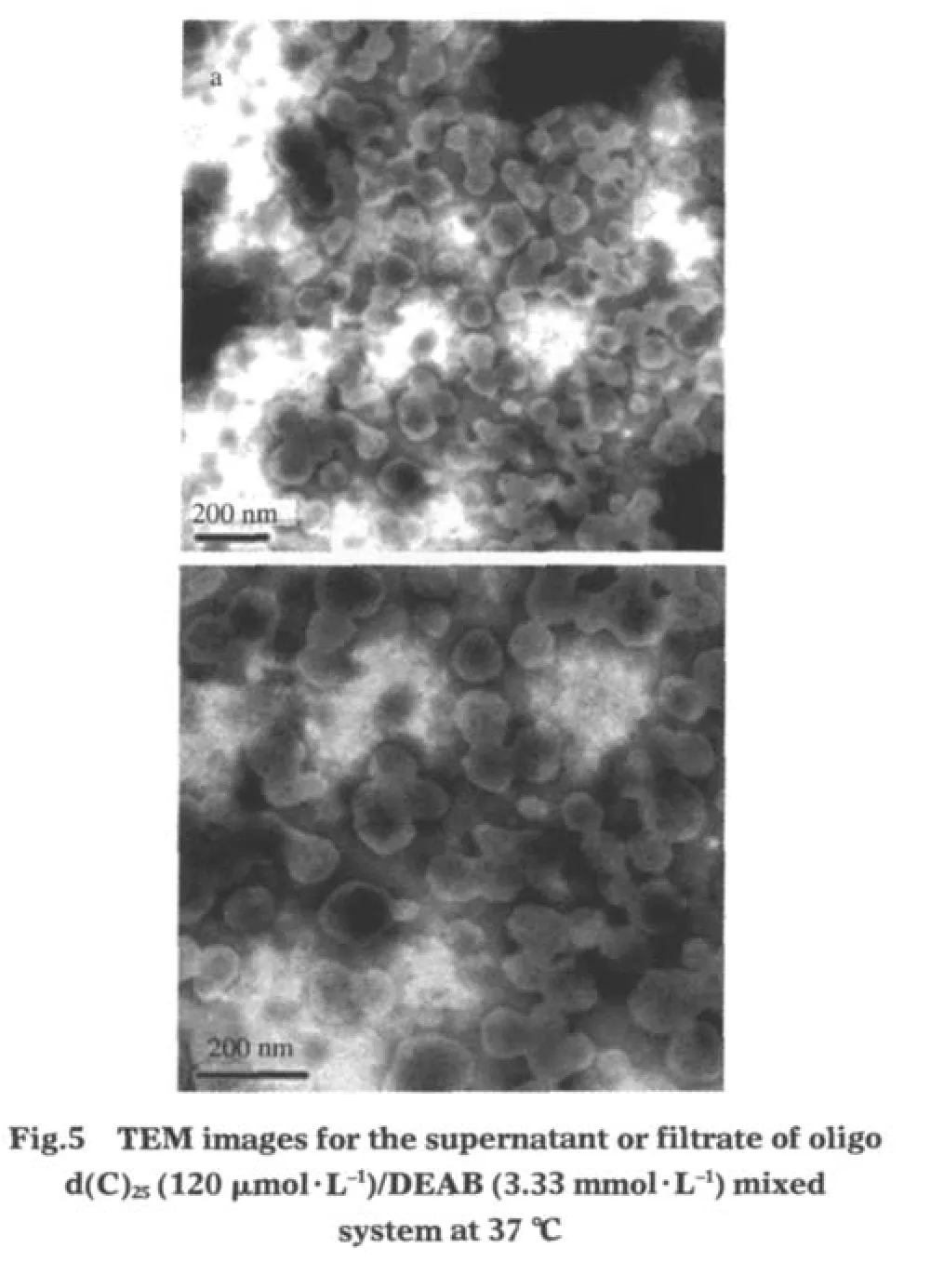
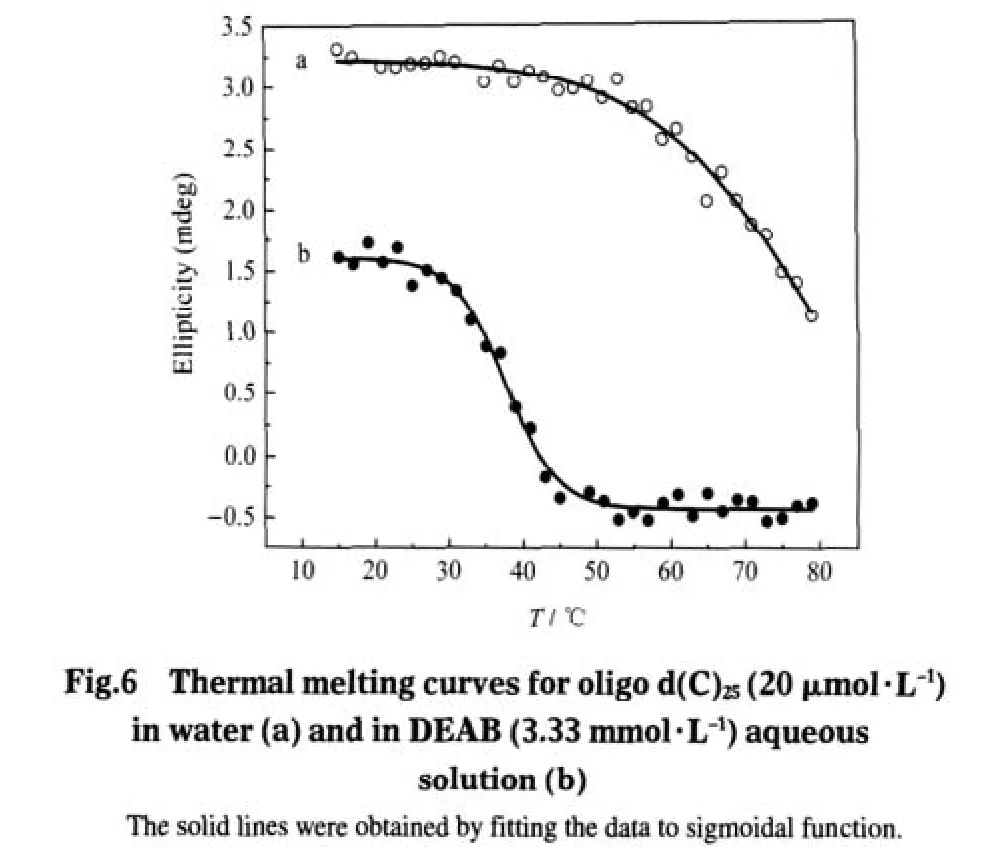
Fig.6 presents the CD melting curves for oligo d(C)25in water (curve a)and in DEAB aqueous solution(curve b).By comparing curve a with curve b,it can be seen that in DEAB aqueous solution,oligo d(C)25becomes melt at ca 29℃,which means that from 29℃on,oligo d(C)25begins to extend from random-coiled structure.By combining the results from Figs.2-6,it could be concluded that in DEAB aqueous solution,at the temperature higher than 29℃,oligonucleotide becomes less compacted and more efficient for vesicle formation.
It has been well illustrated that when the cationic surfactant concentration is lower than the cmc,DNA-surfactant complex is generally insoluble in water,with the alkyl chain of surfactant going out,and the hydrophobic interaction exists not only among the surfactant molecules but also between the bound surfactant and the hydrophobic DNA core[22,30-31].Fig.3 implies that the oligonucleotide should become more extended in the presence of cationic surfactant.With the addition of the cationic surfactant, the added surfactant could bind to the negatively charged elongated oligonucleotide chain to give more precipitates,or interact with the insoluble oligonucleotide/cationic surfactant complex driven by hydrophobic force.While a definite mechanism for vesicle formation is difficult to be drawn at present,the latter interaction should be attributable to the formation of bilayer membranous structure,which may result in vesicle formation.Figs.4 and 5 together further indicate that more extended oligonucleotide,much easier for vesicle formation,implying the important role of the hydrophobic interaction for vesicle formation since the bases are more exposed in the extended oligonucleotide.
3 Conclusions
With the increase of cationic surfactant concentration,the oligonucleotide/cationic surfactant precipitates could transform into vesicles due to the hydrophobic interaction between the added cationic surfactant and the precipitates.Moreover,more extended oligonucleotide,much easier for vesicle formation.Asfar as we know,this study reported for the first time that the oligonucleotide/cationic surfactant vesicles could be transformed from and coexist with the precipitates.Considering the structure and composition of DNA(including oligonucleotide)/amphiphile complex may play a key role in its application,such as in gene transfer,this study should be expected to provide helpful information for its efficient application.
1 Fendler,J.H.Membrane mimetic chemistry.New York:Wiley, 1982:110-125
2 Holowka,E.P.;Pochan,D.J.;Deming,T.J.J.Am.Chem.Soc., 2005,127:12423
4 Wang,Y.;Guo,X.;Guo,R.J.Colloid Interface Sci.,2008,317: 568
5 de Lima,M.C.P.;Simoes,S.;Pires,P.;Faneca,H.;Duzgunes,N. Adv.Drug Delivery Rev.,2001,47:277
6 Pontius,B.W.;Berg,P.Proc.Natl.Acad.Sci.U.S.A.,1991,88: 8237
7 Geck,P.;Nasz,I.Anal.Biochem.,1983,135:264
8 Allers,T.;Lichten,M.Nucleic Acids Research,2000,28:e6
9 McLoughlin,D.M.;O′Brien,J.;Canus,J.J.;Gorelov,A.V.; Dawson,K.A.Bioseparation,2000,9:307
10 Lander,R.J.;Winters,M.A.;Meacle,F.J.;Buckland,B.C.;Lee, A.L.Biotechnol.Bioeng.,2002,79:776
11 Bell,P.C.;Bergsma,M.;Dolbnya,I.P.;Brass,W.;Stuart,M.C. A.;Rowan,A.E.;Feiters,M.C.;Engberts,J.B.F.N.J.Am.Chem. Soc.,2003,125:1551
12 Vijayanathan,V.;Thoma,T.;Thomas,T.J.Biochemistry,2002, 41:14085
13 Mel′nikov,S.M.;Sergeyev,V.G.;Yoshikawa,K.J.Am.Chem. Soc.,1995,117:2401
14 Zhu,D.M.;Evans,R.K.Langmuir,2006,22:3735
15 Clamme,J.P.;Bernacchi,S.;Vuilleumier,C.;Duportail,G.;Mely, Y.Biochimica et Biophysica Acta,2000,1467:347
16 Mel′nikov,S.M.;Sergeyev,V.G.;Yoshikawa,K.;Takahashi,H.; Hatta,I.J.Chem.Phys.,1997,107:6917
17 Sergeyev,V.G.;Mikhailenko,S.V.;Pyshkina,O.A.;Yaminsky,I. V.;Yoshikawa,K.J.Am.Chem.Soc.,1999,121:1780
18 Ghirlando,R.;Wachtel,E.J.;Arad,T.;Minsky,A.Biochemistry, 1992,31:7110
19 Zhou,S.;Liang,D.;Burger,C.;Yeh,F.;Chu,B. Biomacromolecules,2004,5:1256
20 Krishnaswamy,R.;Mitra,P.;Raghunathan,V.A.;Sood,A.K. Europhys.Lett.,2003,62:357
21 Hsu,W.L.;Chen,H.L.;Liou,W.;Lin,H.K.;Liu,W.L. Langmuir,2005,21:9426
22 Karlsson,L.;van Eijk,M.C.P.;S?derman,O.J.Colloid Interface Sci.,2002,252:290
23 Pizzey,C.L.;Jewell,C.M.;Hays,M.E.;Lynn,D.M.;Abbott,C. L.J.Phys.Chem.B,2008,112:5849
24 Guo,X.;Li,H.;Zhang,F.M.;Zheng,S.Y.;Guo,R.J.Colloid Interface Sci.,2008,324:185
25 Guo,X.;Cui,B.;Li,H.;Gong,Z.;Guo,R.J.Polym.Sci.A,2009, 47:434
26 Spink,C.H.;Chaires,J.B.J.Am.Chem.Soc.,1997,119:10920
27 Zhang,Z.;Huang,W.;Tang,J.;Wang,E.;Dong,S.Biophys. Chem.,2002,97:7
28 Marck,C.;Thiele,D.Nucleic Acids Research,1978,5:1017
29 Ivanov,V.I.;Minchenkova,L.E.;Schyolkina,A.K.;Poletayev,A. I.Biopolymers,1973,12:89
30 Dias,R.S.;Magno,L.M.;Valente,A.J.M.;Das,D.;Prasanta,K.; Maiti,S.;Miguel,M.G.;Lindman,B.J.Phys.Chem.B,2008, 112:14446
31 Hayakawa,K.;Santerre,J.P.;Kwak,J.C.T.Biophys.Chem., 1983,17:175
寡聚核苷酸/單鏈陽離子表面活性劑囊泡與沉淀共存
郭 霞*李 華 郭 榮
(揚州大學化學化工學院,江蘇揚州 225002)
DNA(包括寡聚核苷酸)和陽離子表面活性劑可形成難溶復合物.本文通過濁度測試和透射電子顯微鏡觀察,發現單鏈陽離子表面活性劑可以誘使寡聚核苷酸/單鏈陽離子表面活性劑沉淀轉變成為寡聚核苷酸/單鏈陽離子表面活性劑囊泡,且寡聚核苷酸/單鏈陽離子表面活性劑囊泡可以與寡聚核苷酸/單鏈陽離子表面活性劑沉淀共存.在寡聚核苷酸/單鏈陽離子表面活性劑沉淀向囊泡的轉變過程中,表面活性劑和沉淀之間的疏水作用力發揮了重要作用.此外,當體系溫度達到寡聚核苷酸開始融解的溫度后,寡聚核苷酸/單鏈陽離子表面活性劑體系更容易形成囊泡.因此,寡聚核苷酸的鏈越伸展,越易于寡聚核苷酸/單鏈陽離子表面活性劑囊泡的生成.據我們所知,有關寡聚核苷酸/陽離子表面活性劑囊泡的報道尚不多見.因此,考慮到DNA(包括寡聚核苷酸)/兩親分子體系在醫學、生物學、藥學和化學中的重要性,該研究應該有助于我們進一步了解該體系并對其進行更合理有效的應用.
表面活性劑;自組裝;囊泡;寡聚核苷酸;透射電子顯微鏡
O648
Received:March 1,2010;Revised:May 5,2010;Published on Web:June 25,2010.
*Corresponding author.Email:guoxia@yzu.edu.cn;Tel:+86-514-87975590-9513;Fax:+86-514-87975244.The project was supported by the National Natural Science Foundation of China(20603031).
國家自然科學基金(20603031)資助項目
?Editorial office of Acta Physico-Chimica Sinica

